Abstract
Aim
To compare the blood glucose-lowering effect of a highly viscous fiber blend (VFB) added to a starchy snack on postprandial glycemia between healthy participants and participants with diabetes mellitus.
Methods
Ten healthy participants (4 men and 6 women, aged 28 ± 2.6 years, body mass index [BMI], 24.3 ± 0.8 kg/m2) and 9 participants with diabetes mellitus type 2 (3 men and 6 women, aged 68 ± 3.8 years, BMI 28.8 ± 1.2 kg/m2) on four separate occasions took either 50 g available carbohydrates as control biscuits, biscuits with 10 g of highly viscous fiber blend, white bread with 12 g of margarine, or white bread alone. Postprandial blood glucose response, glycemic index (GI), and palatability were determined.
Results
Mean (95% confidence interval) GI values of the viscous fiber blend biscuits were 26 (16-36) and 37 (27-47) GI units for healthy participants and participants with diabetes mellitus, respectively. These values were significantly lower than those of white bread, white bread with 12 g of margarine, and control biscuits (P < 0.001, paired t test) both in healthy participants (GI 100, 108 [57-159], and 101 [44-158], respectively) and participants with diabetes mellitus (GI 100, 103 [79-127], and 94 [78-110], respectively). Viscous fiber blend significantly reduced the glycemic index by 74% (7.4 GI units/g of fiber) in healthy participants and by 63% (6.3 GI units/g of fiber) in participants with diabetes. The GI did not differ between control meals in both healthy participants and participants with diabetes. There were no significant differences in palatability among the types of meals, although participants with diabetes found the viscous fiber blend biscuits more palatable (P = 0.002, t test).
Conclusion
Viscous fiber blend is a very potent and palatable soluble fiber addition to a starchy snack, which is able to reduce the glycemic response to a similar extent in both healthy participants and individuals with diabetes mellitus. Biscuits with low GI, and possibly other viscous fiber blend fortified starchy foods, may potentially be a useful replacement of high GI snack foods in the diet.
ClinicalTrials.gov trial registration number
Despite significant achievements in treatment modalities and preventive measures, the incidence of diabetes mellitus has been exponentially increasing (1). Reduction in both fasting and prolonged postprandial glycemia is of paramount importance in the disease prevention and the delay of diabetic complications (2-5). Blood glucose concentration can be reduced by dietary means and may be influenced by factors such as type and amount of carbohydrate, nature of starch, quantity of protein and fat, dietary fiber content, method of food processing, particle size, and food form (2). Foods with a low glycemic index, such as beans, lentils, and chickpeas, as opposed to highly refined foods with high glycemic index, including white bread, biscuits, or breakfast cereals, will slow absorption of glucose from the diet. Prospective studies have shown that people consuming diets with a lower glycemic index are less likely to develop diabetes compared with those with a high glycemic index diets (6,7). Glycemic index is a measure of the blood glucose-raising ability of the available carbohydrate in foods and is proposed as an additional tool in the management of diabetes (2). Although evidence is not conclusive (8) and individual differences occur, many prospective studies and clinical trials have shown that low-glycemic index diets may reduce the risk of developing diabetes and cardiovascular disease, improve glycemic control in diabetes, increase insulin sensitivity, and regulate body weight (3-5). In some countries, including the United Kingdom and Australia, nutritional advice for people with diabetes specifically promotes the consumption of foods with a low glycemic index (9). The World Health Organization concluded that low glycemic index diets are a possible factor in helping to reduce the risk of type 2 diabetes mellitus and obesity (10). High postprandial plasma glucose level can increase severity of diabetes mellitus (3) and it is generally agreed that the foods which do not raise the blood glucose level greatly, at least for a given carbohydrate content, are most suitable for individuals with type 2 diabetes mellitus (8). Lower postprandial glycemia or consumption of low glycemic index foods may also be of benefit for healthy individuals.
Similarly, high fiber diets have been recommended for the general population (11) and for the nutritional management of patients with type 2 diabetes mellitus (8). Soluble dietary fiber retards digestion and absorption of the associated dietary carbohydrate, thus flattening the postprandial rise in plasma glucose and insulin concentrations (12). Some foods, like beta-glucan fiber-containing oats and barley and soluble fiber isolates such as pectin, guar, psyllium, or glucomannan, added to starchy foods, due to a high level of viscosity, have the greatest blood glucose- or glycemic index-lowering effect (13). Fiber itself does not have a glycemic index value as it does not contain any available carbohydrate, but the addition of fiber to carbohydrate-containing foods can affect the glycemic index of the food. To quantify this effect, the measuring of Glycemic Reduction Index Potential (GRIP) has been proposed. GRIP predicts the reduction in glycemic index units/gram of fiber, which is to be expected when fiber is added to a food (14). For example, the GRIP of beta-glucan fiber from oats was found to be 4, ie, 1 g of added beta-glucan will reduce the glycemic index of various starchy foods by 4 glycemic index units (14).
The highly viscous fiber blend added to the studied biscuit formulation is a blend of complementary highly viscous soluble fibers (polysaccharides) that act synergistically to develop an induced viscosity that is several times higher than the viscosity of any other known fiber in nature, including viscosity of the individual fibers in the viscous fiber blend formulation. One of the main components of the viscous fiber blend is glucomannan, a glucose-mannose polysaccharide obtained by grinding the tuber root of Amorphophallus Konjac C. Koch, a plant that has been used as food and remedy for thousands of years in the Far East. Highly refined glucomannan is approximately 3 times more viscous than guar and approximately 7 times more viscous than psyllium or pectin. The viscosity of the viscous fiber blend is further amplified by the combination of soluble fibers to a viscosity 3-5 times higher than glucomannan alone (15). Earlier research has shown that the higher viscosity in vitro directly corresponded to lower blood glucose (15,16). Supplementation of the diet with viscous fiber blend demonstrated reductions in blood lipids, systolic blood pressure, and improvements in long-term blood glucose regulation in type 2 diabetes mellitus and metabolic syndrome participants (17,18). Although these results were encouraging, they were confined to a research setting only, as viscous fiber supplements were not readily available and translation into practical applications was therefore limited. However, recently interest in supplementation has re-emerged as commercially produced, viscous fiber products are again becoming available. Whether this particular viscous fiber blend could potentially be beneficial for others besides those with diabetes is unknown.
Twenty years ago we conducted a study with the aim to investigate whether viscous fiber blend incorporated into a common snack food would reduce the postprandial blood glucose response equally in healthy participants and individuals with type 2 diabetes mellitus. This question seems especially relevant today in light of the ever increasing burden of diabetes worldwide (1) and where readily available, high-fiber, low-glycemic index snack foods may therefore be a useful addition to the armamentarium for the treatment and prevention of disease.
Subjects and methods
Subjects
Two groups of experiments were conducted: in healthy participants and participants with type 2 diabetes mellitus. The two groups were selected as examples of extremes in glucose tolerance status, ie, one group included healthy, young individuals with efficient glucose metabolism as opposed to the other group with individuals who were older, overweight or obese and with severely impaired glucose tolerance status. The study took place in 1989 at the Diabetes Day Care Unit of St. Michael’s Hospital, Toronto, Canada. Subjects were recruited from an existing database of individuals interested in participating in nutritional research and by advertisement. The group of healthy participants included four men and six non-pregnant women aged (mean ± SEM) 28 ± 2.6 years, with a body mass index (BMI) of 24.3 ± 0.8 kg/m2. Individuals with BMI>30 kg/m2, known history of kidney disease, hepatitis, diabetes mellitus, gastrointestinal disease or a heart disease, participants using medications or fiber supplements, or those who could not comply with the experimental procedures were excluded. The other group included three men and six non-pregnant women with documented type 2 diabetes mellitus of at least 6-month duration without clinically manifest complications, aged 68 ± 3.8 years, with BMI 28.8 ± 1.2 kg/m2. Individuals with liver or kidney disease, BMI>30 kg/m2, gastrointestinal problems, using fiber supplements, or those who could not comply with the experimental procedures were excluded. Six individuals with diabetes were taking insulin and three were controlled with oral agents.
The number of participants in this study provides a reasonable degree of power and precision for most purposes of measuring glycemic index (19), which was the primary outcome of this study. The study was approved by the Human Subjects Review Committee of the University of Toronto and written informed consent was obtained from all participants before the beginning of the study.
Study design
The study used a randomized, controlled, single-blind, four-arm crossover design in which participants acted as their own controls within each group. Subjects in both groups each underwent four treatments on separate days with at least three days between consecutive testing sessions. On each test day, participants came to the Day Care Unit at St Michael’s Hospital in the morning after a 12-hour overnight fast. The volunteers with type 2 diabetes mellitus took their normal morning insulin dose or oral agents approximately 5-10 minutes before eating. After being weighed and having a fasting blood sample obtained by finger-prick, participants consumed a test meal over 10-15 minutes with 200 or 400 mL tea or coffee containing 30 or 60 mL of 2% butterfat milk, depending on preferences. The type and amount of the beverage was kept constant for each participant. Further blood samples were obtained at 15, 30, 45, 60, and 90 minutes after the start of the meal in healthy volunteers and at 30-minute intervals for three hours in participants with diabetes. The difference in timing of the blood samples is a reflection of the time for the blood glucose levels to return to baseline in the two populations and is based on the typical glycemic index protocol described previously (2,14). After the last blood sample was obtained, the participants were offered a snack and then permitted to leave.
Test meals
The test meals consisted of portions of the test food (viscous fiber blend biscuit containing 9.9 g of the viscous fiber blend) or control foods containing 50 g available carbohydrate (defined as total carbohydrate minus dietary fiber). Viscosity of the viscous fiber blend was estimated to be 700 poise, as measured with a viscometer (Brookfield R.V.T. viscometer; DW Brookfield Ltd, Cooksville, Canada) at a concentration of 1% and a shear rate of 1/30 seconds with a spindle type “F.” The total fiber content of viscous fiber blend is 95% (approximately 70% glucomannan and 30% xanthan), of which 98% is water soluble. Viscous fiber blend is a proprietary fiber blend (Vuksan’s US patent 7 326 404), and the prototype of the commercially available viscous fiber blend called PGX® (PolyGlycopleX, InovoBiologic Inc., Calgary, Canada). The control foods included control biscuits, which were identical to the test biscuits except that they did not contain any viscous fiber blend, standard white bread plus 11.9 g of margarine, and on three occasions, standard white bread alone. The white bread with 12 g of margarine test meal was added as a positive control to address the possible effect on postprandial glycemia by the additional fat in the test cookies compared with white bread alone. Test and control biscuits were produced by Dicofarm Spa (Roma, Italy). White bread was used for glycemic index calculations. The bread was prepared in a bread maker in the experimental kitchen of the nutrition department, such that each bread portion contained 66.8 g white flour (Monarch all purpose flour, Maple Leaf Mills, Toronto, Canada).
The portion sizes were calculated based on the results of macronutrient analysis. The macronutrient content of the test and control foods (Table 1) was determined by standard methods (20).
Table 1.
Weight and macronutrient profiles of test foods
| Parameter | Control white bread | White bread and margarine | Control biscuits | Viscous fiber blend biscuits |
|---|---|---|---|---|
| Portion weight (g) | 105 | 116 | 69 | 82 |
| Energy (kcal) | 237 | 324 | 310 | 315 |
| Total carbohydrate (g) | 52.6 | 52.6 | 51.8 | 63.1 |
| Available carbohydrate (g) | 50.0 | 50.0 | 50.0 | 50.0 |
| Dietary fiber (g) | 2.5 | 2.5 | 1.7 | 11.6 |
| Fat (g) | 0.4 | 10.0 | 10.2 | 10.9 |
| Protein (g) | 8.3 | 8.4 | 4.5 | 4.3 |
Palatability of each meal was assessed at 30 minutes using a bi-polar 7 point ordinal scale from -3 (unpleasant) to +3 (very desirable), with 0 being neutral (21). Therefore, the higher the number, the higher is the perceived palatability of the product.
Blood glucose analysis
Finger-prick blood samples (2-3 drops each) were collected into fluoro-citrate tubes. Blood was immediately stored at -20°C before analysis. Analysis for glucose was conducted within three days from collection by the glucose oxidase method (22) using YSI analyzer model 27 (Yellow Springs Instruments, Yellow Springs, OH, USA).
Statistical analysis
The primary outcome measured was the glycemic index. The glycemic index was calculated by expressing each participant’s glucose incremental area under the curve (AUC) for the test food as a percentage of the same participant’s average response after reference white bread (19). Incremental AUC for blood glucose were calculated geometrically using the trapezoid rule and ignoring the area beneath the baseline. Results are reported as mean ± standard error of the mean (SEM) or 95% confidence intervals (CI). The significance of differences was calculated using a paired t test within the groups and t test between the groups. Differences were considered significant at P < 0.05. In addition, the GRIP of the fiber was calculated for both subject groups.
Results
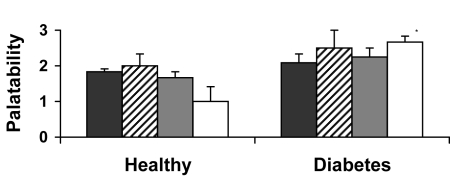
Generally, all test meals were well accepted (Figure 1). No differences in palatability between the types of meals were significant within each subject group. Subjects with diabetes found the viscous fiber blend biscuits more palatable than did the healthy individuals (P = 0.002, t test).
Figure 1.
Palatability of the viscous fiber blend biscuits and control meals using a bi-polar 7-point ordinal scale ranging from -3 to +3 (21). Data are expressed as mean ± standard error of the mean. *P < 0.05 (t test, compared with healthy participants). Closed bars – control white bread biscuits; striped bars – control white bread and margarine biscuits, gray bars – control biscuits; open bars – fiber blend biscuits.
No significant differences were found between mean fasting blood glucose values before any of the test meals. The average values in healthy individuals were 4.4 (95% CI, 4.2-4.6) mmol/L for white bread, 4.5 (95% CI, 4.1-4.9) mmol/L for viscous fiber blend, 4.4 (95% CI, 4.0-4.8) mmol/L for control biscuits, 4.4 (95% CI, 4.2-4.6) mmol/L for white bread with 12 g of margarine (P from 0.635 to 0.943). In participants with diabetes, the average values were 9.8 (95% CI, 8.2-11.4) mmol/L for white bread, 10.8 (95% CI, 8.6-13.0) mmol/L for viscous fiber blend, 9.6 (95% CI, 8.0-11.2) mmol/L for control biscuits, and 10.1 (95% CI, 8.3-11.9) mmol/L for white bread with 12 g of margarine (P from 0.402 to 0.862).
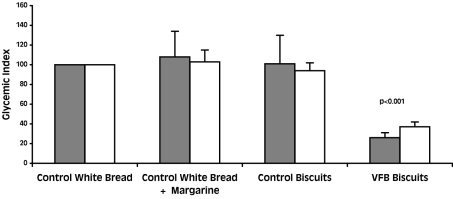
Mean glycemic index values of the viscous fiber blend biscuits were 26 (95% CI, 16-36) and 37 (95% CI, 27-47) for healthy participants and participants with diabetes, respectively. These values were significantly lower than for the white bread, white bread with 12 g of margarine, and control biscuits (P < 0.001, paired t test) both in healthy participants and participants with diabetes (Figure 2). The glycemic index of control biscuits and white bread with 12 g of margarine was not different from white bread both in healthy individuals (108 [95% CI, 57-159] for white bread with 12 g of margarine and 101 [95% CI, 44-158] for control biscuits) and type 2 diabetes mellitus (103 [95% CI, 79-127] for white bread with 12 g of margarine and 94 [95% CI, 78-110] for control biscuits) indicating that fat, at the level found in the biscuits, had no effect on the glycemic response.
Figure 2.
Glycemic index of the viscous fiber blend (VFB) biscuits and control meals in control subjects (closed bars) and patients with diabetes (open bars). Data are expressed as mean ± standard error of the mean. P value was calculated using paired t test (compared with control white bread).
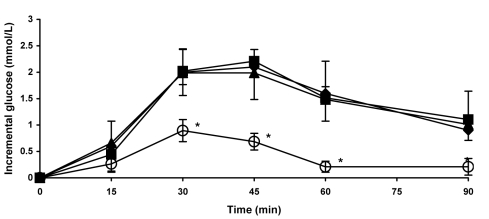
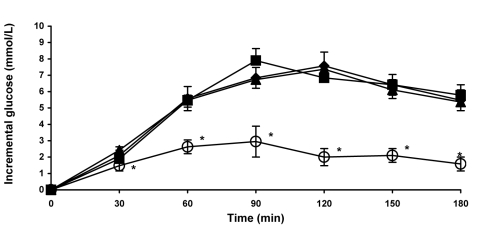
The blood glucose response to viscous fiber blend was significantly lower than to white bread in both healthy volunteers (Figure 3) and participants with diabetes (Figure 4). Differences were significant at 30, 45, 60, and 90 minutes in healthy participants (Figure 3) and at 30, 60, 90, 120, 150, and 180 minutes in participants with diabetes (Figure 4). There were no differences in blood glucose response between white bread, white bread with 12 g of margarine, and control biscuits in either group.
Figure 3.
Postprandial blood glucose response to the viscous fiber blend (VFB) biscuits and control meals in healthy participants. Data are expressed as mean ± standard error of the mean. Rhombs – control white bread; squares – control white bread plus margarine; triangles – control biscuits: circles – VFB biscuits. *P < 0.05 (compared with control white bread). P value calculated using paired t test.
Figure 4.
Postprandial blood glucose response to the viscous fiber blend (VFB) biscuits and control meals in participants with type 2 diabetes mellitus. Data are expressed as mean ± standard error of the mean. Rhombs – control white bread; squares – control white bread plus margarine; triangles – control biscuits: circles – VFB biscuits. *P < 0.05 (compared to control white bread). P value calculated using paired t test.
Discussion
Our study demonstrated that the highly viscous fiber blend added to biscuits had 2-fold benefits to its consumers. It increased dietary fiber intake and reduced the glycemic index value of the snack to a similar extent in both healthy and diabetic participants. Viscous fiber blend reduced the glycemic index by 74% in healthy participants and by 63% in participants with diabetes, which corresponds to a GRIP of 7.4 and 6.3 glycemic index units/g of fiber, respectively, compared with white bread control. This is a considerably stronger effect on a gram basis than has been reported for other soluble fibers such as psyllium, glucomannan alone, beta-glucan, guar, pectin, and flax (14,23-26). These findings are similar to our results from another study where GRIP of the novel viscous polysaccharide, PGX® was determined when added to white bread.
Using the classification of Brand-Miller (27), the viscous fiber blend enriched biscuits could be classified as a “low glycemic index” food (glycemic index ≤55), while the control biscuits are classified as “high glycemic index,” even though they are identical in composition except that control biscuits do not contain viscous fiber blend.
One of the limitations of our study is the date the study was carried out. The population characteristics may have changed since 1989, however, one could speculate that the shift would have been mainly in the healthy group, which may now be heavier and some participants would perhaps have impaired glucose tolerance status. If anything, this would have made the groups more similar. The strength of the study lies in the fact that the characteristics of the two subjects groups are so different. The two subject groups were selected to reflect individuals with very different glucose tolerance status to assess applicability of the viscous fibers blend in different population groups. This study, therefore, demonstrates that despite the difference in age, body weight, and glucose tolerance status between the two subject groups, the blood glucose-lowering effect was comparable, indicating that the viscous fiber blend enriched snack can be used universally. Not all studies have reported similarity in blood glucose lowering-effect of low glycemic index foods between healthy participants and participants with diabetes, but some have (28,29). Exaggerated postprandial glycemia puts healthy individuals at greater risk of developing diabetes and cardiovascular disease. In large prospective observational studies, higher 2-hour postprandial blood glucose predicted cardiovascular mortality and morbidity in individuals without diabetes (30). These findings imply that the glycemic nature of dietary carbohydrates is also relevant for healthy participants.
Many clinical studies have evaluated the acute effects of supplemental fiber on glycemic control (31). Oat beta-glucan, a well recognized cardio-protective food, decreases the glycemic index of different carbohydrate foods in type 2 diabetes mellitus on average by 4 glycemic index units per gram of fiber (14), which is considerably less than GRIP of 7 per gram of viscous fiber blend added in this study. In another study, purified glucomannan (1 g), taken before performing the oral glucose tolerance test (OGTT), has been shown to lower the rise of blood glucose from 1 to 2 hours by modest 10% in comparison with the placebo (25). Enzymatically induced guar gum (5 g) caused a 20% reduction in a relative glycemic response in 3-hour meal glucose tolerance tests in nondiabetic participants (24), and peak blood glucose values as well as area under the curve were improved by ingestion of flax fiber (15 g) in healthy participants (26). However, not all soluble fibers can affect postprandial glucose. It has been shown that in healthy adults, agar and pectin have no impact on the postprandial glucose response, although they can delay gastric emptying (23).
There is no sufficient information to conclude that low-glycemic load diets reduce the risk for diabetes, but low-glycemic index foods that are rich in fiber and other important nutrients are to be encouraged (8). A meta-analysis of randomized controlled trials (4) has shown that choosing low-glycemic index foods in place of conventional or high-glycemic index foods has a clinically useful effect on medium-term glycemic control in patients with diabetes, with the incremental reduction of glycated proteins by 7.4%, a benefit which is similar to pharmacological agents, such as Acarbose. The larger the divergence of glucose metabolism from the norm, the larger the effect of lower glycemic index and glycemic load interventions (32). A systematic review of cohort studies (33) demonstrated that consumption of dietary fiber and grain was inversely correlated and glycemic index and glycemic load were positively correlated with the incidence of type 2 diabetes mellitus. Furthermore, it has been shown that a low-glycemic index diet can alter beta cell function, lipid profile, and hypercoagulability (34). In addition, low glycemic meals can promote a postprandial metabolic milieu that is favorable for reduced food consumption, which may be advantageous in the control of obesity and insulin resistance (35).
Among the various classes of processed starchy foods, wheat-based products exhibit a wide range of glycemic and insulinemic responses. Garsetti et al (36) showed that the glycemic index and insulinemic index of plain sweet biscuits were correlated with in vitro starch digestibility and are dependent on the type of processing. However, it has also been shown that it is not the processing but rather the addition of fiber that has an evident effect on the glycemic index of biscuits (37). The processing techniques used to manufacture the biscuits for our study did not have a significant effect on their glycemic response, as indicated by the similar glycemic index values for bread with margarine and the control biscuits. Thus, processing was unlikely to contribute to the low glycemic responses of the viscous fiber blend enriched biscuits, as shown by some other authors (38).
Soluble dietary fibers have been shown to alter food texture, structure, and viscosity, and, therefore, the rate of starch degradation and digestion (39), which is related to the regulation of postprandial glucose levels. It has been shown that viscous fibers, as a result of their rheological properties, or high viscosity, form a gel with the food and human digesta and consequently reduce peaks in postprandial plasma glucose concentrations in both healthy and diabetic participants, in positive relation to their level of viscosity (15,16,40,41). Insoluble fibers, such as cellulose and wheat bran, have little effect (42). Viscous fiber exerts its effect by delaying digestion and absorption of glucose to the blood (43). In addition, the rate of diffusion of glucose from glucose/fiber mixtures in vitro is proportional to the viscosity of the fiber solution (13). Thus, it is to be expected that fibers in a higher-viscosity blend would have a marked blood glucose-lowering effect.
Dietary fat has been shown to reduce postprandial glycemia; this effect has been suggested to be due to either delayed gastric emptying (43) or the “ileal brake” mechanism (44). However, the amount of fat required to achieve reduced gastrointestinal motility is large, approximately 50% of calories or more. As the fat content of the biscuits was less than that, fat is unlikely to have contributed to the low glycemic response of the viscous fiber blend-enriched biscuits. This is confirmed by the lack of differences between the glycemic response to white bread and to white bread with 12 g of margarine or the control biscuits. Thus, it is likely that the low glycemic index of the viscous fiber blend biscuits was due to the formation of a complex physical link between starch and viscous fiber blend added (45).
Current dietary recommendations for type 2 diabetes mellitus (8) emphasize increasing the carbohydrate content of the diet to 50% of energy, dietary fiber to over 25 g/d, and reducing fat to 30%. It is also recognized that sucrose, in moderate amounts, is not necessarily contraindicated. The viscous fiber blend biscuits, with a fat content of 30% and 10 g of viscous dietary fiber are, therefore, in line with these recommendations. In view of their moderate fat content and very low glycemic response, they may be used to replace fiber depleted high fat snack foods.
Since the postprandial glucose is an independent risk factor of cardiovascular disease, snack foods containing viscous fiber blend, which is able to reduce the magnitude of postprandial glucose rise, and at the same time is rich in dietary fiber, may be helpful in reducing the risk of cardiovascular disease. We have previously shown that viscous fiber blend added to conventional treatment may ameliorate blood lipid profile, long-term glycemic control, and systolic blood pressure in high-risk diabetic individuals, possibly improving the effectiveness of conventional treatment in type 2 diabetes mellitus (17). A diet rich in viscous fiber blend also improved lipid profile and long-term glycemic control in the insulin resistance syndrome, suggesting its therapeutic potential (18).
The emphasis on the importance of fibers in the diet is pressuring food companies to develop fiber-enriched products. However, adding considerable amounts of fiber to a food is sometimes associated with reduction of palatability (46) and the challenge to the food industry is to accomplish this goal without sacrificing the organoleptic appeal. No significant change in palatability of the viscous fiber blend biscuits was observed in our study between the types of biscuits. Interestingly, participants with diabetes found the biscuits with viscous fiber blend more palatable than did healthy individuals. This may have been, however, a reflection of the 20-year age difference between the two groups with the older, diabetic individuals, giving generally higher scores. Nevertheless, the acceptability of the product points toward the possibility of developing viscous fiber blend-enriched products tailored specifically for people with diabetes.
While the small sample size and the acute nature of the study limit the generalizability of the results, future research with an expanded sample size and the inclusion of overweight and obese participants, focusing on examining the caloric intake and satiety subsequent to meals containing viscous fiber blend, could yield important new insights into preventive dietary strategies.
In conclusion, viscous fiber blend is a potent soluble fiber which is able to reduce the glycemic response in both healthy individuals and participants with type 2 diabetes mellitus. The fat content of the viscous fiber blend biscuits is below 30%, which complies with the level currently recommended for the diets of patients with diabetes. In view of their very flat glycemic responses, these biscuits and possibly other products fortified with viscous fiber blend, may have an advantage in replacing other snack foods in the diet of individuals with type 2 diabetes mellitus. Finally, foods enriched with viscous fiber blend may also be a useful addition to other strategies aimed to prevent development of disease in the healthy population.
Acknowledgments
We extend sincere thanks to Dicofarm Spa (Roma, Italy) for providing biscuits for this project.
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29:1263–8. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–6. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–61. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 4.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261–7. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 5.Rizkalla SW, Taghrid L, Laromiguiere M, Huet D, Boillot J, Rigoir A, et al. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care. 2004;27:1866–72. doi: 10.2337/diacare.27.8.1866. [DOI] [PubMed] [Google Scholar]
- 6.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–7. doi: 10.1001/jama.277.6.472. [DOI] [PubMed] [Google Scholar]
- 7.Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–50. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et alNutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 200831Suppl 1S61–78. 10.2337/dc08-S061 [DOI] [PubMed] [Google Scholar]
- 9.Connor H, Annan F, Bunn E, Frost G, McGough N, Sarwar T, et al. The implementation of nutritional advice for people with diabetes. Diabet Med. 2003;20:786–807. doi: 10.1046/j.1464-5491.2003.01104.x. [DOI] [PubMed] [Google Scholar]
- 10.FAO/WHO. Diet, nutrition and prevention of chronic diseases. In: WHO Technical Report Series 916. Geneva: World Health Organization; 2003. [PubMed]
- 11.Dietary guidelines for Americans. 6th ed. Washington (DC): US Department of Agriculture and Department of Health and Human Services; 2005. [Google Scholar]
- 12.Jenkins DJ, Goff DV, Leeds AR, Alberti KG, Wolever TM, Gassull MA, et al. Unabsorbable carbohydrates and diabetes: Decreased post-prandial hyperglycaemia. Lancet. 1976;2:172–4. doi: 10.1016/S0140-6736(76)92346-1. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins DJ, Jenkins MJ, Wolever TM, Taylor RH. Slow release carbohydrate: mechanism of action of viscous fibers. Journal of Clinical Nutrition and Gastroenterology. 1986;1:237–41. [Google Scholar]
- 14.Jenkins AL, Jenkins DJ, Zdravkovic U, Wursch P, Vuksan V. Depression of the glycemic index by high levels of beta-glucan fiber in two functional foods tested in type 2 diabetes. Eur J Clin Nutr. 2002;56:622–8. doi: 10.1038/sj.ejcn.1601367. [DOI] [PubMed] [Google Scholar]
- 15.Kim E, Wong E, Vuksan V. The relationship between viscosity of soluble fibers and their hypoglycemic effects. Korean Journal of Nutrition. 1996;29:615–21. [Google Scholar]
- 16.Vuksan V, Sievenpiper JL, Xu Z, Wong EY, Jenkins AL, Beljan-Zdravkovic U, et al. Konjac-Mannan and American ginsing: emerging alternative therapies for type 2 diabetes mellitus. J Am Coll Nutr. 2001;20(5) Suppl:370S–80S. doi: 10.1080/07315724.2001.10719170. [DOI] [PubMed] [Google Scholar]
- 17.Vuksan V, Jenkins DJ, Spadafora P, Sievenpiper JL, Owen R, Vidgen E, et al. Konjac-mannan (glucomannan) improves glycemia and other associated risk factors for coronary heart disease in type 2 diabetes. A randomized controlled metabolic trial. Diabetes Care. 1999;22:913–9. doi: 10.2337/diacare.22.6.913. [DOI] [PubMed] [Google Scholar]
- 18.Vuksan V, Sievenpiper JL, Owen R, Swilley JA, Spadafora P, Jenkins DJ, et al. Beneficial effects of viscous dietary fiber from Konjac-mannan in subjects with the insulin resistance syndrome: results of a controlled metabolic trial. Diabetes Care. 2000;23:9–14. doi: 10.2337/diacare.23.1.9. [DOI] [PubMed] [Google Scholar]
- 19.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, et al. Glycaemic index methodology. Nutr Res Rev. 2005;18:145–71. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- 20.Williams S, editor. Official methods of analysis. 14th ed. Arlington: Association of Official Analytical Chemists Inc; 1984. [Google Scholar]
- 21.Jenkins DJ, Wolever TM, Thorne MJ, Jenkins AL, Wong GS, Josse RG, et al. The relationship between glycemic response, digestibility, and factors influencing the dietary habits of diabetics. Am J Clin Nutr. 1984;40:1175–91. doi: 10.1093/ajcn/40.6.1175. [DOI] [PubMed] [Google Scholar]
- 22.Clark LC Jr. A polarographic enzyme electrode for the measurement of oxidase substrates. In: Kessler M, Bruley DF, Leland CC, Lubbers DW, Silver IA, Strass J, editors. Oxygen supply. Munich: Urban & Schwarzenberg; 1977. p. 120-8. [Google Scholar]
- 23.Sanaka M, Yamamoto T, Anjiki H, Nagasawa K, Kuyama Y. Effects of agar and pectin on gastric emptying and post-prandial glycaemic profiles in healthy human volunteers. Clin Exp Pharmacol Physiol. 2007;34:1151–5. doi: 10.1111/j.1440-1681.2007.04624.x. [DOI] [PubMed] [Google Scholar]
- 24.Wolf BW, Wolever TM, Lai CS, Bolognesi C, Radmard R, Maharry KS, et al. Effects of a beverage containing an enzymatically induced-viscosity dietary fiber, with or without fructose, on the postprandial glycemic response to a high glycemic index food in humans. Eur J Clin Nutr. 2003;57:1120–7. doi: 10.1038/sj.ejcn.1601652. [DOI] [PubMed] [Google Scholar]
- 25.Chearskul S, Sangurai S, Nitiyanant W, Kriengsinyos W, Kooptiwut S, Harindhanavudhi T. Glycemic and lipid responses to glucomannan in Thais with type 2 diabetes mellitus. J Med Assoc Thai. 2007;90:2150–7. [PubMed] [Google Scholar]
- 26.Dahl WJ, Lockert EA, Cammer AL, Whiting SJ. Effects of flax fiber on laxation and glycemic response in healthy volunteers. J Med Food. 2005;8:508–11. doi: 10.1089/jmf.2005.8.508. [DOI] [PubMed] [Google Scholar]
- 27.Brand-Miller J, Wolever TM, Foster-Powell K, Colagiuri S. The new glucose revolution. The authoritative guide to the glycemic index – the dietary solution for lifelong health. New York (NY): Marlowe and Co; 1999. [Google Scholar]
- 28.Jenkins DJ, Wolever TM, Taylor RH, Ghafari H, Jenkins AL, Barker H, et al. Rate of digestion of foods and postprandial glycaemia in normal and diabetic subjects. BMJ. 1980;281:14–7. doi: 10.1136/bmj.281.6232.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolever TM, Jenkins DJ, Josse RG, Wong GS, Lee R. The glycemic index: similarity of values derived in insulin-dependent and non-insulin-dependent diabetic patients. J Am Coll Nutr. 1987;6:295–305. doi: 10.1080/07315724.1987.10720191. [DOI] [PubMed] [Google Scholar]
- 30.Dickinson S, Brand-Miller J. Glycemic index, postprandial glycemia and cardiovascular disease. Curr Opin Lipidol. 2005;16:69–75. doi: 10.1097/00041433-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Marlett JA, McBurney MI, Slavin JL, American Dietetic Association. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2002;102:993–1000. doi: 10.1016/S0002-8223(02)90228-2. [DOI] [PubMed] [Google Scholar]
- 32.Howlett J, Ashwell M. Glycemic response and health: summary of a workshop. Am J Clin Nutr. 2008;87:212S–6S. doi: 10.1093/ajcn/87.1.212S. [DOI] [PubMed] [Google Scholar]
- 33.Murakami K, Okubo H, Sasaki S. Effect of dietary factors on incidence of type 2 diabetes: a systematic review of cohort studies. J Nutr Sci Vitaminol (Tokyo) 2005;51:292–310. doi: 10.3177/jnsv.51.292. [DOI] [PubMed] [Google Scholar]
- 34.Roberts SB, Pittas AG. The role of glycemic index in type 2 diabetes. Nutr Clin Care. 2003;6:73–8. [PubMed] [Google Scholar]
- 35.Barkoukis H, Marchetti CM, Nolan B, Sistrun SN, Krishnan RK, Kirwan JP. A high glycemic meal suppresses the postprandial leptin response in normal healthy adults. Ann Nutr Metab. 2007;51:512–8. doi: 10.1159/000112309. [DOI] [PubMed] [Google Scholar]
- 36.Garsetti M, Vinoy S, Lang V, Holt S, Loyer S, Brand-Miller JC. The glycemic and insulinemic index of plain sweet biscuits: relationships to in vitro starch digestibility. J Am Coll Nutr. 2005;24:441–7. doi: 10.1080/07315724.2005.10719489. [DOI] [PubMed] [Google Scholar]
- 37.Casiraghi MC, Garsetti M, Testolin G, Brighenti F. Post-prandial responses to cereal products enriched with barley beta-glucan. J Am Coll Nutr. 2006;25:313–20. doi: 10.1080/07315724.2006.10719541. [DOI] [PubMed] [Google Scholar]
- 38.Wolever TM, Jenkins DJ, Kalmusky J, Giordano C, Giudici S, Jenkins AL, et al. Glycemic response to pasta: effect of surface area, degree of cooking, and protein enrichment. Diabetes Care. 1986;9:401–4. doi: 10.2337/diacare.9.4.401. [DOI] [PubMed] [Google Scholar]
- 39.Brennan CS. Dietary fibre, glycaemic response, and diabetes. Mol Nutr Food Res. 2005;49:560–70. doi: 10.1002/mnfr.200500025. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins DJ, Wolever TM, Leeds AR, Gassull MA, Haisman P, Dilawari J, et al. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. BMJ. 1978;1:1392–4. doi: 10.1136/bmj.1.6124.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards CA, Blackburn NA, Craigen L, Davison P, Tomlin J, Sugden K, et al. Viscosity of food gums determined in vitro related to their hypoglycemic actions. Am J Clin Nutr. 1987;46:72–7. doi: 10.1093/ajcn/46.1.72. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins DJ, Newton C, Leeds AR, Cummings JH. Effect of pectin, guar gum, and wheat fibre on serum-cholesterol. Lancet. 1975;1:1116–7. doi: 10.1016/S0140-6736(75)92503-9. [DOI] [PubMed] [Google Scholar]
- 43.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–81. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 44.Welch IM, Bruce C, Hill SE, Read NW. Duodenal and ileal lipid suppresses postprandial blood glucose and insulin responses in man: possible implications for the dietary management of diabetes mellitus. Clin Sci (Lond) 1987;72:209–16. doi: 10.1042/cs0720209. [DOI] [PubMed] [Google Scholar]
- 45.Wolever TM, Katzman-Relle L, Jenkins AL, Vuksan V, Josse RG, Jenkins DJ. Glycemic index of 102 complex carbohydrate foods in patients with diabetes. Nutr Res. 1994;14:651–69. doi: 10.1016/S0271-5317(05)80201-5. [DOI] [Google Scholar]
- 46.Ellis PR, Dawoud FM, Morris ER. Blood glucose, plasma insulin and sensory responses to guar-containing wheat breads: effects of molecular weight and particle size of guar gum. Br J Nutr. 1991;66:363–79. doi: 10.1079/BJN19910041. [DOI] [PubMed] [Google Scholar]