Abstract
Two types of supramolecular transformations, wherein a self-assembled Pt(II)-pyridyl metal-organic polygon is controllably converted into an alternative polygon, have been achieved through the reaction between cobalt carbonyl and the acetylene moiety of a dipyridyl donor ligand. A [6+6] hexagon is transformed into two [3+3] hexagons and a triangle-square mixture is converted into [2+2] rhomboids. 1H and 31P NMR spectra are used to track the transformation process and evaluate the yield of new self-assembled polygons. Such transformed species are identified by electrospray ionization (ESI) mass spectrometry. This new kind of supramolecule-to-supramolecule transformations provides a viable means for constructing, and then converting, new self-assembled polygons.
The past two decades have witnessed the tremendous development of coordination-driven self-assembly that serves as a powerfully versatile means of constructing supramolecular polygons and polyhedra,1 which can be functionalized on their interior2 or exterior3 surfaces and be potentially employed as precursors of electrical,3d,4 catalytic2c,5 and/or photophysical materials.6 By virtue of the common bonding geometries of transition metals—typically octahedral, square planar or tetrahedral, dative metal-ligand bonding can precisely dictate the formation of desired coordination skeletons when coupled with rigid angular donor ligands, thus generating a pre-programmed library of discrete 2-D and 3-D supramolecular structures. Similarly, numerous examples of self-assembled bio-supramolecules are found throughout nature, many of which are capable of achieving a variety of biological functions through conformational changes induced by the incitation of external inputs such as coordinating metal ions, pH, redox potential, magnetic field and light irradiation.7 Inspired by nature, we envisioned that coordination-driven self-assembled supramolecules may also be able to undergo conformational transitions to generate different structures, thereby providing a new strategy to construct supramolecular architectures by means of supramolecule-to-supramolecule transformations, in contrast to the bottom-up approach by using a wide range of building blocks. Up to now, the structural transformations of discrete self-assembled supramolecules are rarely studied.8
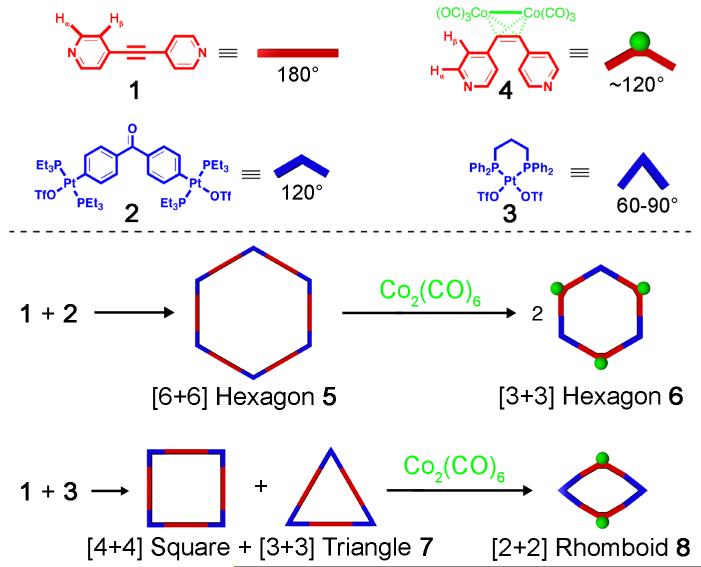
Acetylene moieties are well known to react with metal-carbonyl, thus changing their linear geometry to a tetrahedral M2C2 core (Scheme 1).9 Furthermore, acetylene units are extensively incorporated into many donor and acceptor building blocks utilized in coordination-driven self assembly due to their rigid linear conformation.1a-1c We therefore hypothesize that the reaction of acetylenes with metal-carbonyl complexes may be employed as a probe to implement conformational conversions in supramolecular assembiles. Herein, we demonstrate homotype and heterotype transformations of self-assembled polygons, a [6+6] hexagon to two [3+3] hexagons and a triangle-square mixture to rhomboids, through varying the angle between bonding sites of donor ligand from 180° to 120° upon the bonding of Co2(CO)6 with acetylene moiety.
Scheme 1.
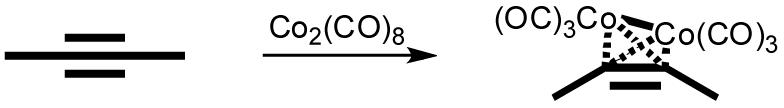
Schematic Representation of C≡C Conformational Transition upon the Bonding of Cobalt Carbonyl.
To date, two main approaches have been developed for the coordination-driven self-assembly of planar hexagons that are based upon the geometries of ditopic transition-metal acceptors and ditopic organic donor ligands with predesigned angles between their bonding sites. Complementary 120° and 180° building blocks give rise to a [6+6] hexagon while 120° and 120° units result in [3+3] hexagonal structures.10 With this in mind, we expect that the linear donor ligand bis(4-pyridyl)acetylene (1) will react with 120° di-Pt(II) acceptor (2) to form a [6+6] hexagon and anticipate that addition of cobalt carbonyl will induce 1 to adopt a 120° geometry, thus causing a transformation from a [6+6] hexagon to two [3+3] hexagons (Scheme 2).
Scheme 2.
Graphical Representation of Two Types of Transformations: Hexagon-to-Hexagon and Triangle-Square-to-Rhomboid.
The reaction of 1 with an equimolar amount of 120° building block 2 in thoroughly degassed CD2Cl2 yields a clear pale-yellow solution of 5. Multinuclear NMR (1H and 31P) analysis of the reaction mixture indicates the formation of a discrete supramolecule with high symmetry (Figure 1a and Figure S2). The 31P{1H} NMR showed a sharp singlet at δ = 13.47 ppm, upfield shifted by roughly 8.4 ppm as compared with the starting acceptor ligand 2 (δ = 21.9 ppm) as a result of the coordination of pyridine moiety. Electrospray ionization (ESI) mass spectrometry confirms the formation of the expected [6+6] hexagon (m/z = 1676.0 for [M - 5OTf]5+, Figure S1), which is analogous to a reported [6+6] hexagon composed of 4,4′-bis(trans-Pt(PPh3)2(OTf))benzophenone and 4,4′-bipyridine.10a
Figure 1.
Partial 1H (left) and 31P (right) NMR spectra of hexagon-to-hexagon transformation (300MHz, CD2Cl2, 298K): (a) [6+6] hexagon 5; (b) titration with 0.20 equivalents Co2(CO)8 stock solution (25 mg in 1mL degassed CD2Cl2); (c) 0.40 equiv; (d) 0.60 equiv; (e) 0.80 equiv; (f) 1.00 equiv; (g) independently prepared [3+3] hexagon 6.
Following the addition of 0.20 equivalents of Co2(CO)8 in degassed CD2Cl2 (25 mg/mL) to self-assembly 5, a new phosphorous peak at δ = 13.78 ppm in 31P{1H} NMR spectrum appears along with a new doublet β-H peak of the pyridine ring at δ = 7.97 ppm in 1H NMR, relative to the original doublet β-H peak at δ = 8.06 ppm in the [6+6] hexagon 5 (Figure 1(b)). Gradually increasing the amount of Co2(CO)8 from 0.20 to 1.00 equiv results in a diminishing of the original phosphorous peak characteristic for [6+6] hexagon 5 and the simultaneous increase of the renascent peak at δ = 13.78 ppm. One side-product can be identified in approximate 5% yield at δ = 16.13 ppm in 31P{1H} NMR spectrum. As shown in Figure 1a-f, both α- and β-H peaks of the pyridine ring experience upfield shifts of 0.03 and 0.10 ppm, respectively, in agreement with the fact that the Co2(CO)6 chelation to the carbon-carbon triple bond has a stronger electron-donating effect on the proximal β-hydrogen of pyridine ring. The 31P and 1H NMR spectra following the addition of 1.0 equiv of Co2(CO)8 (Figure 1f) affirm the completeness of the transformation with high efficiency and the formation of a new species, although the proton NMR spectrum is broadened due to the influence of paramagnetic cobalt complex in the mixture.
ESI mass spectrometry was then employed to identify the new species in the solution corresponding to the NMR spectrum shown in Figure 1f. Consistent with previous reports that examine the interaction of acetylene units with Co2(CO)6,9 the stoichiometry of the new species formed upon addition of Co2(CO)8 to [6+6] hexagon 5 should be [2 + (4-C5H4N)2C≡CCo2(CO)6]n, with n = 3 in the case of expected [3+3] hexagon. The ESI mass spectrometry analysis of the solution following the stoichiometric addition of Co2(CO)8 shows two peaks at m/z = 2560.6 and 1206.1 corresponding to two charged species [M - 2OTf]2+ and [M - 4OTf]4+, respectively, where M represents an intact self-assembled cyclic supramolecular polygon. These results indicate that the cyclic polygon is, in fact, [3+3] hexagon 6. The two mass peaks of [3+3] hexagon were isotopically resolved and are in a good agreement with theoretical distribution, as shown in Figure 2.
Figure 2.

Theoretical (red) and experimental (blue) electrospray ionization (ESI) mass spectrum of two charged species of [3+3] hexagon following the addition of 1.0 equiv Co2(CO)8 to [6+6] hexagon 5.
To further elucidate the outcome and efficiency of the transformation of [6+6] hexagon 5 to [3+3] hexagon, we synthesized the adduct ligand (4-C5H4N)2C2Co2(CO)6 4 via the reaction between donor ligand 1 and Co2(CO)8,11 and carried out its self-assembly with acceptor ligand 2 as a reference. The resulting dark-red CD2Cl2 solution was characterized by 31P and 1H NMR spectra (Figure 1g and Figure S3), revealing a sharp singlet at 13.77 ppm with concomitant 195Pt satellites and a pair of α- and β-H doublets located at the same position as transformed species, respectively. Its ESI mass spectrum also displayed two peaks at m/z = 2560.6 and 1206.1, corresponding to [M - 2OTf]2+ and [M - 4OTf]4+ of [3+3] hexagon 6, respectively. It is noteworthy that the same phosphorous peak at δ = 16.10 ppm that was observed in transformation solution (Figure 1f) appears also in the 31P NMR spectra of self-assembly [3+3] hexagon 6 (Figure 1g), suggestive of the consistent existence of one minor side-product between 120° acceptor 2 and cobalt carbonyl complex 4. Consequently, the above observations clearly establish that the [6+6] hexagon 5 can be chemically induced to transform into [3+3] hexagon 6 in response to conformational changes in donor ligand 1, substantiating the occurrence of a supramolecule-to-supramolecule transformation.
By analogy to fluxional bio-supramolecules that can, upon coordination of metal ions, be induced to adopt a single conformation,7 we further attempted to translate such selective conformational change in an equilibrium mixture of coordination-driven self-assembled polygons. Previous studies involving the flexible ditopic Pt(II) acceptor ligand (dppp)Pt(OTf)2 3 (dppp = bis(diphenylphosphino)propane) have shown that it is capable of serving as 90° and 60° angular acceptors and, together with linear dipyridyl ligands, gives rise to an equilibrium between square and triangular metallacycles in solution.12 This phenomena appears as well in the self-assembly between 3 and the linear ligand 1 in CD2Cl2 solution, resulting in mixture 7. The 31P{1H} NMR study clearly reveals two sharp singlets at δ = -14.96 and -15.23 ppm, indicating the co-existence of two self-assembled polygons (Figure S4). The corresponding proton NMR spectrum shows a 2-fold set of signals with similar splitting patterns at δ = 8.99 and 8.91 ppm, which can be ascribed to α-H atoms on the pyridine rings of two different polygons. In addition, two isotopically well-resolved mass peaks at m/z = 1298.6 and 1479.3 resulting from [square - 3OTf]3+ and [triangle - 2OTf]2+ were observed in the ESI mass spectrum (Figure S1).
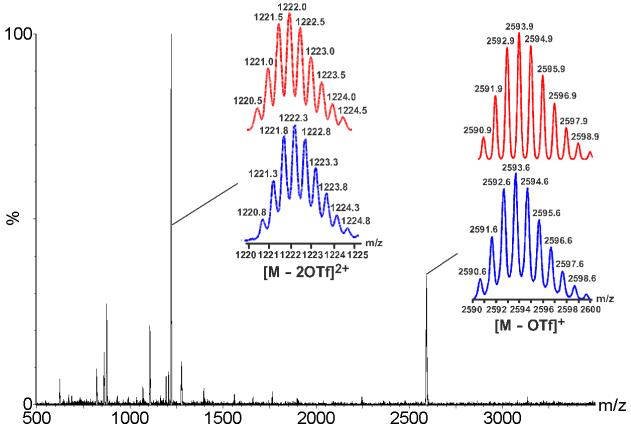
The addition of Co2(CO)8 to triangle-square mixture 7 will likely induce a supramolecular transformation as the Co2(CO)6 bonds to the acetylene moiety of 1. It is expected that conformational transition of 1 will lead to the formation of [2+2] rhomboid, a geometry that can accommodate a variety of tuning angles of the di-Pt(II) acceptor, which may be beneficial given the flexibility of acceptor ligand 3. Tracking the stepwise addition of Co2(CO)8 (in degassed CD2Cl2) into the triangle-square mixture via NMR spectroscopy encountered much difficulty because the nuclear resonance signals are highly unstable. Therefore, 1.0 equiv of cobalt carbonyl CD2Cl2 solution was added directly to 7 and the mixture was allowed to equilibrate for one day under N2 gas, thereby producing a red solution with a small amount of dark-red precipitate. Two sharp singlets at δ = -14.49 and 7.98 ppm can be discerned in the phosphorous NMR spectrum (Figure S5). Both α- and β-H peaks of pyridine rings, which appear as two doublets, shift upfield by Δδ = 0.17-0.25 and 0.3 ppm, respectively. Two pairs of 1H NMR signals corresponding to the phenyl rings of dppp can be differentiated in 4:1 ratio, however one showed no significant shift in comparison with those in triangle-square mixture while the other converged to a more “central” position at δ = 7.55 ppm. In the ESI mass spectrum of this solution, two peaks of high intensity were observed at m/z = 2593.6 and 1222.3 and can be attributed to the loss of triflate anions, [M - OTf]+ and [M - 2OTf]2+, respectively, for a self-assembled [2+2] polygon composed of 3 and (4-C5H4N)2C2Co2(CO)6. As illustrated in Figure 3, these two peaks were isotopically resolved and agree very well with their corresponding theoretical distribution.
Figure 3.
Full ESI mass spectrum of the transformed mixture resulting from adding 1.0 equiv Co2(CO)8 into triangle-square mixture 7. Insets are isotopically resolved peaks of [2+2] rhomboid.
Subsequent self-assembly by mixing acceptor ligand 3 with (4-C5H4N)2C2Co2(CO)6 4 yielded a standard NMR (Figure S5) and ESI mass spectra of [2+2] rhomboid 8 for comparison. A sharp singlet at -14.52 ppm in the 31P{1H} NMR spectrum nearly matches the observed phosphorous peak at -14.49 ppm of the transformed mixture, strongly suggestive that a majority of triangle-square polygonal supramolecules have been transformed into [2+2] rhomboids. Similarly, the chemical shift differences of α- and β-H signals between the pyridine rings of the transformed mixture and the standard rhomboid 8 are negligible. Although the yield of the [2+2] rhomboid as converted from the triangle-square mixture (7) is roughly 80%, possibly due to the flexibility of acceptor ligand 3 and the precipitate of a partial pyridine-cobalt carbonyl adduct (see Supporting Information), the conformational change of pyridine donor ligand 1 indeed drives the triangle-square equilibrium to the formation of [2+2] rhomboid.
In conclusion, the aforementioned results demonstrate a viable means for constructing new self-assembled polygons through supramolecule-to-supramolecule transformations. The interaction between acetylenes and cobalt carbonyl produces conformational transitions of dipyridyl ligand 1 from linear to tetrahedral, thus facilitating two types of supramolecular transformations: (1) from a [6+6] hexagon to two [3+3] hexagons and (2) from a triangle-square mixture to [2+2] rhomboids. The transformation process involves the breakage of dynamic Pt-N bonds along with the interaction of Co2(CO)6 with acetylene units and subsequent re-formation of new Pt-N bonds to generate new self-assembled metallacycles. In both cases, the transformations impelled by the Co2(CO)6 is accompanying by an increase in number and decrease in size of the resulting polygons (see Supporting Information), which likely contributes to the completeness of the transformations in view of increased entropy. Efforts to extend this strategy to other polygons and polyhedra and to take advantage of other organic and/or inorganic reaction motifs to produce novel, controllable supramolecular architectures are underway.
Supplementary Material
Acknowledgement
P.J.S. thanks the NIH (Grant GM-057052) and the NSF (Grant CHE-0306720) for financial support. B.H.N. thanks the NIH (Grant GM-080820) for financial support.
References
- (1).(a) Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502. [Google Scholar]; (b) Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (c) Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (d) Holliday BJ, Mirkin CA. Angew. Chem., Int.Ed. 2001;40:2022. [PubMed] [Google Scholar]; (e) Cotton FA, Lin C, Murillo CA. Acc. Chem. Res. 2001;34:759. doi: 10.1021/ar010062+. [DOI] [PubMed] [Google Scholar]; (f) Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:371. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (g) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:351. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (h) Severin K. Chem. Commun. 2006:3859. doi: 10.1039/b606632c. [DOI] [PubMed] [Google Scholar]; (i) Pitt MA, Johnson DW. Chem. Soc. Rev. 2007;36:1441. doi: 10.1039/b610405n. [DOI] [PubMed] [Google Scholar]
- (2).(a) Tominaga M, Suzuki K, Murase T, Fujita M. J. Am. Chem. Soc. 2005;127:11950. doi: 10.1021/ja054069o. [DOI] [PubMed] [Google Scholar]; (b) Sato S, Iida J, Suzuki K, Kawano M, Ozeki T, Fujita M. Science. 2006;313:1273. doi: 10.1126/science.1129830. [DOI] [PubMed] [Google Scholar]; (c) Murase T, Sato S, Fujita M. Angew. Chem., Int. Ed. 2007;46:1083. doi: 10.1002/anie.200603561. [DOI] [PubMed] [Google Scholar]; (d) Murase T, Sato S, Fujita M. Angew. Chem., Int. Ed. 2007;46:5133. doi: 10.1002/anie.200700793. [DOI] [PubMed] [Google Scholar]
- (3).(a) Yang H-B, Das N, Huang F, Hawkridge AM, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2006;128:10014. doi: 10.1021/ja063377z. [DOI] [PubMed] [Google Scholar]; (b) Yang H-B, Hawkridge AM, Huang SD, Das N, Bunge SD, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2007;129:2120. doi: 10.1021/ja066804h. [DOI] [PubMed] [Google Scholar]; (c) Yang H-B, Ghosh K, Northrop BH, Zheng Y-R, Lyndon MM, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2007;129:14187. doi: 10.1021/ja073744m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yang H-B, Ghosh K, Zhao Y, Northrop BH, Lyndon MM, Muddiman DC, White HS, Stang PJ. J. Am. Chem. Soc. 2008;130:839. doi: 10.1021/ja710349j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Cotton FA, Lin C, Murillo CA. J. Am. Chem. Soc. 2001;123:2670. doi: 10.1021/ja004149m. [DOI] [PubMed] [Google Scholar]
- (5).Yoshizawa M, Takeyama Y, Kusukawa T, Fujita M. Angew. Chem., Int. Ed. 2002;41:1347. doi: 10.1002/1521-3773(20020415)41:8<1347::aid-anie1347>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- (6).Balzani V, Bergamini G, Campagna S, Puntoriero F. Top. Curr. Chem. 2007;280:1. [Google Scholar]
- (7).Dublin SN, Conticello VP. J. Am. Chem. Soc. 2008;130:49. doi: 10.1021/ja0775016. [DOI] [PubMed] [Google Scholar]
- (8).(a) Sun S-S, Anspach JA, Lees AJ. Inorg. Chem. 2002;41:1862. doi: 10.1021/ic010998e. [DOI] [PubMed] [Google Scholar]; (b) Sun S-S, Stern CL, Nguyen ST, Hupp JT. J. Am. Chem. Soc. 2004;126:6314. doi: 10.1021/ja037378s. [DOI] [PubMed] [Google Scholar]
- (9).(a) Abel EW, Stone FGA, Wilkinson G. Comprehensive Organometallic Chemistry II. Vol. 8. Pergamon; Oxford: 1995. Chapter 1. [Google Scholar]; (b) Chung M-C, Sakurai A, Akita M, Moro-oka Y. Organometallics. 1999;18:4684. [Google Scholar]
- (10).(a) Stang PJ, Persky NE, Manna J. J. Am. Chem. Soc. 1997;119:4777. [Google Scholar]; (b) Leininger S, Schmitz M, Stang PJ. Org. Lett. 1999;1:1921. doi: 10.1021/ol9911425. [DOI] [PubMed] [Google Scholar]; (c) Baxter PNW, Khoury RG, Lehn JM, Baum G, Fenske D. Chem. Eur. J. 2000;6:4140. doi: 10.1002/1521-3765(20001117)6:22<4140::aid-chem4140>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]; (d) Yang H-B, Das N, Huang F, Hawkridge AM, Diaz DD, Arif AM, Finn MG, Muddiman DC, Stang PJ. J. Org. Chem. 2006;71:6644. doi: 10.1021/jo0608117. [DOI] [PubMed] [Google Scholar]; (e) Coronado E, Galan-Mascaros JR, Gavina P, Marti-Gastaldo C, Romero FM, Tatay S. Inorg. Chem. 2008;47:5197. doi: 10.1021/ic8000569. [DOI] [PubMed] [Google Scholar]
- (11).Song L-C, Jin G-X, Wang H-T, Zhang W-X, Hu Q-M. Organometallics. 2005;24:6464. [Google Scholar]
- (12).Schweiger M, Seidel SR, Arif AM, Stang PJ. Inorg. Chem. 2002;41:2556. doi: 10.1021/ic0112692.and references therein.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.