Abstract
SecA is an ATPase and motor protein that drives protein translocation across the bacterial plasma membrane. In Escherichia coli SecA levels are regulated by the secretion needs of the cell utilizing secM, which encodes a secreted protein. Previous studies demonstrated that this regulation requires a translational pause within secM, whose duration regulates the accessibility of the secA Shine-Dalgarno sequence on secM secA mRNA. Here we provide evidence that translocon “pulling” of nascent SecM is what regulates the duration of the secM translational pause, and thus secA expression levels, thereby providing direct support for this model.
In bacteria the Sec pathway is responsible for the translocation of most secretory proteins as well as the biogenesis of a number of integral membrane proteins (1, 6). Chaperones such as SecB protein as well as signal recognition particle contribute to the initial membrane targeting mechanism (7, 19). These pathways converge at the translocon, which consists of the core component SecYE, the proposed translocation channel, along with the accessory proteins SecDF and SecG, which enhance the basic process but are nonessential (4, 9, 14). The peripherally associated SecA ATPase is a motor protein that has been proposed to drive protein translocation via successive cycles of translocon insertion and retraction that are coupled to its ATPase activity, thereby promoting the stepwise translocation of proteins (5, 24). Since SecA appears to initiate the first committed step in protein translocation, its level and activity are likely to be carefully regulated.
In Escherichia coli SecA level is regulated by the secretion needs of the cell such that secA derepression occurs when protein translocation is inhibited by either genetic or physiological means (e.g., in sec-defective mutants or in the presence of sodium azide, an inhibitor of SecA ATPase) (15, 17). This regulation was previously shown to (i) occur at the translational level; (ii) require an upstream gene, secM, which encodes a nonessential and rapidly degraded periplasmic protein; and (iii) utilize a repressor helix on secM-secA mRNA that regulates the accessibility of the secA Shine-Dalgarno sequence (10, 13, 18, 23). Recently the basis of this secretion-responsive regulation has come to light with the discovery of the existence of a natural translational pause at the end of secM that regulates formation of the repressor helix (13). The presence of a nonfunctional secM signal sequence, sec-defective alleles, or azide addition was all shown to lengthen the secM translational pause and result in secA derepression (13, 16). These observations lead to the proposal that the duration of the secM translational pause is controlled by the rate of secretion of nascent SecM protein through a coupling of its translation and secretion.
Additional studies have defined the secM translational pause site and have implicated this sequence and portions of the peptide exit channel of the ribosome in promoting the observed translational pause (12, 21). By contrast, the mechanism regulating the release of the secM translational pause remains less defined. Certainly in this regard, one attractive model is that the “pulling” action of the translocon and the use of SecA motor protein directly contribute to the dissociation of those regions of SecM that interact with the ribosomal peptide exit channel and are responsible for pausing. However, alternative models can certainly be entertained as well, such as the requirement for a trans-acting factor for pause release: e.g., a Sec protein or other factor whose activity would correlate with that of the Sec machinery.
In this study we have devised an experimental strategy to directly test the translocon pulling model of secA regulation by secM. We have engineered a stop transfer-membrane anchor sequence into secM prior to its translational pause site in order to stop the pulling action of the translocon distal to the anchor sequence. In this way we were able to uncouple secM translation from its secretion prior to the translocon reaching the pause site. In this communication we demonstrate that, if the secretion of SecM is prematurely terminated by its conversion into a stable integral membrane protein, then the normal release mechanism of secM translational pausing is circumvented, giving rise to constitutive secA derepression.
We made use of standard recombinant DNA methodology as described previously in order to construct the appropriate plasmid-bearing strains (20). A synthetic double-stranded oligonucleotide (Integrated DNA Technologies, Inc., Coralville, Iowa) was designed that encoded four tandem repeats of the hydrophobic peptide Leu-Ala-Leu-Val along with appropriate “sticky” ends for insertion into the BstBI site at codon 74 of secM (Fig. 1). Repeats of this peptide of 16 amino acid residues or longer have been employed previously to construct functional stop transfer-membrane anchor sequences (2). After treatment of the oligonucleotide with polynucleotide kinase and ATP and subsequent annealing, it was inserted into the BstBI site of pPhIF, which is a pBR322 derivative plasmid carrying the secM secA operon that also contains a secA-lacZ translational fusion (10). In order to enrich for plasmids that contained the oligonucleotide, the ligation mixture was treated with BstBI prior to transformation. A transformant that contained the plasmid with the membrane anchor sequence in the correct orientation, pPhIF-MA, was identified by DNA sequence analysis (University of Pennsylvania DNA sequence facility) and was selected for further study.
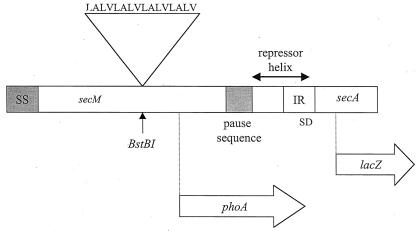
FIG. 1.
Creation of an artificial membrane anchor sequence in secM. The membrane anchor peptide sequence is shown along with a diagram of the secM secA operon that indicates the location of the atypical secM signal (SS) and translational pause sequences (codons 1 to 37 and 150 to 166, respectively) and the repressor helix that blocks the secA Shine-Dalgarno sequence (SD) (12, 22). The sites of insertion of the membrane anchor sequence within secM (BstBI at codon 74), the secM-phoA fusion joint (codon 157), and the secA-lacZ fusion joint (codon 67) are indicated. Only the 5′ portion of secA is shown.
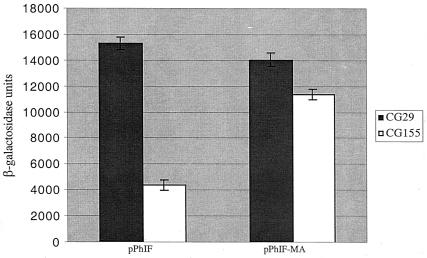
In order to study secretion-responsive secA regulation, the parental plasmid and its derivative with the membrane anchor sequence were transformed into an isogenic set of strains, CG155 and CG29, where secretion-proficient and secretion-defective conditions can be induced by a temperature shift (10). β-Galactosidase assays (Fig. 2) indicated that secA expression was correctly regulated in pPhIF but that it was essentially constitutively derepressed in pPhIF-MA. In order to normalize, if necessary, for differences in mRNA levels that might be caused by introduction of the membrane anchor sequence into secM, we also constructed secA-lacZ transcriptional fusion derivatives with or without the membrane anchor sequence. For this purpose we utilized pOF, a pBR322 derivative containing the secM secA operon with a secA-lacZ transcriptional fusion (10). An 0.9-kb KpnI-NcoI DNA fragment carrying secM and the beginning of secA was isolated from pPhIF or pPhIF-MA and used to replace the comparable fragment of pOF, generating either pOF again or pOF-MA, containing the membrane anchor sequence. Analysis of these strains showed that the β-galactosidase activity of CG155(pOF-MA) was 37% lower than that of CG155(pOF), indicating that the membrane anchor sequence was achieving its effect on secA regulation primarily at the translational level. This result may also explain why there was a modest reduction in β-galactosidase activity of CG155(pPhIF-MA) compared to that of CG29(pPhIF or pPhIF-MA) (i.e., because of a somewhat lower mRNA level in the former case) (Fig. 2).
FIG. 2.
Effect of the secM membrane anchor sequence on secA regulation. CG155 (MC1000 recA) and CG29 [MC1000 secD1(Cs) phoR recA1 srl::Tn10] containing pPhIF or pPhIF-MA were grown in Luria broth containing 100 μg of ampicillin per ml at 39°C to mid-logarithmic phase when the cultures were shifted to 23°C for 4 h. β-Galactosidase assays were performed in duplicate for each of two duplicate cultures as described previously (11). The average result is given with the error bar indicating the standard deviation.
In order to provide proof that the membrane anchor was functioning correctly, we made secM-phoA fusion derivatives with or without the membrane anchor sequence and performed alkaline phosphatase activity assays on the relevant strains. It has been previously shown that alkaline phosphatase, the product of the phoA gene, is enzymatically active only when it is translocated to the trans side of the plasma membrane, and therefore it is a good indicator of membrane topology (8). For this purpose, it was important to utilize a secM-phoA fusion where the fusion joint was downstream of the membrane anchor sequence and the remainder of secM (particularly the translational pause site) was deleted (Fig. 1). pSS1, a pBR322 derivative plasmid containing a secM-phoA fusion at codon 157 of secM that lacks the rest of the secM gene (22), was employed to construct an isogenic plasmid containing the membrane anchor sequence, pSS1-MA, utilizing the same strategy as described above (i.e., insertion of the oligonucleotide linker at the BstBI site of pSS1). Alkaline phosphatase activity assays of the appropriate strains, CC118 [MC1000 phoA20 rpsE rpoB argE(Am) recA1] containing pSS1 or pSS1-MA, showed that the membrane anchor mutant had only 15% of the activity of the wild-type strain (266 versus 41 U, respectively). Addition of 1 mM (final concentration) iodoacetamide to the cultures at the end of growth and in the assay buffer did not affect these results, ruling out artificial in vitro activation of alkaline phosphatase activity (3). The low but appreciable level of alkaline phosphatase activity in the membrane anchor mutant may be due to some leakiness in the function of the membrane anchor as a stop transfer sequence, given that it is shorter than many membrane anchors, which often consist of 20 or more hydrophobic amino acid residues (25).
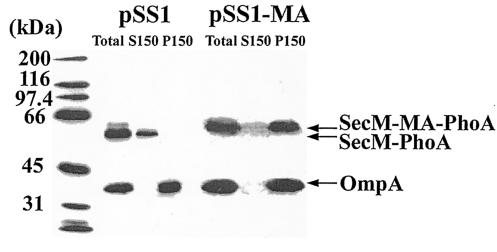
In order to demonstrate that the observed difference in alkaline phosphatase activity was due to its cellular location, a subcellular fractionation experiment was performed on these strains. The results showed that the wild-type SecM-PhoA chimera was present in the soluble fraction, consistent with its predicted periplasmic location, while the SecM-PhoA chimera containing the membrane anchor was present in the membrane fraction, consistent with the presence of a functional membrane anchor sequence (Fig. 3). The membrane location of OmpA in both cases provided assurance of the fidelity of the fractionation methodology employed here. There was approximately a twofold difference in the steady-state levels of the two SecM-PhoA chimeras, indicating that the severe reduction in alkaline phosphatase activity of the chimera containing the membrane anchor was mostly due to its inactivity. The modest reduction in the steady-state level of this latter chimera was anticipated, given the inability of alkaline phosphatase on the cis side of the plasma membrane to acquire disulfide bonds and assemble properly, thus making it a target for proteolysis (3).
FIG. 3.
Subcellular location of the SecM-PhoA chimeras. CC118(pSS1) and CC118(pSS1-MA) were grown in Luria broth containing 100 μg of ampicillin per ml at 30°C to an optical density at 600 nm of 1.0, when cells were harvested by sedimentation at 7,000 × g for 5 min at 4°C. Cell pellets were resuspended in 0.02 volumes of TKMDP (25 mM TrisOAc [pH 7.5], 25 mM KCl, 1 mM MgOAc, 1 mM dithiothreitol, 0.5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 100 μM leupeptin, 1 μM pepstatin, and 0.3 μM aprotinin) and broken by two passages at 8,000 lb/in2 in the French pressure cell. Unbroken cells were removed by two successive sedimentations at 13,000 × g for 10 min at 4°C, giving rise to the total cleared lysate (Total). Soluble (S150) and membrane (P150) fractions were obtained by sedimentation at 150,000 × g for 3 h at 4°C. The P150 fraction was resuspended in the original volume of TKMDP. A 28-μl [CC118(pSS1)] or 56-μl [CC118(pSS1-MA)] quantity of each fraction was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western blotting with alkaline phosphatase and OmpA antiserum and visualization with enhanced chemiluminescence as described by the manufacturer (Perkin-Elmer). The positions of the wild type (SecM-PhoA) and the SecM-PhoA chimera containing the membrane anchor (SecM-MA-PhoA) are given along with that of OmpA. Molecular mass markers are provided at the left.
Finally, in order to show that the conversion of SecM into an integral membrane protein per se was insufficient to perturb secA regulation, an experiment in which a membrane anchor sequence was inserted downstream of the secM translational pause site was also considered. However, since the translational pause site is near the end of secM and the inserted sequence would disrupt the repressor helix on secM-secA mRNA (since the 5′ end of the repressor helix overlaps the 3′ end of the pause site) (12, 21), this option was not pursued further.
Our results provide direct support for a model where translocon pulling of the nascent SecM polypeptide chain provides the necessary force to dislodge the paused SecM sequence within the ribosomal peptide exit channel. We assume that this action by SecA and the translocon could distort an unusual secondary or tertiary structure of the secM translational arrest sequence, FXXXXWIXXXXXGIRAGP (where X indicates any amino acid residue), which appears to interact with key residues of 23S rRNA and L22 protein at the narrowest constriction site of the peptide exit channel of the ribosome to effect the initial pause (12). Release of the secM translational pause followed by translational termination would allow reformation of the repressor helix on secM-secA mRNA and restore secA repression (12, 21). This model provides a simple explanation for how secA expression is tied to translocon function (i.e., secretion-responsive regulation). Presumably in the secretion-defective state the translocon would be largely unavailable to interact with nascent SecM signal peptide and unable to provide the necessary force during translocation to promote the release of the translationally arrested SecM complex. This defect would in turn prevent reformation of the secA repressor helix (since the arrested ribosome occupies sequences that comprise the 5′ end of the repressor helix) (12, 21) and would promote secA derepression. Our results are not consistent with a whole class of trans-acting models, where a diffusible factor in the cytosol or the membrane would provide the linkage between translocon activity and the secM translational pause release step. Clearly additional studies of this intriguing system are warranted in order to decipher the details of this complex regulatory paradigm.
Acknowledgments
We thank Shameema Sarker for guidance during the earlier portions of the study.
This work was supported by grant GM42033 from the National Institutes of Health to D.B.O.
REFERENCES
- 1.Danese, P. N., and T. J. Silhavy. 1998. Targeting and assembly of periplasmic and outer-membrane proteins in Escherichia coli. Annu. Rev. Genet. 32:59-94. [DOI] [PubMed] [Google Scholar]
- 2.Davis, N. G., and P. Model. 1985. An artificial anchor domain: hydrophobicity suffices to stop transfer. Cell 41:607-614. [DOI] [PubMed] [Google Scholar]
- 3.Derman, A. I., and J. Beckwith. 1995. Escherichia coli alkaline phosphatase localized to the cytoplasm slowly acquires enzymatic activity in cells whose growth has been suspended: a caution for gene fusion studies. J. Bacteriol. 177:3764-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duong, F., and W. Wickner. 1997. Distinct catalytic roles of the SecYE, SecG, and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 16:2756-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Economou, A., and W. Wickner. 1994. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell 78:835-843. [DOI] [PubMed] [Google Scholar]
- 6.Fekkes, P., and A. J. M. Driessen. 1999. Protein targeting to the bacterial cytoplasmic membrane. Microbiol. Rev. 63:161-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Froderberg, L., E. Houben, J. C. Samuelson, M. Chen, S.-K. Park, G. J. Phillips, R. Dalbey, J. Luirink, and J.-W. de Gier. 2003. Versatility of inner membrane protein biogenesis in Escherichia coli. Mol. Microbiol. 47:1015-1027. [DOI] [PubMed] [Google Scholar]
- 8.Manoil, C., J. J. Mekalanos, and J. Beckwith. 1990. Alkaline phosphatase fusions: sensors of subcellular location. J. Bacteriol. 172:515-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manting, E. H., C. van der Does, H. Remigy, A. Engel, and A. J. M. Driessen. 2000. SecYEG assembles into a tetramer to form the active protein translocation channel. EMBO J. 19:852-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNicholas, P., R. Salavati, and D. Oliver. 1997. Dual regulation of Escherichia coli secA translation by distinct upstream elements. J. Mol. Biol. 265:128-141. [DOI] [PubMed] [Google Scholar]
- 11.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 12.Nakatogawa, H., and K. Ito. 2002. The ribosomal exit tunnel functions as a discriminating gate. Cell 108:629-636. [DOI] [PubMed] [Google Scholar]
- 13.Nakatogawa, H., and K. Ito. 2001. Secretion monitor, SecM, undergoes self-translation arrest in the cytosol. Mol. Cell 7:185-192. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama, K.-I., T. Suzuki, and H. Tokuda. 1996. Inversion of the membrane topology of SecG coupled with SecA-dependent preprotein translocation. Cell 85:71-81. [DOI] [PubMed] [Google Scholar]
- 15.Oliver, D., R. Cabelli, K. Dolan, and G. Jarosik. 1990. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc. Natl. Acad. Sci. USA 87:8227-8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver, D., J. Norman, and S. Sarker. 1998. Regulation of Escherichia coli secA by cellular protein secretion proficiency requires an intact gene X signal sequence and an active translocon. J. Bacteriol. 180:5240-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver, D. B., and J. Beckwith. 1982. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell 30:311-319. [DOI] [PubMed] [Google Scholar]
- 18.Rajapandi, T., K. M. Dolan, and D. Oliver. 1991. The first gene in the Escherichia coli secA operon, gene X, encodes a nonessential secretory protein. J. Bacteriol. 173:7092-7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randall, L. L., and S. J. S. Hardy. 2002. SecB, one small chaperone in the complex milieu of the cell. Cell. Mol. Life Sci. 59:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Sarker, S., and D. Oliver. 2002. Critical regions of secM that control its translation and secretion and promote secretion-specific secA regulation. J. Bacteriol. 184:2360-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarker, S., K. E. Rudd, and D. Oliver. 2000. Revised translation start site of secM defines an atypical signal peptide that regulates Escherichia coli secA expression. J. Bacteriol. 182:5592-5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt, M., and D. B. Oliver. 1989. SecA protein autogenously represses its own translation during normal protein secretion in Escherichia coli. J. Bacteriol. 171:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Wolk, J. P. W., J. G. de Wit, and A. J. M. Driessen. 1997. The catalytic cycle of the Escherichia coli SecA ATPase comprises two distinct preprotein translocation events. EMBO J. 16:7297-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Heijne, G. 1997. Getting greasy: how transmembrane polypeptide segments integrate into the lipid bilayer. Mol. Microbiol. 24:249-253. [DOI] [PubMed] [Google Scholar]