Abstract
Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) are ubiquitous human pathogens which infect their hosts for life and reactivate to cause disease at or near the initial site of infection. As the incidence of genital HSV-1 infections increase, there is an increased demand for valid viral typing diagnostics. In this report, we reconsidered and developed a triple-phase, immune-typing procedure that compares differences in electrophoretic mobilities of viral ICP4, ICP27, and VP22 proteins between HSV-1 and HSV-2 strains. We isolated and immunotyped five primary HSV-1 strains derived from oro-facial, ocular, and genital areas along with two primary HSV-2 strains from the genital area. Advantages of this methodology include its general technical simplicity, sensitivity, and ability to definitively type HSV. It is anticipated that this methodology will be useful in distinguishing viruses obtained in clinical cultures.
Keywords: HSV-1, HSV-2, diagnostics, viral protein, immunoblotting
1. Introduction
Herpes simplex viruses cause widespread infections in the human population. In the case of HSV-1, the severity can range from simple cold sores to fatal encephalitis (Markoulatos et al., 1997). Vertical transmission of HSV-2 can result in disseminated neonatal infections which can have devastating consequences (Corey, 1994; Coyle et al., 1999). In each situation, the virus is spread by intimate oral/sexual contact, requiring penetration of the virus through skin or breaks in the mucous membrane. Once infection has initiated, the virus may migrate through innervating neuronal axons to the host ganglia where it lies dormant until reactivation (Dawkins, 1990). During a productive lytic infection in cultured cells, HSV replication follows a highly ordered program that involves tight transcriptional regulation which proceeds in a cascade fashion (Roizman, 2001). HSV gene expression begins with the immediate-early (IE) genes whose transcription occurs in the absence of de novo protein synthesis (Batterson and Roizman, 1983; Honess and Roizman, 1974; Honess and Roizman, 1975). The IE gene products, which include infected cell protein (ICP) 4 and 27, cooperatively act to regulate all kinetic classes of viral gene expression (Roizman, 2001). Synthesized next are the early (E) gene products, which encode proteins that are mainly associated with viral DNA synthesis (Boehmer and Lehman, 1997). The late (L) gene products, such as the major tegument virion protein (VP) 22, are the last set of viral proteins produced and are principally involved in virion assembly and structure (Enquist et al., 1998). Completion of the viral replication cycle ultimately results in the destruction of infected cells.
Advances in the field of molecular pathology are leading to developments in a number of polymerase chain reaction (PCR)-based differentiation techniques (Espy et al., 2000; Kessler et al., 2000; Kimberlin et al., 1996; Lakeman and Whitley, 1995; van Doornum et al., 2003). PCR-based restriction fragment length polymorphism (RFLP), in particular, is a sensitive method for typing HSV in the clinic (Madhavan, Priya, and Bagyalakshmi, 2003; Marshall et al., 2001). Although these assays require instrumentation, experienced technical support, and isolated work areas to prevent contamination, the detection of HSV DNA in cerebral spinal fluid is considered the "gold standard" for diagnosing HSV encephalitis in the clinical virology laboratory {reviewed in (Tyler, 2004; Whitley, 2006)}.
Detection of HSV from the skin and mucosa by PCR methods has been used routinely for over a decade (Hobson et al., 1997). More recently, quantitative real time PCR tests have significantly improved the sensitivity and selectively of these assays (Adelson et al., 2005; Espy et al., 2000; Filen et al., 2004; Jerome et al., 2002; Lai et al., 2005). While this argues strongly for HSV PCR to be the standard for all HSV diagnostics, virus isolation from mucosa and skin in shell cultures is still utilized in many clinical settings. Unfortunately, virus growth in cultured cells cannot distinguish between HSV-1 and HSV-2 so distinctions are often made based on clinical presentation.
In this study, we reconsidered the use of viral protein immunoblotting for differentiating herpes simplex viruses. The original HSV specific monoclonal antibodies were directed against HSV-1 antigens. It was almost immediately recognized that these new reagents cross-reacted with polypeptides from HSV-2 and it was proposed that they might serve as a means to differentiate HSV types, if used for western blotting (Pereira et al., 1976; Pereira et al., 1977). In our hands, immunoblot typing has been extremely useful for gene identification when combined with a panel of HSV-1 X HSV-2 intertypic recombinant strains (Blaho, Mitchell, and Roizman, 1994). We now present the definitive immunotyping five HSV-1 and two HSV-2 clinical isolates using the viral protein immunoblot technique. This method should complement and, perhaps, assist in validating current DNA based assays for clinical virus typing.
2. Materials and methods
2.1 Cell Culture
Vero cells were obtained from the American Type Culture Collection (Rockville, Md.) and were maintained in Dulbecco’s modified Eagle’s medium containing 5% fetal bovine serum (FBS), 100 µg/ml penicillin, and 100 µg/ml streptomycin. Unless otherwise noted, all cell culture reagents were purchased from Life Technologies. HSV-1(F) and HSV-2(G) are standard laboratory strains (Ejercito, Kieff, and Roizman, 1968). HSV-2(333) is another laboratory passaged virus which has been routinely used as a standard strain (Duff and Rapp, 1971; Oram et al., 2000). To obtain high titer virus stocks, confluent Vero monolayer cells (approximately 2×107) were inoculated with a multiplicity of infection (MOI) of 0.01 for 2 h at 37°C in medium containing 5% newborn calf serum (NBCS). The inoculum was then removed, fresh 5% NBCS was added, and the cells were incubated at 37°C in 5% CO2. Virus stocks were prepared once the infection reached a cytopathic effect of 100%. The infected cells were lysed by freezing at−80°C. After allowing them to warm to room temperature and transferring to a tube containing 3 ml of sterile 9% milk (Carnation nonfat, dry), extracts were sonicated (3 × 10 sec with cooling periods) using a Branson Sonifier 250 (output level 4) on ice to release the virus particles. Virus stocks were aliquotted and stored at −80°C. The virus titers were established from the number of plaque-forming units (PFU) detected with Vero cells following serial dilutions. An Olympus CK2 inverted phase-contrast microscope was used to count and pick viral plaques.
2.2 Isolation of HSV
HSV was isolated from clinical lesions as follows. After disinfecting the scab and surrounding area with ethanol, the scab was then removed by scraping with the end of a sterile swab. The lesion was then swabbed and the swab used to immediately inoculate 3 ml of 5% NBCS, which in turn was used to inoculate a confluent layer of Vero cells in a 25 cm2 flask (approximately 4 × 106 cells). The virus and cells were incubated with rocking at 37°C after which time the medium was aspirated and replaced with 3 ml of fresh 5% NBCS, followed by incubation at 37°C in 5% CO2 until the infection reached a cytopathic effect of approximately 100% (four to nine days). An original virus stock was prepared and its titer determined as described above. The isolation of all viruses in this study were performed in accordance with the guidelines of our Institutional Review Board.
2.3 Plaque purifications
Serial dilutions of the HSV stocks were made in 5% NBCS (3 ml) and used to inoculate a series of 25 cm2 flasks containing confluent Vero cells. The cells were incubated with rocking at 37°C for 2 h, the medium was replaced with fresh containing 2.5 µg/ml pooled human immunoglobulin (Sigma), followed by incubation at 37°C in 5% CO2 for approximately 2 d. After this time, an appropriate dish containing well separated plaques was selected, the medium aspirated, and the cells overlaid with 1% agarose in sterile phosphate-buffered saline (PBS). A number of plaques were picked and used to inoculate separate tubes of 5% NBCS/ sterile 9% milk (1:1, 2 ml) which were subject to sonication for 30 seconds (on ice) using a Branson Sonifier 250 (output level 4). From each of these viral suspensions, 0.5 ml was taken and used to infect individual 75 cm2 flasks of confluent Vero cells and high titer virus stocks were prepared as above. One of these stocks was selected and designated plaque purified, passage A.
2.4 Whole cell extracts
Confluent 25 cm2 flasks of Vero cells were infected with viruses at an MOI of 5 in 5% NBCS. For mock infections, the same procedure was followed but without addition of virus to the NBCS. After a 2 h adsorption on a rocker at 37°C, the medium was aspirated, replaced with 3 ml of fresh 5% NBCS, and incubated at 37°C in 5% CO2. The cells were harvested using a scraper to detach the cells from the surface of the flask and the suspension was transferred to a tube prior to centrifugation at 800 × g for 3 min. After aspirating the supernatant, the cell pellet was resuspended in cold PBS containing 10 µM of each of the protease inhibitors N-tosyl-L-phenyl-alanine-chloromethylketone (TPCK), phenylmethylsulphonyl fluoride (PMSF), and tosyl-L-lysine-chloromethylketone (TLCK). The cells were then pelleted by gentle microcentrifugation, the supernatant aspirated, and the cells resuspended in lysis solution (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.4% Triton X-100, 60 µM TPCK, 60 µM PMSF, and 60 µM TLCK). After sonication (3 × 10 sec with cooling periods) to disrupt the cells, protein concentrations were determined by a modified Bradford protein assay (Bio-Rad). All other biochemical reagents were obtained from Sigma.
2.5 Denaturing gel electrophoresis and electrical transfer of polypeptides to membranes
Protein samples (approximately 50 µg) were separated on sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) cross-linked with N,N’-diallyltartadiamide (DATD) using a 6% stacking gel (Blaho and Roizman, 1998). Samples individually probed for ICP4, ICP27, or VP22 were run on a 9.3%, 15%, or 17.5% polyacrylamide gel, respectively. Samples probed for all 3 viral proteins at the same time were run on a 15% polyacrylamide gel. Prestained molecular mass markers (GibcoBRL) were also loaded and run on all gels. Electrical transfer (100V for 2 h at 4°C) to Protran nitrocellulose membranes (Schleicher and Schuell) using a tank apparatus (Bio-Rad). The blots were then blocked in 5% nonfat dried milk (Carnation) in PBS for 1 h at 25°C.
2.6 Immunoblotting
After blocking, membranes were washed in PBS to remove excess milk before incubating in primary antibody at 25°C for 1 h. After rinsing 3 times in 100 mM NaCl, 10 mM Tris-HCl, pH 7.5, 0.1% Tween 20 (TBST 0.1%), the blots were then incubated in secondary antibody at 25°C for 1 h. After rinsing 3 times in TBST 0.1% and rinsing again 3 times in 100 mM NaCl, 10 mM Tris-HCl, pH 7.5 (TBS), the blots were washed once for 5 min in 100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 5 mM MgCl2. The blot was then incubated in developer (p-nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate; Gibco) until the protein bands could be visualized. All immunoblots were digitized at 600 pixels per square inch using an AGFA Arcus II scanner linked to a Macintosh G3 PowerPC workstation and organized using Adobe Photoshop and Adobe Illustrator. All blots remain unmodified graphically.
2.7 Antibodies
The primary antibodies used were as follows. The anti-ICP4 antibody was a mouse monoclonal antibody, H1114 (Goodwin Institute for Cancer Research). The anti-ICP27 antibody was a mouse monoclonal antibody, H113 (Goodwin Institute for Cancer Research). The anti-VP22 antibody was a rabbit polyclonal antibody, RGST49, raised against a GST-VP22 fusion protein (Blaho, Mitchell, and Roizman, 1994; Pomeranz and Blaho, 1999). Each of these primary antibodies were raised against antigen derived from HSV-1 polypeptides. Primary antibodies were used at a dilution of 1:1000 in a solution of 1% BSA in PBSA. Alkaline phosphatase (AP)-conjugated goat anti-mouse or anti-rabbit secondary antibodies (Southern Biotech, Birmingham, Ala.) were used at a dilution of 1:1000 in 5% milk in PBSA.
2.8 Microscopy
Visualization of viral plaques was enhanced by staining of the cells with Geisma stain (Gibco), following washing of the cells with PBS, and fixation in 100% methanol (Pomeranz and Blaho, 2000). Photographs of plaques were taken using a Sony DKC-5000 digital camera mounted on an Olympus IX70/IX-FLA inverted microscope and processed using Photoshop on a PowerMac computer.
3. Results
3.1 Distinct electrophoretic mobilities of ICP4, ICP27, and VP22 proteins derived from HSV-1and HSV-2
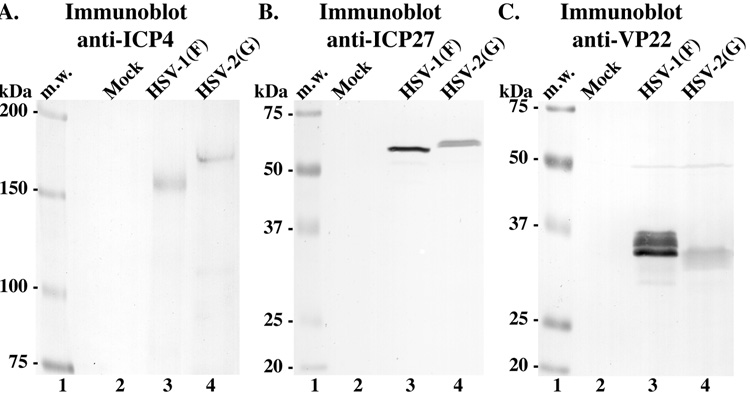
We set out to develop a rapid experimental procedure that would conveniently and convincingly discriminate between HSV-1 and HSV-2 strains. As a method for typing HSV, we examined differences between the electrophoretic mobilities of HSV-1 viral proteins and their homologues produced by HSV-2. In previous studies (Blaho, Mitchell, and Roizman, 1993; Blaho, Mitchell, and Roizman, 1994; Blaho and Roizman, 1991), such electrophoretic differences were utilized to identify novel modified proteins synthesized by HSV-1 and HSV-2. Confluent cultures of Vero cells were mock-infected or infected with either HSV-1(F) or HSV-2(G) (MOI). At 18 hours post infection (hpi), whole cell extracts were prepared, equal amounts of infected cell proteins were separated in 9.3%, 15%, or 17.5% denaturing gels, transferred to nitrocellulose and immunostained for ICP4 (~175 kDa), ICP27 (~63 kDa), or VP22 (~38 kDa), respectively, as described in Materials and methods. The results (Fig. 1) were as follows.
Fig. 1.
Immunoblots of HSV-1 and HSV-2 ICP4, ICP27, and VP22 proteins. Lysates of mock-, HSV-1(F)-, and HSV-2(G)-infected cells were separated in three independent denaturing gels (9.3, 15, and 17.5%, respectively), transferred onto nitrocellulose, and probed for (Panel A) ICP4, (Panel B) ICP27, and (Panel C) VP22. Locations (kDa) of molecular mass markers (m.w.) are indicated in the left margins.
In each case, distinct immune reactivity signals were detected for each protein with both HSV-1 and HSV-2 (Fig. 1). This is an important initial finding since each of the antibodies used were raised against the HSV-1 proteins and it, therefore, demonstrates that these reagents successfully cross-react with the HSV-2 homologues. As expected (Blaho, Mitchell, and Roizman, 1994), the HSV-1 immune reactive ICP4 (Fig. 1A, lane 3), ICP27 (Fig. 1B, lane 3), and VP22 (Fig. 1C, lane 3) species had mobilities of approximately 175,000, 63,000, and 38,000 molecular weight, respectively. Consistent with earlier findings (Blaho, Mitchell, and Roizman, 1994; Pomeranz and Blaho, 1999), the VP22 protein derived from HSV-1 migrated as multiple electrophoretic forms (Fig. 1C, lane 3). Furthermore, all three of the HSV-2 homologues had mobilities which were different from that of their corresponding HSV-1 protein and could be readily distinguished from such. In the cases of ICP4 and ICP27, the HSV-2 forms migrated more slowly than that of HSV-1 (compare lane 3 with 4 in Panel 1A and 1B). For VP22, the HSV-2 form migrated faster than the HSV-1 species (Panel 1C, compare lane 3 and 4). Based on these findings, we conclude the following. (i) The three antibodies that were used in this study recognize viral proteins from both HSV-1 and HSV-2. Since each antibody was raised against specific HSV-1 antigens, reactivities with polypeptides derived from HSV-2 represent cross-reactivities. It is conceivable that other such antibodies exist which may also be used for these types of comparisons. (ii) As outlined, our method may be used to distinguish between HSV-1 and HSV-2 viruses.
3.2 Isolation of HSV(TC)
The results in Fig. 1 showed that immunoblotting might serve as a method for HSV differentiation based on an analysis of common laboratory strains. As our next goal, we undertook to isolate a primary sample of HSV. In this initial case, the patient was a 37 year old male who had suffered from oral herpes since childhood and presented with a lesion on his lip which was remising and covered in a dry scab at the time of sampling. After disinfecting the area of the cold sore by wiping with ethanol, the scab was removed, and a swab taken of the resulting discharge was used to inoculate growth medium of Vero cells, as described in Materials and methods. The specific strategy for obtaining virus is outlined in Fig 2. This initial virus stock required 9 days to achieve 100% CPE, reflecting the low number of infectious particles present in the remising cold sore lesion. Single virus isolates were plaque purified and one randomly chosen isolate was expanded and determined to have a titer of 8.3 × 108 PFU/ml. We designated this virus HSV(TC) after its human host. The HSV(TC) strain represents a limited-passage, nontyped, primary HSV isolate. Primary HSV isolates from ocular and genital regions (below) were obtained from other consenting adults in a similar manner.
Fig. 2.
Schematic representation of the protocol for obtaining clinical isolates (Top Panel). Specific details of the procedure used for obtaining the HSV clinical isolates used in this study are outlined in the text. The complete pathways (filled arrowheads) yields a plaque-purified isolate, while the detoured open arrowhead route bypasses this step. Photographic images of plaques produced by HSV-1, HSV-2 and HSV(TC) viruses (A–C). Confluent Vero cells were infected with (Panel A) HSV-1(F), (Panel B) HSV-2(G), or (Panel C) HSV(TC) at 37°C for approximately 2 days before staining with Geisma dye. Comparison of the three photographs demonstrates similar plaque morphologies.
As an initial characterization of the HSV(TC) virus, we documented its growth properties in cultured cells using two separate strategies. In the first analysis, we determined the time required for HSV-1(F), HSV-2(G), and HSV(TC) to reach maximum CPE. When Vero cells were infected with each virus at an MOI of 5, it was observed that HSV(TC) infected cells reached 100% CPE at approximately 19 hpi, which was the same time period required with HSV-1(F). In contrast, HSV-2(G)-infected cells showed 100% CPE at approximately 12 hpi. In the second study, we evaluated the plaque morphologies of each virus. Vero cells were infected at an MOI of 0.01 with HSV-1(F), HSV-2(G), or HSV(TC) and at 48 hpi, the cells were fixed, stained (Pomeranz and Blaho, 2000), and photographed as described in Materials and methods. Representative low magnification images of plaques from all three viruses are shown in Fig. 2. As expected, the HSV-1(F) and HSV-2(G) plaques displayed typical cytopathic morphologies. Close inspection of the HSV(TC) plaque indicated that it shared features with that of HSV-1(F) and HSV-2(G), including loss of central cells and darkly-stained, rounded cells at the periphery of the plaque (compare Fig. 2C with 2A and 2B). Taking these observations together, we conclude that HSV(TC) indeed represents an alphaherpesvirus strain, inasmuch as its plaque phenotype in cell culture is indistinguishable from HSV-1(F) and HSV-2(G).
3.3 Immunoblot differentiation of HSV(TC)
The goal of this section was to apply the immunoblot typing method (Fig. 1) to determine the alphaherpesvirus subfamily to which HSV(TC) belongs. Whole cell extracts were prepared from mock-, HSV-1(F)-, HSV-2(G)-and HSV(TC)-infected (MOI=5) Vero cells as above. Two separate strategies were employed to analyze the infected cell proteins. The first approach was identical to that shown in Fig. 1 where the samples were separated on three different polyacrylamide gels, transferred to nitrocellulose, and probed, as appropriate, for ICP4, ICP27, and VP22. The results of this study (Fig. 3A–C) showed that the ICP4, ICP27 and VP22 proteins produced by HSV(TC) had electrophoretic mobilities identical to the proteins derived from HSV-1(F) (Fig. 3A–C, compare lane 5 with 3).
Fig. 3.
ICP4, ICP27, and VP22 immunoblot typing of HSV(TC). Lysates of mock-, HSV-1(F)-, HSV-2(G)-, and HSV(TC)-infected cells were separated in three independent denaturing gels (9.3, 15, and 17.5%, respectively), transferred onto nitrocellulose, and probed for (Panel A) ICP4, (Panel B) ICP27, and (Panel C) VP22. Locations (kDa) of molecular mass markers (m.w.) are indicated in the left margins.
In the second detection system, infected cell polypeptides were separated in a single 15% denaturing gel prior to transferring to a single nitrocellulose membrane. This membrane was then cut twice and each piece was probed for a single viral protein. The results (Fig. 4) confirmed the findings in Fig. 3A–C that the HSV(TC) proteins migrated with the same mobilities as the HSV-1(F) forms (Fig. 4, compare lane 5 with 3). Based on these data, we can conclude that the HSV(TC) isolate is a member of the human simplex virus 1 subfamily of the alphaherpesviridae. In addition, the results in Fig. 4 show that the separation of the HSV-2(G) homologues from the corresponding HSV-1(F) proteins on a single gel proved successful, giving the same result as achieved with the individual gels (Fig. 3A–C). One additional modification of this method which would yield identical results involves mixing the anti-ICP4, -ICP27, and-VP22 antibodies together and probing the intact single membrane (Bowles and Blaho, data not shown). This then demonstrates a "short cut" technique for differentiating HSV-1 and HSV-2 by immunoblotting using these three diagnostic proteins.
Fig. 4.
Immunoblot differentiation of HSV(TC) (D). In this case, mock-, HSV-1(F)-, HSV-2(G)-, and HSV(TC)-infected cell lysates were run on a single 15% denaturing gel, transferred onto nitrocellulose which was cut into three pieces, and immunoblotted for ICP4, ICP27, and VP22. Locations (kDa) of molecular mass markers (m.w.) are indicated in the left margin.
3.4 Immunoblot typing of ocular HSV isolates
The experiments described above were performed using a single, primary HSV-1 isolated from the oro-facial region. We next used our immunodetection assay to characterize additional patient-derived virus isolates. HSV(MS) and HSV(MB) were obtained from individuals with skin/conjunctiva lesions in the ocular region. Polypeptides extracted from cells infected with these two nontyped viruses were immunoblotted using the differentiation protocol as described in Fig. 4. HSV-1(F) and HSV-2(G) were used as type-specific virus controls. The results (Fig. 5) showed that the mobilities of the ICP4 and ICP27 proteins derived from both of these isolates closely resembled that of HSV-1(F) (compare lane 4, 6 with 2). Based on these findings, we conclude that HSV(MS) and HSV(MB) are HSV-1 isolates.
Fig. 5.
Immunoblot typing of ocular samples (A). Lysates of mock-, HSV-1(F)-, HSV-2(G)-, HSV(MS)-, and HSV(MB)-infected cells were run on a single 15% denaturing gel, transferred onto nitrocellulose, cut into two pieces, and probed for ICP4 and ICP27. Locations (kDa) of molecular mass markers (m.w.) are indicated in the left margin.
3.5 Immunoblot typing of genital HSV isolates
As a final step, we characterized four viruses isolated from individuals with genital herpes lesions. Extracts from cells infected with nontyped HSV(C2), HSV(C3), HSV(C4), HSV(C9), and control HSV-1(F), HSV-1(TC), HSV-2(G), and HSV-2(333) were analyzed by immunoblot differentiation. The results (Fig. 6) indicated that the mobilities of ICP4, ICP27, and VP22 of HSV(C2) and HSV(C9) resembled that of HSV-2(G). However, the mobilities of these viral proteins from HSV(C3) and HSV(C4) were similar to that of HSV-1(F) and HSV-1(TC). Traditionally, isolates derived from patients with diagnosed genital herpes disease would be type 2 (Cone et al., 1994; Fleming et al., 1997; Ndjoyi-Mbiguino et al., 2003). However, the finding that half of the herpes simplex virus isolates we characterized in this very small analysis ended up being type 1 is consistent with recent studies noting increases in genital HSV-1 infections (Adelson et al., 2005; Gupta, Warren, and Wald, 2007). Based on this, we conclude that the HSV(C2) and HSV(C9) isolates are HSV-2, while HSV(C3) and HSV(C4) are HSV-1.
Fig. 6.
Immunoblot typing of genital samples (B). Lysates of mock-, HSV-1(F)-, HSV-1(TC)-, HSV-2(G)-, HSV-2(333)-, HSV(C2)-, HSV(C3)-, HSV(C4)-, and HSV(C9)-infected cells were run on a single 15% denaturing gel and immunoblotted for ICP4, ICP27, and VP22. Locations (kDa) of molecular mass markers (m.w.) are indicated in the right margin.
3.6 Plaque purification of HSV-2(333) based on immunoblot typing
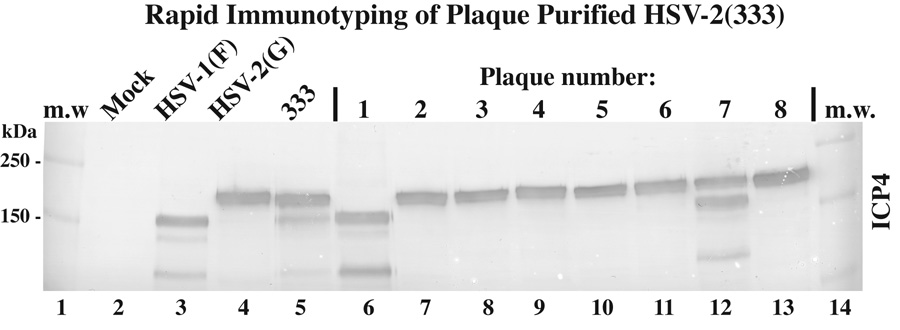
Close inspection of the HSV-2(333) immunoblot above (Fig. 6) revealed that while the majority of its ICP4 migrated with that of HSV-2(G), a detectable immune reactive band was present that had a mobility similar to that of ICP4 from HSV-1(F) and HSV-1(TC) (Fig. 6, compare lane 6 with 3–5). However, the majority of the ICP27 and VP22 forms from HSV1-2(333) had mobilities resembling that of the two HSV-1 controls. Thus, it appeared that this particular stock may be a mixed population of type 2 and type 1 viruses. It should be noted that ICP4 is routinely used as standard marker for HSV infection. In the event that only ICP4 is used, it is conceivable that the contaminating HSV-1 in the 333 stock might be dismissed as simply a degradation product. We therefore performed a plaque purification by limiting dilution of the original 333 stock, isolated eight plaques, and produced high titer stocks from each as described in Materials and methods. Extracts from cells infected with each plaque-pure stock, as well as control HSV-1(F), HSV-2(G), and the original 333 stock were immunoblotted for ICP4 (Fig. 7). The results showed that one plaque (lane 12) remained a mixture while one was separated out as an unknown HSV-1 strain (lane 6) and the remaining six were HSV-2. It should be emphasized that this mixture is likely unique to the particular 333 stock used in this study and should not be inferred to be a feature of all 333 stocks. The source of the HSV-1 contamination remains unknown but its presence emphasizes the need for proper plaque purification of HSV isolates.
Fig. 7.
Immunoblot typing of plaque purified HSV-2(333) (C). Lysates of mock-, HSV-1(F)-, HSV-2(G)-, HSV(333)-, and eight isolated plaque-infected cells were immunoblotted for ICP4. Locations (kDa) of molecular mass markers (m.w.) are indicated in the left margin.
4. Discussion
Both HSV-1 and HSV-2 can cause genital and facial lesions, with the presentations being clinically indistinguishable. Although genital herpes is classically considered to be caused by HSV-2, in recent years an increasing proportion of genital herpes cases have been found to be caused by the type 1 virus (Engelberg et al., 2003; Lekstrom-Himes, Pesnicak, and Straus, 1998; Mertz, 1993), particularly in the UK (Scoular, Leask, and Carrington, 1990; Slomka et al., 1998). Indeed, in Northern Ireland HSV-1 has become the predominant genital type in women (Coyle et al., 1999). Identification of the HSV type responsible for such infections is of clinical importance {reviewed in (Gupta, Warren, and Wald, 2007)}. Currently, there is no licensed vaccine or cure for HSV and, once infected, the patient remains infected for life. HSV-2 causes symptomatic recurrences at a much higher frequency than HSV-1 (Corey et al., 1983; Dawkins, 1990; Madhavan, Priya, and Bagyalakshmi, 2003). Acyclovir can alleviate the symptoms of herpes infection and speed the healing process. However, it does not decrease the frequency of subsequent recurrences (Baker, 1992; Corey and Holmes, 1983). In terms of evaluating the infection, particularly in the context as a sexually transmitted disease, typing HSV is of prognostic importance (Corey and Holmes, 1983; Engelberg et al., 2003).
Historically, a number of techniques have been evaluated for typing HSV in the laboratory after initial growth and isolation. They have had varying amounts of success and required varying degrees of scientific expertise. Early successful antigenic differentiation was achieved by comparing pock size on embryonated egg chorioallantoic membrane (CAM) after inoculation with virus, with HSV-2 producing characteristically larger pocks than HSV-1(Nahmias et al., 1968). Distinguishable histological differences between HSV-1 and HSV-2 pocks are also evident. Another reliable biological marker used for differentiating HSV-1 and HSV-2 was the ability of the viruses to form plaques in primary chick embryo cells, with HSV-1 virus plaques being morphologically distinct from those produced by HSV-2 (Lowry, Melnick, and Rawls, 1971). Specifically, HSV-2 replicates well, forming distinct plaques, whereas HSV-1 either forms no plaques or only small plaques (producing low quantities of infectious virus). Other biological markers investigated have included differences in temperature sensitivity {HSV-1 can grow at a higher temperature than HSV-2 (Ratcliffe, 1971)}, heparin sensitivity (Marks-Hellman and Ho, 1976), and (E)-5-(2-bromovinyl)-2’-deoxyuridine (BVDU) sensitivity (Zheng, Mayo, and Hsiung, 1983).
In the past, immunological methods such as microneutralization, immunofluorescence, enzyme immunoassay, and ELISA often proved troublesome in successfully differentiating between HSV types due to cross-reactivity of HSV-1 and HSV-2 antibodies (Garcia-Corbeira et al., 1999; Zheng, Mayo, and Hsiung, 1983). Improvements in antibody production in recent years providing monospecific polyclonal and monoclonal antibodies has increased the efficiency of typing (Coyle et al., 1999; Markoulatos et al., 1997). Specifically, antibodies raised against type specific envelope glycoproteins are allowing for a much more accurate diagnosis, providing better sensitivity and specificity (Liljeqvist, Svennerholm, and Bergstrom, 1999; Slomka et al., 1998). Similarly, immobilized glycoproteins are being used in commercial ELISA kits for serological testing (Nishimura et al., 2001). Surprisingly however, only assays based on the viral glycoprotein G appear to have acceptable accuracy in the clinical setting (Strick and Wald, 2004).
Our experiments actually rely on the inherent immunological cross-reactivities that exist between HSV-1 and HSV-2 proteins. Thus, that which is bane to the development of certain antibody-based diagnostics is a benefit to our study. By comparing the protein bands on the immunoblots of our primary isolates to those of standard virus strains, the primary viruses were unequivocally typed. Thus, we demonstrated that HSV-1 and HSV-2 could be unambiguously distinguished on the basis of the electrophoretic mobility of each of three viral proteins, ICP4, ICP27, and VP22. Similar strategies have been employed earlier for such virus strain comparisons (Blaho, Mitchell, and Roizman, 1994; Pereira et al., 1976; Pereira et al., 1977). Advantages of this method include its ability to definitively type HSV, its high degree of sensitivity, and its general technical simplicity (since we were able to probe for all three viral proteins on one immunoblot). Our resurrection and development of an immunoblot-based differentiation methodology will be useful for the continued characterization of clinical HSV isolates.
Acknowledgments
We thank Elise Morton for expert cell culture technical assistance, Penny Asbell (MSSM-Ophthalmology) and Betsy Herold (MSSM - Pediatrics) for assistance in obtaining primary virus isolates. These studies were supported by grants from the U.S. Public Health Service (AI 38873 and AI 48582).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelson ME, Feola M, Trama J, Tilton RC, Mordechai E. Simultaneous detection of herpes simplex virus types 1 and 2 by real-time PCR and Pyrosequencing. J Clin Virol. 2005;33(1):25–34. doi: 10.1016/j.jcv.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Baker DA. Herpes simplex virus infections. Curr Opin Obstet Gynecol. 1992;4(5):676–681. [PubMed] [Google Scholar]
- Batterson W, Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983;46(2):371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho JA, Mitchell C, Roizman B. Guanylylation and adenylylation of the alpha regulatory proteins of herpes simplex virus require a viral beta or gamma function. J Virol. 1993;67(7):3891–3900. doi: 10.1128/jvi.67.7.3891-3900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho JA, Mitchell C, Roizman B. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J Biol Chem. 1994;269(26):17401–17410. [PubMed] [Google Scholar]
- Blaho JA, Roizman B. ICP4, the major regulatory protein of herpes simplex virus, shares features common to GTP-binding proteins and is adenylated and guanylated. J Virol. 1991;65(7):3759–3769. doi: 10.1128/jvi.65.7.3759-3769.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho JA, Roizman B. Analyses of HSV proteins for posttranslational modifications and enzyme functions. In: Brown SM, Maclean AR, editors. Methods in Molecular Medicine: Herpes Simplex Virus Protocols. Vol. 10. Totowa: Human Press Inc.; 1998. pp. 237–256. [DOI] [PubMed] [Google Scholar]
- Boehmer PE, Lehman IR. Herpes simplex virus DNA replication. Annu Rev Biochem. 1997;66:347–384. doi: 10.1146/annurev.biochem.66.1.347. [DOI] [PubMed] [Google Scholar]
- Cone RW, Hobson AC, Brown Z, Ashley R, Berry S, Winter C, Corey L. Frequent detection of genital herpes simplex virus DNA by polymerase chain reaction among pregnant women. Jama. 1994;272(10):792–796. [PubMed] [Google Scholar]
- Corey L. The current trend in genital herpes. Progress in prevention. Sex Transm Dis. 1994;21(2 Suppl):S38–S44. [PubMed] [Google Scholar]
- Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98(6):958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- Corey L, Holmes KK. Genital herpes simplex virus infections: current concepts in diagnosis, therapy, and prevention. Ann Intern Med. 1983;98(6):973–983. doi: 10.7326/0003-4819-98-6-973. [DOI] [PubMed] [Google Scholar]
- Coyle PV, Desai A, Wyatt D, McCaughey C, O'Neill HJ. A comparison of virus isolation, indirect immunofluorescence and nested multiplex polymerase chain reaction for the diagnosis of primary and recurrent herpes simplex type 1 and type 2 infections. J Virol Methods. 1999;83(1–2):75–82. doi: 10.1016/s0166-0934(99)00108-1. [DOI] [PubMed] [Google Scholar]
- Dawkins BJ. Genital herpes simplex infections. Prim Care. 1990;17(1):95–113. [PubMed] [Google Scholar]
- Duff R, Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971;233(36):48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- Ejercito PM, Kieff ED, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Engelberg R, Carrell D, Krantz E, Corey L, Wald A. Natural history of genital herpes simplex virus type 1 infection. Sex Transm Dis. 2003;30(2):174–177. doi: 10.1097/00007435-200302000-00015. [DOI] [PubMed] [Google Scholar]
- Enquist LW, Husak PJ, Banfield BW, Smith GA. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- Espy MJ, Uhl JR, Mitchell PS, Thorvilson JN, Svien KA, Wold AD, Smith TF. Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol. 2000;38(2):795–799. doi: 10.1128/jcm.38.2.795-799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filen F, Strand A, Allard A, Blomberg J, Herrmann B. Duplex real-time polymerase chain reaction assay for detection and quantification of herpes simplex virus type 1 and herpes simplex virus type 2 in genital and cutaneous lesions. Sex Transm Dis. 2004;31(6):331–336. doi: 10.1097/00007435-200406000-00002. [DOI] [PubMed] [Google Scholar]
- Fleming DT, McQuillan GM, Johnson RE, Nahmias AJ, Aral SO, Lee FK, St Louis ME. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337(16):1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- Garcia-Corbeira P, Hogrefe W, Aguilar L, Garcia-de-Lomas J, Gil A, Bayas JM, Vilella A, Dal-Re R. Whole cell lysate enzyme immunoassays vs. recombinant glycoprotein G2-based immunoassays for HSV-2 seroprevalence studies. J Med Virol. 1999;59(4):502–506. doi: 10.1002/(sici)1096-9071(199912)59:4<502::aid-jmv13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- Hobson A, Wald A, Wright N, Corey L. Evaluation of a quantitative competitive PCR assay for measuring herpes simplex virus DNA content in genital tract secretions. J Clin Microbiol. 1997;35(3):548–552. doi: 10.1128/jcm.35.3.548-552.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40(7):2609–2611. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler HH, Muhlbauer G, Rinner B, Stelzl E, Berger A, Dorr HW, Santner B, Marth E, Rabenau H. Detection of Herpes simplex virus DNA by real-time PCR. J Clin Microbiol. 2000;38(7):2638–2642. doi: 10.1128/jcm.38.7.2638-2642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin DW, Lakeman FD, Arvin AM, Prober CG, Corey L, Powell DA, Burchett SK, Jacobs RF, Starr SE, Whitley RJ. Application of the polymerase chain reaction to the diagnosis and management of neonatal herpes simplex virus disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1996;174(6):1162–1167. doi: 10.1093/infdis/174.6.1162. [DOI] [PubMed] [Google Scholar]
- Lai KK, Cook L, Krantz EM, Corey L, Jerome KR. Calibration curves for real-time PCR. Clin Chem. 2005;51(7):1132–1136. doi: 10.1373/clinchem.2004.039909. [DOI] [PubMed] [Google Scholar]
- Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1995;171(4):857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- Lekstrom-Himes JA, Pesnicak L, Straus SE. The quantity of latent viral DNA correlates with the relative rates at which herpes simplex virus types 1 and 2 cause recurrent genital herpes outbreaks. J Virol. 1998;72(4):2760–2764. doi: 10.1128/jvi.72.4.2760-2764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeqvist JA, Svennerholm B, Bergstrom T. Typing of clinical herpes simplex virus type 1 and type 2 isolates with monoclonal antibodies. J Clin Microbiol. 1999;37(8):2717–2718. doi: 10.1128/jcm.37.8.2717-2718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry SP, Melnick JL, Rawls WE. Investigation of plaque formation in chick embryo cells as a biological marker for distinguishing herpes virus type 2 from type 1. J Gen Virol. 1971;10(1):1–9. doi: 10.1099/0022-1317-10-1-1. [DOI] [PubMed] [Google Scholar]
- Madhavan HN, Priya K, Bagyalakshmi R. Phenotypic and genotypic methods for the detection of herpes simplex virus serotypes. J Virol Methods. 2003;108(1):97–102. doi: 10.1016/s0166-0934(02)00264-1. [DOI] [PubMed] [Google Scholar]
- Markoulatos P, Fountoucidou P, Marinakis G, Krikelis V, Spyrou N, Vamvakopoulos N, Moncany ML. Clear detection and typing of herpes simplex virus types 1 and 2 by an indirect ELISA assay: comparison with three different combined methods--capture ELISA, restriction enzymes, and polymerase chain reaction. J Clin Lab Anal. 1997;11(3):146–153. doi: 10.1002/(SICI)1098-2825(1997)11:3<146::AID-JCLA5>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks-Hellman S, Ho M. Use of biological characteristics to type Herpesvirus hominis types 1 and 2 in diagnostic laboratories. J Clin Microbiol. 1976;3(3):277–280. doi: 10.1128/jcm.3.3.277-280.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DS, Linfert DR, Draghi A, McCarter YS, Tsongalis GJ. Identification of herpes simplex virus genital infection: comparison of a multiplex PCR assay and traditional viral isolation techniques. Mod Pathol. 2001;14(3):152–156. doi: 10.1038/modpathol.3880273. [DOI] [PubMed] [Google Scholar]
- Mertz GJ. Epidemiology of genital herpes infections. Infect Dis Clin North Am. 1993;7(4):825–839. [PubMed] [Google Scholar]
- Nahmias AJ, Dowdle WR, Naib ZM, Highsmith A, Harwell RW, Josey WE. Relation of pock size on chorioallantoic membrane to antigenic type of herpesvirus hominis. Proc Soc Exp Biol Med. 1968;127(4):1022–1028. doi: 10.3181/00379727-127-32861. [DOI] [PubMed] [Google Scholar]
- Ndjoyi-Mbiguino A, Ozouaki F, Legoff J, Mbopi-Keou FX, Si-Mohamed A, Onas IN, Avoune E, Belec L. Comparison of washing and swabbing procedures for collecting genital fluids to assess cervicovaginal shedding of herpes simplex virus type 2 DNA. J Clin Microbiol. 2003;41(6):2662–2664. doi: 10.1128/JCM.41.6.2662-2664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Ayabe M, Shoji H, Hashiguchi H, Eizuru Y, Kawana T. Differentiation of Herpes simplex virus types 1 and 2 in sera of patients with HSV central nervous system infections by type-specific enzyme-linked immunosorbent assay. J Infect. 2001;43(3):206–209. doi: 10.1053/jinf.2001.0881. [DOI] [PubMed] [Google Scholar]
- Oram RJ, Marcellino D, Strauss D, Gustafson E, Talarico CL, Root AK, Sharma PL, Thompson K, Fingeroth JD, Crumpacker C, Herold BC. Characterization of an acyclovir-resistant herpes simplex virus type 2 strain isolated from a premature neonate. J Infect Dis. 2000;181(4):1458–1461. doi: 10.1086/315387. [DOI] [PubMed] [Google Scholar]
- Pereira L, Cassai E, Honess RW, Roizman B, Terni M, Nahmias A. Variability in the structural polypeptides of herpes simplex virus 1 strains: potential application in molecular epidemiology. Infect Immun. 1976;13(1):211–220. doi: 10.1128/iai.13.1.211-220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Wolff MH, Fenwick M, Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Pomeranz LE, Blaho JA. Modified VP22 localizes to the cell nucleus during synchronized herpes simplex virus type 1 infection. J Virol. 1999;73(8):6769–6781. doi: 10.1128/jvi.73.8.6769-6781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz LE, Blaho JA. Assembly of infectious Herpes simplex virus type 1 virions in the absence of full-length VP22. J Virol. 2000;74(21):10041–10054. doi: 10.1128/jvi.74.21.10041-10054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe H. The differentiation of herpes simplex virus type 1 and type 2 by temperature markers. J Gen Virol. 1971;13(1):181–183. doi: 10.1099/0022-1317-13-1-181. [DOI] [PubMed] [Google Scholar]
- Roizman B, Knipe DM. Herpes simplex viruses and their replication. In: Knipe aPMHDM., editor. Virology. 4th ed. Philadelphia, Pa: Lippincott-Raven; 2001. pp. 2399–2459. [Google Scholar]
- Scoular A, Leask BG, Carrington D. Changing trends in genital herpes due to Herpes simplex virus type 1 in Glasgow, 1985–88. Genitourin Med. 1990;66(3):226. doi: 10.1136/sti.66.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomka MJ, Emery L, Munday PE, Moulsdale M, Brown DW. A comparison of PCR with virus isolation and direct antigen detection for diagnosis and typing of genital herpes. J Med Virol. 1998;55(2):177–183. [PubMed] [Google Scholar]
- Strick L, Wald A. Type-specific testing for herpes simplex virus. Expert Rev Mol Diagn. 2004;4(4):443–453. doi: 10.1586/14737159.4.4.443. [DOI] [PubMed] [Google Scholar]
- Tyler KL. Update on herpes simplex encephalitis. Rev Neurol Dis. 2004;1(4):169–178. [PubMed] [Google Scholar]
- van Doornum GJ, Guldemeester J, Osterhaus AD, Niesters HG. Diagnosing herpesvirus infections by real-time amplification and rapid culture. J Clin Microbiol. 2003;41(2):576–580. doi: 10.1128/JCM.41.2.576-580.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71(2–3):141–148. doi: 10.1016/j.antiviral.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Zheng ZM, Mayo DR, Hsiung GD. Comparison of biological, biochemical, immunological, and immunochemical techniques for typing herpes simplex virus isolates. J Clin Microbiol. 1983;17(3):396–3992. doi: 10.1128/jcm.17.3.396-399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]