Abstract
Listeriosis is a rare disease that causes mild maternal illness, but can be devastating to the fetus. Listeria’s rare microbiologic features make it a difficult infection to diagnose and treat: it is an intracellular organism that hides within host cells. Because of the potentially severe consequences, it is important that obstetricians are familiar with the diagnosis, treatment, and prevention of listerial infection.
Key words: Listeriosis, Intracellular transmission, Fetal listerial infection, Neonatal listerial infection
Listeriosis is a rare infection, but is about 20 times more common in pregnant women than in the general population.1 Pregnant women account for 27% of all listerial infections,2 which can cause mild illness in mothers, but can be devastating to the fetus, in some cases leading to severe disease or fetal death.3 Pregnant women may be able to reduce risk of listerial infection by following dietary guidelines recommended by the Centers for Disease Control and Prevention (CDC) (see Table 3). National food agencies, such as the United States Department of Agriculture (USDA) and the Food and Drug Administration (FDA) have also lowered risk of listerial infection by monitoring potential sources of contamination.4 Because of the potentially severe consequences, it is important that practicing obstetricians are familiar with the diagnosis, treatment, and prevention of listerial infection.
Table 3.
Centers for Disease Control and Prevention Recommendations on Listeriosis Prevention
| • Do not eat hot dogs and luncheon meats unless they are reheated until steaming hot. |
| • Avoid cross-contaminating other foods, utensils, and food preparation surfaces with fluid from hot dog packages, and wash hands after handling hot dogs. |
| • Do not eat soft cheeses such as feta, brie, and camembert cheeses; blue-veined cheeses; and Mexican-style cheeses such as queso blanco fresco. Cheeses that may be eaten include hard cheeses; semi-soft cheeses such as mozzarella; pasteurized processed cheeses such as slices and spreads; cream cheese; and cottage cheese. |
| • Do not eat refrigerated pâtés or meat spreads. Canned or shelf-stable pâtäs and meat spreads may be eaten. |
| • Do not eat refrigerated smoked seafood, unless it is contained in a cooked dish, such as a casserole. Canned or shelf-stable smoked seafood may be eaten. |
| • Do not drink raw (unpasteurized) milk or eat foods that contain unpasteurized milk. |
Data from Centers for Disease Control and Prevention.2
Bacteriology
Listeria monocytogenes (Figure 1) is a common organism in nature and can be easily isolated from soil, dust, water, processed foods, raw meat, and the feces of animals and humans.1 It is an aerobic, motile rod that grows well on most routine culture media.5
Figure 1.
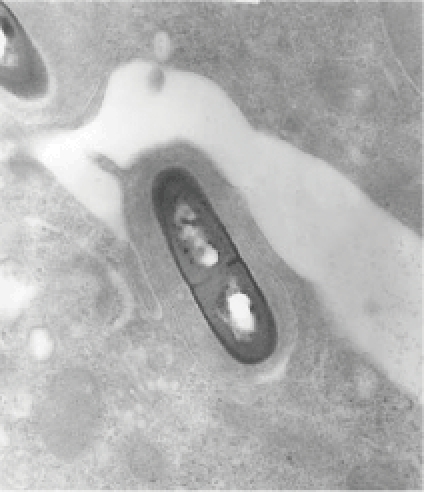
Listeria monocytogenes. Image courtesy of the Centers for Disease Control and Prevention Public Health Image Library (http://phil.cdc.gov/phil/home.asp).
Listeria is a unique pathogen because it has an intracellular life cycle. Once contaminated food has been ingested, Listeria can be phagocytosed by gastrointestinal cells and can enter the host without disrupting the integrity of the gastrointestinal tract.1 Once the organism has reached the host cytoplasm, it rapidly divides and pushes up against the cell membrane; it can then be ingested by adjacent cells. Through this series of steps, Listeria can multiply and spread without being exposed to antibodies, neutrophils, or antibiotics in the extracellular fluid (see Figure 2).5 This explains why maternal listerial illness can be mild or even asymptomatic. Cell-mediated immunity is the host’s defense against Listeria, and any condition that reduces cell-mediated immunity, such as pregnancy, can predispose to listerial infection.1
Figure 2.
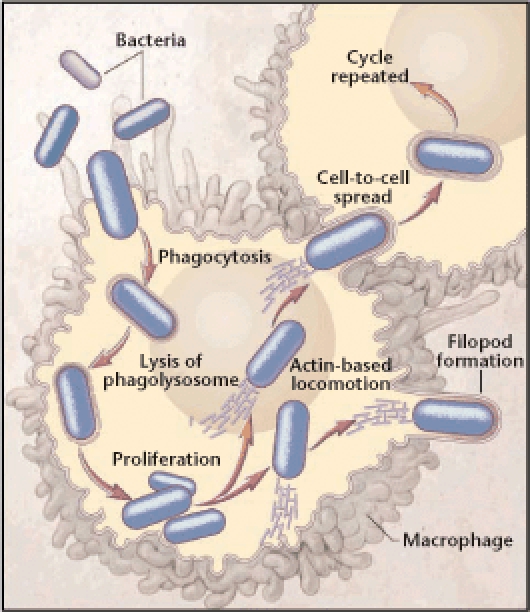
The intracellular transmission of Listeria monocytogenes. Listeria can multiply and spread without exposure to antibodies, neutrophils, or antibiotics in the extracellular fluid. Reproduced with permission from Southwick FS and Purich DL, © 1996 Massachusetts Medical Society. All rights reserved.
Listeria’s intracellular transmission pattern likely explains its ability to cross the placental barrier. Listeria can also cross the blood-brain barrier, leading to meningitis or encephalitis.6
Epidemiology
The incidence of listeriosis in pregnancy is 12 per 100,000, compared with a rate of 0.7 per 100,000 in the general population.2 The CDC monitors cases of listerial infection, and estimates that there were about 800 cases in 2007.7 Cases were spread evenly throughout the United States. Based on known incidences, approximately 200 of those likely occurred during pregnancy.6 The incidence of listeriosis in the newborn is estimated at a rate of 8.6 per 100,000 live births.7
Listeriosis is most often a food-borne illness, and sporadic cases as well as outbreaks have been linked to contaminated food.1 Outbreaks of listeriosis are more common in the summer. Listeria is a resilient organism; it can survive at temperatures ranging from 4°C to 37°C. Listeria maintains its motility best at room temperature, where it can multiply rapidly in a short period of time.6 The incubation period for Listeria has not been well established; according to case reports, the incubation period is from 24 hours to 70 days.1
The FDA and the USDA perform routine screening and surveillance for listerial contamination. In 2008, there have been recalls of prepared chicken, pork, and seafood products in the United States due to concerns of listerial contamination.8
Symptoms and Diagnosis
Although severe maternal illness from listeriosis has been reported, it is rare. In most cases, maternal illness is mild and sometimes even asymptomatic. In the largest collection of 222 reported cases, fever was the most common symptom (Table 1). Fever ranged between 38.2°C and 41.2°C (mean, 38.9°C). Flu-like syndrome was the second most common description of clinical presentation. In this series, white blood cell count ranged from 3900 to 33,800 cells/mm3 with a mean of 16,300 cells/mm3. Listeriosis occurred at all stages of pregnancy. The most common means of diagnosis were culture of blood or placenta.3
Table 1.
Symptoms of Listeriosis
| Symptom | Number of Patients | Percentage |
| Fever | 126 | 65 |
| Flu-like syndrome | 61 | 32 |
| Abdominal or back pain | 41 | 21.5 |
| Vomiting/diarrhea | 14 | 7 |
| Headache | 20 | 10.5 |
| Myalgia | 8 | 4 |
| Sore throat | 7 | 4 |
| None | 55 | 29 |
Data from 191 cases reviewed by Mylonakis and colleagues.3 Patients may have more than 1 symptom.
Patients with comorbidities, such as a history of splenectomy, human immunodeficiency virus (HIV) infection, steroid use, diabetes, or use of immunosuppressive medications, are at increased risk for listerial infection1 due to a decrease in cell-mediated immunity. As expected, pregnant women who are immunosuppressed or using corticosteroids are at higher risk of listeriosis than the general pregnant population. In patients with comorbidities, listerial infection can cause severe maternal illness, usually by spreading to the central nervous system (CNS). Symptoms of listerial CNS infection include seizures, cranial nerve deficits, and tremor. Listeria can also cause endocarditis or brain abscess. In immunocompromised patients with disseminated disease, listeriosis has a case fatality rate of 20% to 50%.9
Although pregnant women with comorbidities are at increased risk of listeriosis, most cases occur in otherwise healthy pregnant women. For example, in a report by Mylonakis and colleagues,3 only 2 of 11 patients (18%) had any comorbidities. This argues that obstetricians should be vigilant about diagnosis and prevention of listeriosis in all pregnant women.
In contrast to maternal illness, fetal and neonatal infection is severe and frequently fatal, with a case fatality rate of 20% to 30%.10 Neonatal listerial infection can cause pneumonia, sepsis, or meningitis. Although presentation can be variable, most neonates present with respiratory distress, fever, rash, jaundice, or lethargy. The pathognomonic finding in neonatal listerial infection is granulomatous infantiseptica, a rare, severe disseminated form of disease characterized by widespread microabscesses and granulomas. In the 222 cases of maternal infection reported in the literature and reviewed by Mylonakis and colleagues,3 94 infants were infected. Of these, 59 (62.8%) recovered completely, 23 (24.5%) died, and 12 (12.7%) had neurologic sequelae or other long-term complications. Meningitis alone or in combination with bacteremia/sepsis or pneumonia was associated with the worst prognosis.
A late neonatal infection has also been described, occurring more than 5 to 7 days after delivery. This often presents as a meningeal illness.11 Infants with late-onset listeriosis are generally full term, healthy at birth, and delivered to mothers who have had uncomplicated pregnancies. The source of listerial infection in this group is not known, but is possibly the mother’s alimentary tract or the environment, as the organism is rarely isolated from the mother’s genital tract.11
Diagnosis of listerial infection can only be made by culturing the organism from a sterile site such as blood, amniotic fluid, or spinal fluid. Vaginal or stool cultures are not helpful in diagnosis because some women are carriers but do not have clinical disease.1 Fecal carriage of Listeria occurs in 1% to 15% of the population; the incidence of women carrying Listeria in the vagina is lower.12 Pregnancy does not affect the fecal, cervicovaginal, or oropharyngeal carriage rate of Listeria, but it has been reported as a possible additional predisposing factor for perinatal listeriosis.13 Gram stain is useful in only about 33% of cases, both because Listeria is an intracellular organism and can be entirely missed,4 and because the organism can resemble pneumococci (diplococci), diphtheroids (Corynebacteria), or Haemophilus species. Informing the microbiologist of suspicion of listerial infection can improve the specificity of Gram stains.14
Some prior studies suggested an association between chronic carriage of Listeria and recurrent abortion, but this has not been a consistent finding. In a small study from Israel, the carriage of Listeria in the genital tract was associated with a history of recurrent abortion, but this was not confirmed in 2 follow-up studies.15,16 In the largest prospective study, conducted from 1979 to 1989, Listeria was not isolated from the cervix or the endometrium of any of the 86 patients with 2 or more fetal losses.17
Because listeriosis in pregnancy is serious and difficult to diagnose, blood cultures should be considered in any pregnant patient presenting with fever, especially if accompanied by flu-like or gastrointestinal symptoms. After obtaining cultures, providers should consider starting treatment if suspicion for listerial infection is high.4
Treatment
Because listeriosis is rare, there are no prospective in vivo studies on antibiotic regimens. Experience from in vitro studies, case reports, and expert opinion can be useful to guide therapy.6 For an antibiotic to be effective against Listeria, the antibiotic must penetrate into the host cell and maintain high intracellular concentrations; it must penetrate into the host cell without significant changes in concentration or pH that might reduce its efficacy; and the antibiotic must bind to the penicillin-binding protein 3 (PBP3) of Listeria, which causes cell death.5 A fourth consideration in pregnancy is that the antibiotic must cross the placenta in adequate concentration.
Penicillin, ampicillin, and amoxicillin have been used most extensively in the treatment of listeriosis.6 These drugs block several PBPs and do penetrate intracellularly. Microbial resistance of Listeria to penicillin or its derivatives has not yet been found under natural conditions.5 High doses are generally used to assure adequate penetration of the umbilical cord and placenta. Some in vitro studies suggest a synergistic effect when gentamicin is added to treatment regimens; however, animal models do not reliably show a synergistic effect. Given the toxicities of gentamicin some clinicians have questioned the value of adding it to the treatment regimen.6
Whichever antibiotic is chosen, dosage is critical. Most experts recommend 6 g or more per day of ampicillin for treatment during pregnancy.6 This dosage provides adequate intracellular penetration and crosses the placenta in adequate amounts. The recommended dosage in many case reports is 2 g every 6 to 8 hours3 (Table 2).
Table 2.
Treatment Options for Listerial Infections
| Infection | Treatment | Duration |
| Bacteremia | First-line: ampicillin ≥ 6 g/d IV; add gentamicin if patient > 50 y, chronic disease, cardiovascular or respiratory compromise | 14 d |
| Second-line: erythromycin 4 g/d, or TMP/SMX 200–320 mg of TMP component, or vancomycin 1 g tid | 14 d | |
| Bacteremia in pregnancy | First-line: ampicillin ≥ 6 g/d IV | 7–14 d; if fetus survives, consider longer treatment |
| Second-line: erythromycin 4 g/d IV | 7–14 d; if fetus survives, consider longer treatment | |
| Acute meningitis | First-line: ampicillin ≥ 6 g/d IV plus gentamicin if patient > 50 y, chronic disease, cardiovascular or respiratory compromise | 21 d |
| Second-line: TMP/SMX 200–320 mg of TMP component | 21 d | |
| Infective endocarditis | First-line: ampicillin ≥ 6 g/d IV; add gentamicin if patient > 50 y, chronic disease, cardiovascular or respiratory compromise | 6 wk for natural valves, 8 wk for prosthetic valves |
| Second-line: vancomycin 1 g tid plus gentamicin 2.5 mg/kg/d | 6 wk for natural valves, 8 wk for prosthetic valves | |
| Brain abscess | First-line: ampicillin 14 g/d plus gentamicin 2.5 mg/kg/d | 4–6 wk |
| Second-line: TMP/SMX 200–320 mg of TMP component | 4–6 wk | |
| Joint/bone infection | First-line: ampicillin ≥ 6 g/d IV; add gentamicin if patient > 50 y, chronic disease, cardiovascular or respiratory compromise | 6 wk if no prosthesis, 2 wk IV with prosthesis, and lifelong oral amoxicillin or TMP/SMX in each case |
IV, intravenous; TMP/SMX, trimethoprim/sulfamethoxazole.
Reproduced with permission from Temple ME and Nahata MC.6
In patients who are allergic to penicillin, treatment with trimethoprim/sulfamethoxazole (TMP/SMX) offers an alternative. Because data on penicillin are much more extensive, steps should be taken to establish and document true penicillin allergy prior to starting this second-line agent.5 However, if true penicillin allergy is present, TMP/SMX is a reasonable alternative.6 Resistance to TMP/SMX in listeriosis cases is rare.5 Again, high doses are needed. Expert opinion and case reports suggest doses of 200 mg to 320 mg of TMP component per day (equivalent of 1–2 tablets by mouth every 6 hours).3,6 Because TMP/SMX has excellent oral bioavailability, either oral or intravenous dosing is acceptable. Some providers recommend checking liver function, kidney function, and platelets weekly while on high-dose TMP/SMX (K. Zachary, personal communication, August 1, 2008). TMP/SMX is best avoided in early pregnancy or in patients at risk for neural tube defects due to the antifolate activity of the trimethoprim component.17
Vancomycin has also been used in case reports of listerial infection.6 Although experience with vancomycin for listeriosis in pregnancy is limited, vancomycin has been used against listerial meningitis via intraventricular injection, and in listerial endocarditis (Table 2).18 Again, listerial resistance to vancomycin is rare.5
Other sources have recommended erythromycin for treatment of listeriosis in pregnancy (Table 2).6 However, transplacental passage of erythromycin has been shown to provide subtherapeutic concentrations in both the amniotic fluid and fetal serum.19 Therefore, many experts recommend using another alternative.
Other antibiotics used in case reports of listeriosis include erythromycin, meropenem, linezolid, and rifampin.3 However, experience with meropenem and linezolid in pregnancy is limited, and resistance has been reported with rifampin monotherapy.5 Cephalosporins are ineffective against Listeria because they do not bind to PBP3.1
Optimal duration of therapy in pregnancy has not been established. In case reports, duration of therapy has varied from 2 weeks to continuous treatment until delivery.3,4,6 Even if a host seems clinically improved, the intracellular concentration of short-course antibiotic treatment may not be sufficient for complete sterilization. Indeed, in immunosuppressed patients, relapses have been reported after 2 weeks of penicillin therapy.9 In pregnancy, there are additional considerations, such as adequate treatment of the placenta, and potential ongoing infection of the fetus and/or placenta. There has been concern that placental infection may not be clinically apparent, but could progress once antibiotic therapy has been withdrawn. For this reason, some experts have suggested at least 3 to 4 weeks of treatment in pregnancy.6
Prevention
Epidemiologic investigations have demonstrated that nearly all types of food can transmit Listeria. Most sporadic cases and all large outbreaks have been associated with manufactured foods.20 Food items implicated in outbreaks include ready-to-eat meats such as turkey deli meat, meat pâté, pork tongue in jelly, and hot dogs.1,3,4 Dairy products, especially soft cheeses, have also been implicated in outbreaks.9,12 Pasteurization eliminates Listeria from dairy products, and most dairy-associated outbreaks are from items that are inadequately pasteurized or contaminated after pasteurization.9
Most cases of listeriosis are sporadic and not associated with an outbreak.3 In these cases, a specific food source of Listeria is rarely found. As stated previously, Listeria is a common organism in nature and can easily be isolated from processed foods, raw meat, and even some prepared vegetables.1 Thus, creating guidelines that will prevent exposure to Listeria is nearly impossible. Certainly, avoiding unpasteurized dairy products will reduce risk because these have clearly been sources of contamination in the past. Cross-contamination is also an important protective strategy: women should wash all utensils and surfaces well after preparing meat dishes or cutting prepared foods (Table 3).
Ultimately, not all listerial exposures can be prevented. Patients should know to contact their provider if they have any of the common symptoms listed in Table 1. Providers should then maintain enough suspicion for listerial infection to draw blood cultures for any woman at risk.
Conclusions
Listeriosis is a rare disease that causes mild maternal illness, but can be devastating to the fetus. Listeria’s rare microbiologic features make it a difficult infection to diagnose and treat: it is an intracellular organism that hides within host cells. Listeria usually causes only mild maternal illness. Although women with comorbidities, such as diabetes, steroid use, or HIV, are at higher risk of listerial infection, most cases occur in healthy pregnant women. Any pregnant woman presenting with fevers and flu-like illness, or other symptoms that raise suspicion for listeriosis, should be tested for this disease. Testing is usually by blood culture. Gram stain is sometimes, but not always, diagnostic. Once listeriosis is diagnosed, high-dose penicillin or ampicillin is the treatment of choice. If the patient has a penicillin allergy, there are other treatment options (Table 2). Ongoing education about the transmission of Listeria, as well as governmental surveillance programs, will likely continue to reduce the incidence of listeriosis in pregnancy.
Main Points.
Listeria monocytogenes is a common organism in nature and can be easily isolated from soil, dust, water, processed foods, raw meat, and the feces of animals and humans.
Listeria is a unique pathogen because it has an intracellular life cycle; this intracellular transmission pattern likely explains its ability to cross the placental barrier.
The incidence of listeriosis in pregnancy is 12 per 100,000, compared with a rate of 0.7 per 100,000 in the general population.
Patients with comorbidities, such as a history of splenectomy, human immunodeficiency virus infection, steroid use, diabetes, or use of immunosuppressive medications, are at an increased risk for listerial infection due to a decrease in cell-mediated immunity.
Whereas maternal illness is mild and sometimes asymptomatic, fetal and neonatal infection is severe and frequently fatal, with a case fatality rate of 20% to 30%.
Penicillin, ampicillin, and amoxicillin have been used most extensively in the treatment of listeriosis. Most experts recommend 6 g or more per day of ampicillin for treatment during pregnancy. This dosage provides adequate intracellular penetration and crosses the placenta in adequate amounts.
Ready-to-eat meats, such as hot dogs, and dairy products, especially soft cheeses, have been implicated in outbreaks of listerial infection. Avoiding cross-contamination is an important protective strategy: all utensils and surfaces should be washed well after preparing meat dishes or cutting prepared foods.
References
- 1.Southwick FS, Purich DL. Intracellular pathogenesis of listeriosis. N Engl J Med. 1996;334:770–776. doi: 10.1056/NEJM199603213341206. [DOI] [PubMed] [Google Scholar]
- 2.Listeriosis. Atlanta: Centers for Disease Control and Prevention; 1999. [Google Scholar]
- 3.Mylonakis E, Paliou M, Hohmann EL, et al. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore) 2002;81:260–269. doi: 10.1097/00005792-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Silver HM. Listeriosis during pregnancy. Obstet Gynecol Surv. 1998;53:737–740. doi: 10.1097/00006254-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Hof H, Nichterlein T, Kretschmar M. Management of listeriosis. Clin Microbiol Rev. 1997;10:345–357. doi: 10.1128/cmr.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temple ME, Nahata MC. Treatment of listeriosis. Ann Pharmacother. 2000;34:656–661. doi: 10.1345/aph.19315. [DOI] [PubMed] [Google Scholar]
- 7.Prevention CfDCa. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, 2007. MMWR. 2008;57:366–370. [PubMed] [Google Scholar]
- 8. [Accessed August 27, 2008];Supreme Cuts announces voluntary recall of small sample of Off The Cob fresh kernel corn [press release] Available at http://www.fda.gov/oc/po/firmrecalls/supremecuts05_08.html.
- 9.Cherubin CE, Appleman MD, Heseltine PNR, et al. Epidemiological spectrum and current treatment of listeriosis. Rev Infect Dis. 1991;13:1108–1114. doi: 10.1093/clinids/13.6.1108. [DOI] [PubMed] [Google Scholar]
- 10.Schwarze R, Bauermeister CD, Ortel S, Wichmann G. Perinatal listeriosis in Dresden 1981–1986: clinical and microbiological findings in 18 cases. Infection. 1989;17:131–138. doi: 10.1007/BF01644011. [DOI] [PubMed] [Google Scholar]
- 11.Skidmor AG. Listeriosis at Vancouver General Hospital, 1965–79. Can Med Assoc J. 1981;125:1217–1221. [PMC free article] [PubMed] [Google Scholar]
- 12.Lennon D, Lewis B, Mantell C, et al. Epidemic perinatal listeriosis. Pedatr Infect Dis. 1984;3:30–34. doi: 10.1097/00006454-198401000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Lamont RJ, Postlethwaite R. Carriage of Listeria monocytogenes and related species in pregnant and non-pregnant women in Aberdeen, Scotland. J Infect. 1986;13:187–193. doi: 10.1016/s0163-4453(86)93121-x. [DOI] [PubMed] [Google Scholar]
- 14.Lorber B. Listeria monocytogenes. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005. [Google Scholar]
- 15.Macnaughton M. Listeria monocytogenes in abortion. Lancet. 1962;2:482–483. doi: 10.1016/s0140-6736(62)90342-2. [DOI] [PubMed] [Google Scholar]
- 16.Ansbacher R, Borchardt KA, Hannegan MW, Boyson WA. Clinical investigation of L. monocytogenes as a possible cause of human fetal wastage. Am J Obstet Gynecol. 1966;94:386–390. doi: 10.1016/0002-9378(66)90660-0. [DOI] [PubMed] [Google Scholar]
- 17.Manganiello PD, Yearke RR. A 10-year prospective study of women with a history of recurrent fetal losses fails to identify Listeria monocytogenes in the genital tract. Fertil Steril. 1991;56:781–782. doi: 10.1016/s0015-0282(16)54617-2. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed August 27, 2008];Reprotox: An information system on environmental hazards to human reproduction and development. doi: 10.1300/J115v27n01_05. Reprotox Web site. http://www.reprotox.org. [DOI] [PubMed]
- 19.Mylonakis E, Hohmann EL, Calderwood SB. Central nervous system infection with Listeria monocytogenes. 33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 1998;77:313–336. doi: 10.1097/00005792-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Heikkinen T, Laine K, Neuvonen PJ, Ekbald U. The transplacental transfer of the macrolide antibiotics erythromycin, roxithromycin and azithromycin. BJOG. 2000;107:770–775. doi: 10.1111/j.1471-0528.2000.tb13339.x. [DOI] [PubMed] [Google Scholar]
- 21.Braden CR. Listeriosis. Pediatr Infect Dis J. 2003;8:745–746. doi: 10.1097/01.inf.0000079439.30496.57. [DOI] [PubMed] [Google Scholar]


