Abstract
It has been suggested that discrepant findings regarding low basal cortisol levels and enhanced suppression of cortisol in response to dexamethasone (DEX) administration in PTSD may reflect individual differences in gender, trauma type, stage of development at trauma occurrence (e.g., childhood vs. adulthood), early pre-traumatic risk factors, or other individual differences. This study examined salivary cortisol levels at 8:00 a.m. and 4:00 p.m. as well as cortisol response to 0.5 mg DEX in 40 female Vietnam nurse veterans who had current, chronic PTSD (Current) vs. 43 who never had PTSD (Never). Repeated measures analyses of covariance did not reveal significant group differences in cortisol levels or cortisol suppression. Given that nurses who served in Vietnam had similar exposures, ages at exposure, and duration since exposure to previously studied male Vietnam combat veterans, the present lack of evidence for low cortisol and cortisol hyper-suppression in nurses with PTSD suggests that previous findings of low cortisol and cortisol hyper-suppression in male Vietnam veterans, females sexually abused as children, and other populations may reflect risk factors beyond simply having PTSD.
Keywords: stress disorders, post-traumatic, dexamethasone, comorbidity, depressive disorder
1. Introduction
As a unifying concept, PTSD was intended to capture a universal response to any life-threatening event. Consequently, studies examining biological alterations in PTSD have not typically included subgroup analyses addressing whether the type of trauma exposure, chronicity of the PTSD, or other individual difference factors might explain heterogenous biological findings (Yehuda, 2002, 2006). And yet, the type of event to which the person has been exposed, is (among other things) a potential source of individual variability that might explain different biological phenotypes in this disorder.
The earliest biological investigations in PTSD were performed in combat veterans, who seemed to be a prototypic PTSD group in that their symptom manifestations appeared to generalize to survivors of other types of traumatic exposures. The first published neuroendocrine study demonstrated significantly lower 24-hr mean urinary cortisol excretion in combat Vietnam veterans with PTSD compared to veterans with other psychiatric disorders (Mason et al., 1986). To determine whether low cortisol levels reflected altered regulation of the hypothalamic-pituitary-adrenal axis, studies using the low dose dexamethasone suppression test (DST) were performed. Most found evidence for an exaggerated suppression of cortisol following 0.50 mg dexamethasone (DEX), reflecting a greater negative feedback inhibition in PTSD (reviewed in Yehuda, 2002).
With subsequent studies, discrepant observations about cortisol levels and, to a lesser degree, the cortisol response to DEX administration, emerged. Generally, evidence for lower basal urinary and plasma cortisol, and increased plasma cortisol suppression on the DST, has come from studies that compared relatively homogeneous PTSD and trauma-exposed groups (e.g., combat veterans, Holocaust survivors, victims of domestic violence, adult survivors of childhood sexual abuse, children exposed to earthquakes) (e.g., Yehuda, 2002, 2005, 2006; Bremner et al. 2007; Heim et al. 2001; Griffin et al., 2005), whereas negative findings were more likely in studies in which more heterogenous or epidemiological samples have been compared to a control group consisting of non-trauma-exposed persons without PTSD (Wessa et al., 2006; Young and Breslau, 2004a, 2004b; de Kloet et al, 2007). It may be that in the latter studies the “noise” associated with individual differences in gender, PTSD duration, type of exposure, and/or age overwhelms the neuroendocrine “signal” associated with PTSD. Alternatively, previously reported neuroendocrine correlates of PTSD might actually reflect specific PTSD risk factors. Consequently, the reliability and generality of cortisol findings is difficult to ascertain due to the sometimes substantive sample differences between studies that produce differing results.
Gender, in particular, is a potentially important mediator/moderator of basal cortisol levels and hyper-suppression of cortisol. There is some evidence suggesting that gender may be an important variable in differentiating different cortisol-related phenotypes. For example, Gill et al. (2005) observed that studies of male veterans have more consistently supported lower cortisol in PTSD than studies of females. However, studies of men have typically involved more chronic PTSD, thereby confounding gender and chronicity. The confounding of gender, trauma heterogeneity and PTSD chronicity makes it difficult to test which of these variables, or their combinations, contribute to cortisol alterations in PTSD.
The present study examined basal cortisol and cortisol suppression in a unique, homogeneous sample of female nurse veterans who served in Vietnam. This group closely paralleled previously studied male Vietnam combat veterans with respect to age at time of trauma (early adulthood), exposure to the culture of the Vietnam War experience, time elapsed since trauma, and duration of PTSD (thirty plus years). We hypothesized that female nurse Vietnam veterans with PTSD would show lower basal cortisol and higher cortisol suppression compared to nurse veterans without PTSD.
2. Methods
2.1. Participants
Mailings about the study were sent out to women all across the United States whose names appeared on the Women’s Vietnam War Memorial database. Interested persons completed the Women’s Wartime Stressor Scale (WWSS; Wolfe et al., 1993) for screening purposes (see Carson et al., 2000) and were flown to the Manchester, VA Medical Center if they met initial inclusion/exclusion criteria. Women were not invited to participate if it was determined during screening that they had current or past schizophrenic, paranoid, bipolar I, melancholic, or psychotic disorder. After a full explanation of study procedures, which were approved by the Manchester VA Medical Center Human Studies Subcommittee, participants gave written informed consent.
The Clinician Administered PTSD Scale: Current and Lifetime Diagnosis Version (CAPS-DX; Blake et al., 1995) was used to determine the presence or absence of PTSD (categorical diagnosis) and to provide a continuous measure of PTSD severity (CAPS total score). Participants also underwent the Structured Clinical Interview for DSM-IV (SCID; First et al., 1994) to confirm the absence of the above exclusionary disorders, and to evaluate presence or absence of allowable comorbidities (e.g., mood and other anxiety disorders). Other exclusion criteria included: a.) past but not current Vietnam-related PTSD (N=43); b.) substance dependence (N=3); and c.) use of steroid medications (N=1). None of the participants had current non-Vietnam related PTSD or an endocrinologic disorder.
2.2.Procedures
Participants completed the low-dose dexamethasone suppression test (LD-DST, 0.5 mg) according to the method of Yehuda et al. (1993). On Day 1, participants collected saliva cortisol samples at 0800h (PRE-DEX morning cortisol) and 1600h (PRE-DEX afternoon cortisol). They then ingested 0.5 mg of dexamethasone at 2300h. On Day 2, they collected saliva samples at 0800h (POST-DEX morning cortisol) and 1600h (POST-DEX afternoon cortisol). Samples were sent to Dr. Yehuda’s laboratory in New York via express mail.
Salivary cortisol was measured using a standard cortisol radioimmunoassay (RIA) kit developed by Clinical Assays. The interassay coefficient of variation is 4.0%. Salivary dexamethasone (morning and afternoon POST-DEX) was also determined by RIA in a procedure that uses 3H-dexamethasone, a commercially available antibody (IgG Corporation). Bound and free dexamethasone were separated by a 20% polyethylene glycol solution. The assay sensitivity limit is 0.1 ng/ml. Data from a total of 5 subjects were eliminated based on pre-specified criteria: a morning DEX level of zero (N = 3, suggesting the subject did not take the DEX) or a far greater cortisol level in the afternoon than in the morning (N = 2, suggesting inversion of morning and afternoon samples). Two participants had an undetectable Day 1 0800h cortisol level (< 5.0 ng/ml). Consequently, their data were missing from the analyses involving 0800h cortisol but were included in the analyses of 4:00 p.m. values. All cortisol values were log transformed prior to analysis due to skewness and kurtosis in the distribution (Yehuda et al., 2004). Group differences in baseline cortisol levels were examined using a two-factor, analysis of covariance for repeated-measures (ANCOVAR), with Group as a between-subjects factor, Time (Day-1 0800h vs. Day-1 1600h) as a within-subjects repeated measure, and body mass index (BMI) as a covariate. Group differences in morning (0800h) and afternoon (1600h) cortisol suppression were examined separately using ANCOVAR with Group as the between-subjects factor, Day (Day-1 vs. Day-2, i.e., pre- vs. post-DEX) as a within-subjects repeated measure, and BMI and DEX level (0800h and 1600h, respectively) as the covariates. Because depression has been found to be associated with higher basal cortisol and non-suppression, these analyses were repeated using depression as an additional covariate. Depression was coded as a dummy variable and reflected the presence or absence of current MDD and/or dysthymia. To identify other potential confounding variables, Pearson correlations between cortisol at each time point and age, military exposure (WWSS scores), and nicotine usage (number of cigarettes smoked in previous 24 hours) were examined. None of these correlations were significant and therefore were not used as covariates in the ANCOVARs.
3. Results
Participants were classified into current PTSD (Current, N = 40) vs. never-had PTSD (Never, N = 43) based on the CAPS results. Current co-morbid Axis I disorders for nurses with current PTSD included 14 major depressive, 4 dysthymic, 5 panic, 2 agoraphobic, 5 social phobic, 6 specific phobic, 1 obsessive compulsive, 1 undifferentiated somatoform, and 2 binge eating. Current disorders for nurses who never met criteria for PTSD included 1 major depressive, 1 dysthymic, 1 social phobic, and 3 specific phobic. (Some participants had more than one current co-morbid disorder.) About half the women with current PTSD had past MDD (N = 21). Thirteen participants in the Never PTSD group also met criteria for past MDD. Demographic and clinical data are presented in Table 1.
Table 1.
Demographic, Psychometric and Biologic Measures in Female Nurse Veterans with and without PTSD.
| Current PTSD (N = 40) | Never PTSD (N =43) | |||||
|---|---|---|---|---|---|---|
| Measures | M | SD | M | SD | t(df) | p |
| Demographic/Clinical | ||||||
| Age | 54 | 3.3 | 54 | 3.8 | 0.1(79) | .95 |
| WWSS | 47.9 | 14.7 | 43.5 | 10.5 | 1.6(81) | .12 |
| CAPS | 60.3 | 15.6 | 9.3 | 10 | 17.8(81) | <.001 |
| Biological | ||||||
| Body Mass Index | 29.7 | 7.1 | 28.7 | 5.9 | 0.7(77) | .49 |
| Cortisol level(ng/dl) | ||||||
| Day-1 (PRE-DEX) | ||||||
| 0800h | 59791.5 | 30798.4 | 52812.6 | 26872.3 | 1.0(79) | .30 |
| 1600h | 23077.0 | 15761.2 | 17812.6 | 11888.3 | 1.9(81) | .07 |
| Day-2 (POST-DEX) | ||||||
| 0800h | 7041.8 | 5201.7 | 5889.5 | 5513.7 | 1.7(79) | .09 |
| 1600h | 10237.7 | 11306.1 | 8938.6 | 17725.7 | 1.4(81) | .16 |
| % Suppression | ||||||
| 0800h | 87.3 | 9.1 | 85.0 | 19.1 | -1.3(79) | .21 |
| 1600h | 51.3 | 44.1 | 47.7 | 70.0 | -0.6(81) | .54 |
Cortisol data are shown as raw values, but the analyses were performed on log-transformed data. The df vary due to missing data. WWSS = Women’s Wartime Stressor Scale; CAPS = Clinician Administered PTSD Scale; DEX = dexamethasone.
Results of ANCOVAR for Day-1 baseline cortisol levels, adjusting for BMI, indicated the expected main effect of Time (F(1,74) = 17.4, p < 0.001). There was a non-significant trend for the Group main effect (F(1,74) = 3.3, p = .07) reflecting that in combining the two time points, there was a tendency for higher salivary cortisol in the Current, compared to Never, PTSD participants. However, there was no Group × Time interaction (F(1,74) < 1) (see Table and Figure). The covariate BMI accounted for a significant portion of the variance in Day-1 baseline cortisol levels (F(1,74) = 4.2, p= .04). When presence or absence of Depression was added as an additional covariate, the trend for the Group main effect was no longer present (F(1,73) = 1.1, p = .29).
Figure.
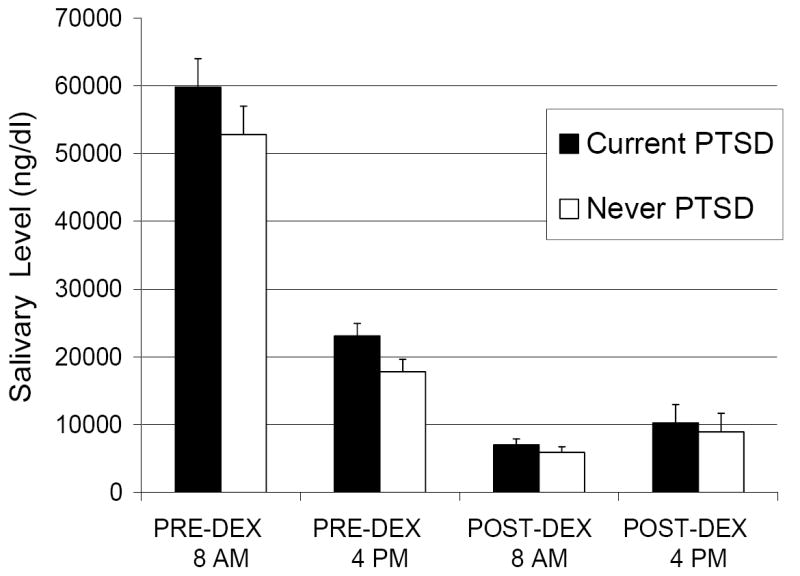
Group Mean (SE) Untransformed Salivary Cortisol Levels (ng/dl) in Female Nurse Vietnam Veterans with N = 40 and without N = 43 PTSD.
Results of ANCOVAR examining morning cortisol suppression, adjusting for BMI and DEX levels, revealed the expected significant effect for Day (F(1,72) = 18.9, p < .001) reflecting lower 0800h POST-DEX cortisol levels across groups (see Table and Figure), but no Group × Day interaction (F(1,72) <1). A non-significant trend for a Group main effect (F(1,72) = 3.3, p = .07), was present but disappeared when depression was added as a covariate (F(1,71)<1). The covariate BMI accounted for a nearly significant portion of the variance in this model (F(1,72) = 3.9, p = .05), but 0800h DEX level did not (F(1,72) < 1).
Results of ANCOVAR examining afternoon cortisol suppression, adjusting for BMI and DEX levels, showed a similar pattern, i.e., a significant Day main effect (F(1,75) = 4.4, p = .04), but no Group × Day interaction (F(1,75) < 1), and a trend level main effect of Group (F(1,75) = 2.9, p < .10) which was reduced by the addition of Depression as a covariate (F(1,74) < 1). In this model, however, BMI and DEX levels did not account for a significant portion of the variance (all p’s > .36). When the data were expressed as percent cortisol suppression, no group differences were observed (Table 1).
4. Discussion
The present study found no significant differences in cortisol levels as measured in morning and afternoon samples in nurses with current PTSD, compared to those without any (current or lifetime) PTSD, and no cortisol hypersuppression following administration of 0.50 DEX. The inclusion of depression as a covariate in the statistical model eliminated the trend for elevated baseline cortisol levels in the PTSD group when the two cortisol samples were considered together. These findings differ from reports of both lower and higher baseline cortisol levels in PTSD (for review, Yehuda, 2006). Insofar as this study is presented, in part, as a de facto examination of gender differences, given that the sample was selected to resemble male combat Vietnam veterans with chronic PTSD, it is important to consider whether the findings represent a true discrepancy between Vietnam nurses and combat veterans, or whether the lack of significant PTSD vs. non-PTSD group differences in this sample could be attributed to other factors, including methodological artifacts. Thus, we consider below possible explanations for the failure to replicate previous observations in other PTSD groups, particularly, male combat Vietnam veterans.
Although it may be argued that there are differences in the quality and severity of their traumatic Vietnam experiences, female Vietnam nurses represent the closest possible comparison group to male combat veterans with respect to the nature and severity of the focal trauma. Importantly, the Vietnam nurses with PTSD demonstrated PTSD symptom severity scores comparable to those reported in the literature for male combat veterans with PTSD. These similarities strongly suggest that the cortisol differences between the Vietnam nurses studied herein and previously studied male Vietnam combat veterans reflect a gender and/or other constitutional differences between women who served in Vietnam as nurses and men exposed to combat in this theatre.
Since deployment-related trauma may not explain the lack of group differences in baseline cortisol and cortisol suppression to DEX in the current study, differences in pre-military experiences may be relevant. Low cortisol levels have been particularly associated with early (childhood) traumatization (Tarullo & Gunnar, 2006, Shea et al., 2005). Therefore, a possible explanation for the failure to observe low cortisol in the current study sample may be that the nurse veterans generally reported their focal traumatic experiences to be limited to those that occurred in Vietnam. Only 3 of 40 PTSD and 2 of 43 non-PTSD subjects reported traumatic events that occurred during childhood. In contrast, childhood trauma has been commonly reported by Vietnam combat veterans who develop combat-related PTSD, (e.g., Bremner et al., 1993; Zaidi and Foy, 1994). This discrepancy may be associated with the fact that nurses had to pass a higher selection threshold (usually a college degree) to become military officers and thereby, are more likely to come from advantaged backgrounds than the male veterans, who were mostly enlisted men.
It is also possible that the absence of group differences in either cortisol levels or cortisol suppression reflects a common influence of trauma exposure in Vietnam across PTSD and non-PTSD groups, rather than PTSD per se. This possibility cannot be addressed in the present study because of the absence of a non-trauma-exposed comparison group. However, unlike early traumatization, which has been demonstrated to mediate lower cortisol levels and hypersuppression on the DST, adult traumatization has not generally been found to result in long-lasting changes in cortisol in the absence of accompanying psychopathology (Young and Breslau, 2004a; Yehuda et al., 2004).
It is important to note that previous studies that have measured cortisol from saliva, as was done in the present study, have not demonstrated group differences in cortisol suppression as often as studies that have measured cortisol from plasma (Lindley et al., 2004; Lipschitz et al., 2003). The failure to observe group differences in the current study may have resulted from using saliva rather than plasma. Interestingly, a comparison of the mean percent suppression observed in the current study with those previously reported (e.g., Yehuda, 2002) suggests that the suppression shown by the nurse veterans without PTSD was greater than that which has been typically observed in normal controls using plasma samples of cortisol in response to 0.50 mg DEX (e.g., in the range of 60-80% for healthy controls compared to a range of 85-95% for PTSD patients). The low dose DST was previously validated and standardized on plasma. However, when suppression in both plasma and saliva for multiple doses of prednisone and dexamethasone have been examined in normal subjects, suppression in saliva is much greater than in plasma. Indeed, there was a 90% suppression of salivary cortisol with 0.50 mg DEX in normal subjects (Pariante et al., 2002), raising the possibility that 0.50 mg DEX is not a low enough dose for a salivary DST in which the objective is to examine hypersuppression, rather than nonsuppression. Results of this study highlight this potential floor effect in a traumatized group. Future studies should consider using even lower doses in PTSD when salivary samples are collected. Related to this methodologic concern, an additional limitation is that the baseline salivary cortisol measure was derived from only one day of morning and afternoon sampling. Because cortisol levels vary from day to day, sampling across several pre-DEX days would have been preferable to provide a more accurate estimate of baseline. Having only one day of pre-DEX sampling can lead to a “noisy” baseline cortisol measure, which could have reduced the power to detect stable individual differences in baseline cortisol between groups.
In conclusion, the findings contribute to our understanding of the neuroendocrine aspects of PTSD by indicating that PTSD alone is insufficient to produce low basal cortisol and/or cortisol hyper-suppression. This suggests that the previously reported low basal cortisol and enhanced HPA axis negative feedback inhibition in the PTSD literature reflect risk factors beyond the mere presence of PTSD. Such factors may include early traumatization; type, timing, and duration of trauma exposure; gender; and the interaction of these factors, as well as factors and interactions still to be discovered.
Acknowledgments
This research was supported by a Department of Veterans Affairs Health Services Research and Development Service Award (Dr. Carson), a Department of Veterans Affairs Medical Research Service Award (Dr. Orr), and National Institute of Mental Health Grant #RO3MH57386 (Dr. Metzger).
The authors wish to thank Heike Croteau, Karen Sheldon, and Pauline Simard for assistance in the implementation of this project. Finally, we would like to express our appreciation to the Vietnam Women’s Memorial Project, Inc. for their support, and to the nurse veterans for their willingness to participate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, Geracioti TD. Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. American Journal of Psychiatry. 2005;162:992–994. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LN, Kaloupek DG, Gusman FD, Charney DS, Keane T. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. American Journal of Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Bremner D, Vermetten E, Kelley ME. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. Journal of Nervous and Mental Disorders. 2007;195:919–927. doi: 10.1097/NMD.0b013e3181594ca0. [DOI] [PubMed] [Google Scholar]
- Carson MA, Paulus LA, Lasko NB, Metzger LJ, Wolfe J, Orr SP, Pitman RK. Psychophysiologic assessment of posttraumatic stress disorder in Vietnam nurse veterans who witnessed injury or death. Journal of Consulting and Clinical Psychology. 2000;68:890–897. [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EG, Westenberg HG. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2007;32:215–226. doi: 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders, Version 2.0. New York: Biometrics Research Department; 1994. [Google Scholar]
- Gill JM, Szanton SL, Page GG. Biological underpinnings of health alterations in women with PTSD. A sex disparity. Biological Research for Nursing. 2005;7:44–54. doi: 10.1177/1099800405276709. [DOI] [PubMed] [Google Scholar]
- Glover DA, Poland RE. Urinary cortisol and catecholamines in mothers of child cancer survivors with and without PTSD. Psychoneuroendocrinology. 2002;27:805–819. doi: 10.1016/s0306-4530(01)00081-6. [DOI] [PubMed] [Google Scholar]
- Griffin MG, Resick PA, Yehuda R. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. American Journal of Psychiatry. 2005;162:1192–1199. doi: 10.1176/appi.ajp.162.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Lindauer RJL, Olff M, van Meijel EPM, Carlier IVE, Gersons BPR. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biological Psychiatry. 2006;59:171–177. doi: 10.1016/j.biopsych.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Lindley SE, Carlson EB, Benoit M. Basal and dexamethasone suppressed salivary cortisol concentrations in a community sample of patients with posttraumatic stress disorder. Biological Psychiatry. 2004;55:940–945. doi: 10.1016/j.biopsych.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Lipschitz DS, Rasmusson AM, Yehuda R, Wang S, Anyan W, Gueoguieva R, Grilo CM, Fehon DC, Southwick SM. Salivary cortisol responses to dexamethasone in adolescents with posttraumatic stress disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;421:1310–1317. doi: 10.1097/01.chi.0000084832.67701.0d. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L, Biondi M, Bosmanse F, Kenis G, Scharpe S. Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta psychiatrica Scandinavica. 1998;98:328–335. doi: 10.1111/j.1600-0447.1998.tb10092.x. [DOI] [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free-cortisol levels in posttraumatic stress disorder patients. Journal of Nervous and Mental Disorders. 1986;174:145–149. doi: 10.1097/00005053-198603000-00003. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Papadopoulos AS, Poon L, Checkley SA, English J, Kerwin RW, Lightman S. A novel prednisolone suppression test for the hypothalamic-pituitary-adrenal axis. Biological Psychiatry. 2002;51:922–930. doi: 10.1016/s0006-3223(01)01314-2. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Lipschitz DS, Wang S, Hu S, Vojvoda D, Bremner JD, Southwick SM, Charney DS. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biological Psychiatry. 2001;50:965–977. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30:162–78. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Brown PJ, Furey J, Levin K. Development of a wartime stressor scale for women. Psychological Assessment. Journal of Consulting and Clinical Psychology. 1993;5:330–335. [Google Scholar]
- Yehuda R. Current status of cortisol findings in posttraumatic stress disorder. The Psychiatric clinics of North America. 2002;25:341–368. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Neuroendocrine aspects of PTSD. Handbook of Experimental Pharmacology. 2005;169:371–403. doi: 10.1007/3-540-28082-0_13. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Annals of the New York Academy of Sciences. 2006;1071:137–66. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Mason JW, Giller EL. Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorders. Biological Psychiatry. 1993;34:18–25. doi: 10.1016/0006-3223(93)90252-9. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004;29:389–404. doi: 10.1016/s0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder. An epidemiologic community study. Archives of General Psychiatry. 2004a;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder: A community epidemiologic study. Biological Psychiatry. 2004b;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]


