Abstract
A comprehensive understanding of human memory requires cognitive and neural descriptions of memory processes along with a conception of how memory processing drives behavioral responses and subjective experiences. One serious challenge to this endeavor is that an individual memory process is typically operative within a mix of other contemporaneous memory processes. This challenge is particularly disquieting in the context of implicit memory, which, unlike explicit memory, transpires without the subject necessarily being aware of memory retrieval. Neural correlates of implicit memory and neural correlates of explicit memory are often investigated in different experiments using very different memory tests and procedures. This strategy poses difficulties for elucidating the interactions between the two types of memory process that may result in explicit remembering, and for determining the extent to which certain neural processing events uniquely contribute to only one type of memory. We review recent studies that have succeeded in separately assessing neural correlates of both implicit memory and explicit memory within the same paradigm using event-related brain potentials (ERPs) and functional magnetic resonance imaging (fMRI), with an emphasis on studies from our laboratory. The strategies we describe provide a methodological framework for achieving valid assessments of memory processing, and the findings support an emerging conceptualization of the distinct neurocognitive events responsible for implicit and explicit memory.
Keywords: EEG, event-related potentials, ERPs, explicit memory, implicit memory, neuroimaging, fMRI
Memory does not refer to a unitary behavioral phenomenon, but instead can be fractionated into a set of component processes that are expressed in different combinations under different circumstances. Analyses of memory in healthy individuals and in patients with memory impairments have revealed various expressions of memory that can be assessed using specialized memory tests. Theoretical schemes used to categorize the memory phenomena measured in these tests have emphasized behavioral, cognitive/representational, neural, and/or subjective criteria (see Gabrieli, 1998; Mayes & Roberts, 2001; Paller, 2001; Squire, 2004). Taxonomies of memory have thus helped to guide research into fundamental questions about memory. Beyond taxonomies, however, we must seek a comprehensive understanding of memory by describing the component processes in both cognitive and neural terms, by clarifying the relationships between cognitive and neural descriptions, and by showing how neurocognitive processing produces memory behavior and conscious experiences.
In order to develop this comprehensive understanding, it is necessary to achieve valid descriptions of component memory processes such that each can be understood individually and in relationship to other processes. Identifying neural substrates of a single, isolated memory process, however, faces a serious methodological challenge because multiple memory processes tend to be operative concurrently. Furthermore, neural substrates of distinct memory subtypes are predominantly identified using very different specialized tests. This situation creates barriers to understanding memory process distinctions, because of possible confounds due to differing task demands, as well as barriers to understanding memory process interactions, because the distinct memory processes are not measured concurrently.
Complexities associated with the fact that multiple memory processes can contribute to performance on any given memory test have been considered for many years (e.g., Jacoby, 1991; Mandler, 1980). One prominent behavioral approach for addressing this issue has been to devise situations in which two memory expressions effectively compete with one another to drive responses in a memory test, such that their independent influences can be estimated. For instance, a process dissociation procedure (Jacoby, 1991) can be implemented by testing memory for words learned as members of two separate lists. An exclusion test requires that only words from one of the two lists are endorsed as old, whereas an inclusion test requires that all old words are endorsed as old. Thus, controlled and automatic memory processes oppose one another in determining behavioral responses in one test and they work together in the other.
Here, we outline a different approach to investigating memory processes. We propose that it is possible to track the simultaneous operation of multiple memory processes by isolating their neural correlates on the basis of a variety of experimental manipulations that produce reliable memory dissociations. Moreover, multiple behavioral measures of memory are also essential for isolating neural correlates of different memory processes. One key advantage of this approach is that it is poised to characterize interactions among memory processes without requiring opposition circumstances as in Jacoby’s exclusion test. We illustrate this approach of concurrently scrutinizing implicit and explicit memory with an emphasis on novel paradigms developed for this purpose in our laboratory.
Before examining these experiments, the terminology of implicit and explicit memory must be fleshed out. Prior neuropsychological investigations provided the basis for our current use of and thinking about these terms. Patients with an amnesic syndrome, such as can result from damage to the hippocampus and surrounding neocortex, exhibit specific impairments in remembering facts and events as assessed in recall and recognition tests. This category of memory, explicit memory (also known as declarative memory), contrasts with other categories of memory phenomena that are not impaired in amnesia (Moscovitch, 1992; Paller, 2002; Schacter, 1987; Schacter & Tulving, 1994; Squire, 2004). Expressions of explicit memory coincide with the potential for making the metamemory judgment that memory is being expressed—the awareness of memory retrieval. For these reasons, explicit memory is usually regarded as fundamentally distinct from other expressions of memory.1
In an explicit memory test, reference to information learned earlier is specifically made or implied. In an implicit test of memory, no reference is made to learning episodes. Rather, implicit memory is demonstrated via a change in performance in a certain task due to a prior event that may or may not be consciously remembered. We would not claim that all implicit and explicit memory tests are pure indicators of one or another type of “memory process.” Indeed, it is a standard assumption that performance in implicit memory tests can potentially be influenced by explicit memory (for instance, in tests of stem-completion priming when stems are completed via explicit retrieval of studied words). Furthermore, some evidence indicates that implicit memory can influence responses in explicit memory tests (Keane, Orlando, & Verfaellie, 2006; Kleider & Goldinger, 2004; Rajaram & Geraci, 2000; Tunney & Fernie, 2007; Verfaellie & Cermak, 1999; Voss, Baym, & Paller, 2008; Wolk et al., 2005). We use the phrase “explicit memory processes” to refer to the neurocognitive processing that supports memory accompanied by the phenomenological awareness of remembering, a central function disrupted in organic amnesia. Likewise, we use the phrase “implicit memory processes” to refer to the neurocognitive processing that supports memory without the concomitant awareness of memory retrieval, as in priming effects that are typically spared in organic amnesia. We do not advocate defining implicit and explicit memory processes solely according to test format, given that (as already noted) explicit memory tests and implicit memory tests are not necessarily process-pure. Although there are other ways to ground memory terminology, the present conceptualization is useful to the extent that it provides a plausible neurocognitive framework from which detailed investigations of memory processes can be launched.
An ongoing research goal is to determine the extent to which implicit memory processes and explicit memory processes overlap. This empirical question must be addressed by comparing and contrasting the processes responsible for each type of memory. We wish to stress, however, that the neurocognitive processing associated with explicit memory and implicit memory may transpire independent from the circumstances of any particular memory test. In other words, implicit memory processes may be engaged in response to a stimulus even if behavioral responses indicative of implicit memory are not emitted (or are emitted but not measured), and explicit memory processes likewise may be engaged in response to a stimulus even in the absence of behavioral responses indicative of explicit memory. This manner of using these two important terms—explicit memory processes and implicit memory processes—thus divorces memory processes from the specific tests chosen to measure them, and instead emphasizes theoretically relevant neurocognitive features of memory processes.
Contrasts between these two categories of memory phenomena have been very prominent in memory research over the past two decades (see Henson, 2003; Roediger, 1990; Schacter & Buckner, 1998). Priming is a specific measure of implicit memory that comprises faster or more accurate behavioral responses on specialized priming tests. The most common types of priming tests are for perceptual priming (also sometimes called repetition priming or item-specific priming). These behavioral effects are often thought to reflect facilitated or more fluent perceptual processing of the physical features of repeated items, distinct from accessing a memory for the full episode in which the item occurred. These tests thus measure perceptual implicit memory. A different set of mechanisms may be responsible for other types of priming (i.e., conceptual priming, novel-information priming, new-association priming, and cross-domain priming), and in some of these cases the implicit memory processing that supports priming may not be preserved in amnesia, although the boundary conditions for preserved priming in amnesia have yet to be precisely defined.
When memory tests are given to healthy individuals, performance may be guided by explicit memory processes, implicit memory processes, or by some combination. In addition to acknowledging that behavioral measures in memory tests can reflect multiple memory processes, it is important to note that neural measures are liable to be influenced by multiple memory processes as well. Moreover, neural measures can reflect memory processes whether or not those processes influence behavioral performance. In either implicit or explicit memory tests, neural measures can reflect both explicit memory processes and implicit memory processes. Experimental parameters that selectively capture the operation of distinct components of memory are thus essential. Otherwise, neuroimaging results cannot be unequivocally associated with one type of memory versus the other.
Explicit memory and perceptual implicit memory
Identifying neural correlates of perceptual implicit memory processes uncontaminated by those of explicit memory processes is problematic because of the difficulty of preventing subjects from recalling prior episodes or recognizing repeated stimuli during priming tests. Similarly, automatic perceptual implicit memory processing may occur during explicit memory tests, even if no indications of perceptual implicit memory are observed in behavior, and this processing can potentially be reflected in neural measures accompanying explicit memory performance.
In order to isolate neural correlates of perceptual implicit memory, Paller, Hutson, Miller, and Boehm (2003) used a condition in which novel faces were encoded only to a minimal extent so as to promote priming in the absence of recognition. Subjects viewed each face for 100 ms at a central location while simultaneously a yellow cross was shown unpredictably in one of the four quadrants 1.8° from fixation. While maintaining central fixation, subjects attempted to discriminate between two subtly different types of yellow crosses, and further stimulus processing of the cross and the face was disrupted via backward masking at 100 ms. A short delay ensued after multiple study trials with different faces. Then, if an explicit memory test was given, recognition of these minimally processed faces was near chance levels. However, if an implicit memory test was given, perceptual priming was exhibited for these faces.
The logic of this experimental design was that brain potentials elicited by these repeated faces could conceivably reflect neural events responsible for perceptual priming, whereas contributions from recognition processes would be negligible. ERPs to these repeated faces were thus compared to ERPs to new faces. Furthermore, other faces also encoded during the study phase were presented for a longer duration without disruptive perifoveal visual discriminations or backward masking. These faces could later be recognized at well above chance levels. The three conditions embedded in the test phase of this experiment thus provided for a direct comparison between ERPs associated with conscious memory for faces (possibly contaminated by priming) and ERPs associated with perceptual priming (not contaminated by explicit memory).
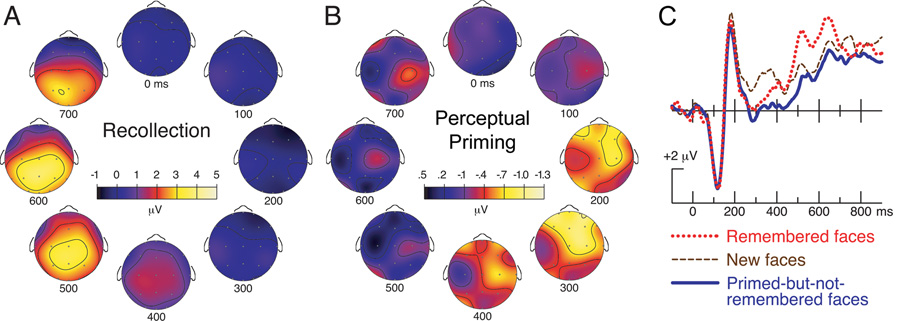
Recognition-related neural signals (based on the contrast between remembered faces and new faces) took the form of positive potentials largest at posterior scalp locations 400–800 ms after face onset (Fig. 1A). These late potentials closely resembled ERPs previously associated with face-cued recollection uncontaminated by perceptual implicit memory, as achieved when ERPs were compared as a function of a study-phase manipulation that influenced explicit memory but did not influence perceptual implicit memory (Paller, Bozic, Ranganath, Grabowecky, & Yamada, 1999). On the other hand, neural signals of perceptual priming (based on the contrast between primed-but-not-remembered faces and new faces) appeared as negative potentials at anterior recording locations from approximately 200–400 ms after face onset (Fig. 1B). Spatiotemporally distinct ERPs of opposite polarity were thus associated with conscious remembering versus perceptual priming (Fig. 1C). This pattern of neuroimaging findings complements neuroanatomical dissociations exhibited by amnesic patients; the results imply that implicit access to memory is supported by neural processing that is qualitatively distinct from that supporting conscious memory access.
Figure 1. ERP correlates of explicit memory and perceptual implicit memory (Paller et al., 2003).
The ERP difference between remembered faces and new faces is displayed in A. The ERP difference between primed-but-not-remembered faces and new faces is displayed in B. Waveform differences are averaged over 100-ms intervals starting at the latency indicated underneath each topographic map. Each map represents amplitudes on the scalp as viewed from above. Light yellow colors indicate positive difference potentials in A and negative difference potentials in B. ERP waveforms recorded from the midline frontal scalp location referenced to averaged mastoids are shown in C for the three conditions (including only trials with reaction times faster than the median reaction time for each subject and each condition, so as to accentuate effects associated with perceptual priming). (Figure adapted from Paller et al., 2003.)
Schott and colleagues (2005) also employed a novel paradigm to produce perceptual priming in the absence of recognition. These investigators capitalized on trial-to-trial variability in the strength of perceptual priming and recognition in order to identify neural correlates of both processes. Their approach involved a two-stage procedure to assess memory. Three-letter word stems were presented in an explicit memory test (i.e., cued recall), but subjects were encouraged to guess if they could not remember a studied word, so that priming might also occur. After each stem was completed, subjects indicated using strict criteria whether they recognized the word from the encoding phase. Although an encoding manipulation was not included to reduce recognition (as in Paller et al., 2003), priming-without-recognition nonetheless occurred for some trials when the subject produced the word at the completion stage but failed to endorse it as an old word. Trials were categorized as remembered when the correct response was made at both stages, and as forgotten if not produced at the completion stage.
Priming-without-recognition for studied words produced less fMRI activity compared to correct rejections of new words in bilateral occipital, inferior temporal, and prefrontal cortex (Schott et al., 2005). Conversely, correct recognition produced more activity than both priming-without-recognition and correct rejections in bilateral parietal, posterior cingulate, and anterior prefrontal cortex. Thus, priming was associated with response reductions in a set of brain regions commonly associated with implicit memory in neuroimaging studies (reviewed in Henson, 2003; Schacter & Buckner, 1998; Schacter, Wig, & Stevens, 2007; Wiggs & Martin, 1998) whereas recognition was associated with response enhancements in a separate set of brain regions that have been identified in many fMRI studies of explicit memory (reviewed in Buckner & Wheeler, 2001; Wagner, Shannon, Kahn, & Buckner, 2005). These findings provide strong support for the notion that perceptual implicit memory and explicit memory are supported by distinct brain networks and fundamentally different neurocognitive processing operations.
Evidence for the independence of implicit and explicit memory can also be derived from contrasts made at the encoding stage via a subsequent-memory or Dm analysis (Paller & Wagner, 2002). That is, neural signals of encoding predictive of later perceptual implicit memory may differ systematically from neural signals of encoding predictive of later explicit memory. Schott and colleagues (2002) used deep/semantic versus shallow/non-semantic encoding conditions, followed by the two-stage procedure described above to study recognition and priming-without-recognition. By analyzing encoding trials as a function of subsequent memory performance, neurophysiological Differences related to subsequent memory (Dm effects) were identified. The Dm for priming-without-recognition was identified by contrasting (a) ERPs to words for which the corresponding stem was completed with the studied word that was nonetheless not recognized, versus, (b) ERPs to words for which the corresponding stem was completed with an unstudied word. This Dm took the form of a relative ERP negativity over central and fronto-central locations approximately 200–400 ms after word onset (resembling ERP correlates of perceptual priming identified during memory testing, e.g. Paller et al., 2003). Furthermore, Dm for priming-without-recognition was distinct from ERP differences between deep versus shallow encoding as well as from Dm for recognition, which both included relatively positive potentials at later intervals with different topographies. ERP subsequent memory effects have also been examined using separate tests of stem-completion priming and stem-cued recall (Paller, 1990). Dm for cued recall took the form of a late positivity, as in many other studies of Dm with explicit memory tests, whereas Dm for priming was nonsignificant (this pattern was replicated with a different priming test by Paller & Kutas, 1992). Collectively, these results indicate that neural events during encoding differentially set the stage for subsequent implicit or explicit memory—these two memory expressions are neurally dissociable during both encoding and retrieval.
Similarly, Schott and colleagues (2006) identified Dm for recognition and for priming-without-recognition using fMRI data from the same experiment in which they studied retrieval (Schott et al., 2005). The recognition Dm included enhanced activity in bilateral hippocampus and parahippocampal gyrus and left prefrontal cortex. In contrast, the priming-without-recognition Dm (based on contrasting items later completed with an unrecognized studied word versus an unstudied word) included reduced activity in bilateral occipital and prefrontal cortex and left fusiform gyrus. These investigators thus found Dm for priming-without-recognition and retrieval effects for priming-without-recognition that were both de-activations. Whereas some of the same regions of prefrontal cortex and fusiform gyrus exhibited de-activations during both encoding and retrieval, ventral processing stream effects were more anterior for encoding than retrieval. Collectively, these results again indicate that, at both encoding and retrieval, perceptual implicit memory and explicit memory are supported by fundamentally different processing operations instantiated by distinct brain networks. Furthermore, these findings enrich previous descriptions of perceptual implicit memory in showing that processing during encoding that predicts later implicit memory (Schott et al., 2006), and processing required for implicit-memory retrieval (Schott et al., 2005), engage similar brain networks. Understanding the precise functional relevance of this apparent recapitulation of activity related to perceptual priming, however, must await future investigation. These findings nonetheless highlight the necessity of characterizing explicit and implicit memory separately, during both encoding and retrieval, in order to accurately characterize the critical neurocognitive processing operations that set them apart.
The studies described above are noteworthy in that they all employed behavioral measures of both implicit and explicit memory. This methodological characteristic is necessary to disambiguate neural correlates of processes responsible for the two types of memory. The majority of neuroimaging studies have not included relevant behavioral measures for both types of memory together. For instance, Rugg and colleagues (1998) attempted to identify ERP correlates of explicit memory and implicit memory during a single recognition test. Recognition hits (old items endorsed as “old”) were compared to correct rejections (new items endorsed as “new”) for the explicit memory contrast, whereas recognition misses (old items incorrectly endorsed as “new”) were compared to correct rejections for the implicit memory contrast. However, this method depends on the questionable assumptions that implicit memory is equivalently operative for all repeated stimuli in the recognition test and that explicit memory processes are not operative for recognition misses. Given that no behavioral measures of implicit memory were included (e.g., from a perceptual priming test), the evidence linking recognition-miss ERPs to implicit memory is equivocal.
On the other hand, more suitable behavioral measures had been previously used in a study with visual words and abstract visual stimuli (Van Petten & Senkfor, 1996). ERP correlates of short-lag repetition priming (approximately 20 seconds) were compared to those of relatively long-delay (several minutes) recognition misses, and qualitatively distinct ERPs for these conditions were identified. Moreover, a recent study examined short-lag subliminal priming during a recognition test for visual word stimuli, and corresponding ERPs were also qualitatively distinct from recognition misses (Woollams, Taylor, Karayanidis, & Henson, 2008). These two studies are novel in their attempt to compare neural correlates of priming with those of failed memory retrieval. However, dissociations between the two memory outcomes would be more convincing if both were studied at similar delays, given that short-delay priming might be functionally distinct from longer-delay priming that could have been operative during the recognition tests. In any case, we argue that appropriate behavioral measures of both implicit and explicit memory are essential. Indeed, this stance applies to perceptual implicit memory as well as to conceptual implicit memory.
Explicit memory and conceptual implicit memory
Conceptual implicit memory can occur when concepts are repeated, and behavioral measures of conceptual implicit memory are similar to those of perceptual implicit memory in that they can occur in the absence of awareness of remembering and typically take the form of faster or more accurate responses to a specific stimulus in a conceptual priming test. These alterations of behavioral responses are thought to reflect facilitated processing of relevant meaning, and potentially support some of the short-term mnemonic operations that are preserved in amnesia, such as language comprehension. Facilitation in both perceptual and conceptual processing is often possible when repeated stimuli are used in priming tests, such that behavioral measures can reflect both perceptual and conceptual priming. Ideally, analyses of conceptual implicit memory are conducted when perceptual implicit memory can be ruled out.
Because conceptual implicit memory processes can potentially unfold regardless of whether behavioral measures of conceptual priming are obtained, neural activity that underlies conceptual implicit memory may occur during recognition testing with meaningful stimuli. Likewise, explicit remembering can occur incidentally during a conceptual priming test. Thus, special consideration is required in order to disambiguate neural correlates of explicit memory and conceptual priming whenever meaningful stimuli are employed in memory tests.
Conceptual implicit memory shares many functional characteristics with a form of explicit memory known as familiarity. Familiarity refers to the memory phenomenon whereby a stimulus is recognized as having been encountered previously, but without the concomitant retrieval of any further detail regarding the initial encounter. Familiarity occurs, for instance, when one recognizes a person from the past but cannot recall the person’s name or when or where the person was previously met (Mandler, 1980). In contrast, memory processing can lead to recognition along with episodic retrieval such that details from an earlier encounter are recovered and support the full-blown experience of remembering, or recollection. Many researchers posit that recollection and familiarity are supported by distinct neurocognitive operations that provide unique cues to stimulus recognition (Yonelinas, 2002). On the other hand, some evidence indicates that recollection and familiarity entail distinct phenomenological experiences that nonetheless derive from the same neurocognitive processing (Squire, Wixted, & Clark, 2007). This controversy may also spur the development of additional ways to measure familiarity memory.
On the basis of current methods used to measure implicit and explicit memory, conceptual implicit memory and familiarity appear to respond in similar ways to a variety of experimental manipulations (Paller, Voss, & Boehm, 2007; Yonelinas, 2002), and so it might seem natural for conceptual implicit memory processes to ultimately result in familiarity experiences and corresponding behavioral manifestations of explicit memory (Mandler, 1980; Rajaram & Geraci, 2000; Whittlesea & Williams, 1998; Wolk et al., 2005; Yonelinas, 2002). Nonetheless, whether explicit memory inferences result from conceptual implicit memory processes remains highly controversial (e.g., Levy, Stark, & Squire, 2004). Neural markers of relevant processes may be helpful for resolving this issue.
Effects on ERPs known as FN400 potentials have been postulated by many experimenters to index familiarity (reviewed in Curran, Tepe, & Piatt, 2006; Mecklinger, 2006; Rugg & Curran, 2007). FN400 potentials are so named because they are maximal over Frontal brain regions, Negative in polarity, and peak at approximately 400 ms after stimulus onset. These potentials are strikingly similar to N400 potentials that have been extensively characterized as neural markers of semantic priming (Kutas & Hillyard, 1980; Kutas, Van Petten, & Kluender, 2006). The primary difference is in the distribution of the effects across the scalp—midfrontal for FN400 potentials and centro-parietal for typical N400 potentials. However, the distribution of N400 correlates of semantic priming vary depending on many factors, including the nature of the stimuli (i.e., more anterior for pictures versus words, reviewed in Kutas et al., 2006). Little effort has been devoted to attempting to directly dissociate FN400 potentials from N400 potentials. Yet, the pervasive position taken in the ERP/memory literature has been that FN400 potentials reflect distinct brain processes aligned with familiarity, not with semantic priming.
FN400 amplitudes are consistently reduced in association with increases in familiarity following word repetition in explicit memory tests (Paller et al., 2007; Rugg & Curran, 2007). However, N400 reductions with word repetition have also been observed in amnesic patients (Olichney et al., 2000), suggesting that FN400 potentials may reflect a form of memory that is not disrupted in amnesia. Although it remains unknown whether FN400 potentials are produced by any of the same neural processes that lead to N400 potentials, Olichney and colleagues (2000) proposed that N400 potentials reflect preserved conceptual priming operative during word repetition. Conceptual priming was not measured in their study. In an earlier ERP study, Bentin, Moscovitch, and Heth (1992) offered a similar suggestion. And yet, for many years this position was overshadowed by interpretations emphasizing familiarity. Based on a recent analysis of published findings on FN400, Paller and colleagues (2007) interpreted the available evidence as consistent with the notion that it is premature to strongly associate FN400 potentials with either familiarity or conceptual priming. A major weakness of previous studies of neural correlates of familiarity is that conceptual priming was seldom taken into account. Given that FN400 potentials could conceivably reflect conceptual priming that occurs concurrently during explicit memory tests, behavioral measures of both conceptual priming and explicit memory are needed to disentangle these memory functions and their neural correlates.
In a recent study, we examined these issues by using celebrity faces to elicit neural correlates of conceptual priming and explicit memory (Voss & Paller, 2006). Conceptual priming was manipulated by presenting a brief snippet of well-known information concerning a celebrity along with the corresponding celebrity visage for just one of two sets of celebrities. Later, electrophysiological recordings were obtained while subjects rapidly discriminated celebrity faces from unknown faces. Evidence for conceptual priming consisted of faster and more accurate responses to celebrity faces previously presented with biographical information, compared to a baseline provided by a counterbalanced set of celebrity faces that were presented in the experiment under comparable conditions but without corresponding biographical information. Electrophysiological responses obtained during the famous face discrimination test were characterized according to whether conceptual priming had or had not been induced with biographical information, and as a function of each subject’s ratings of explicit memory for the celebrities (obtained in the last phase of the experiment). We thus obtained behavioral measures of both conceptual priming and explicit memory, and we used these measures to attempt to disentangle neural correlates of memory processing obtained during a test of conceptual priming.
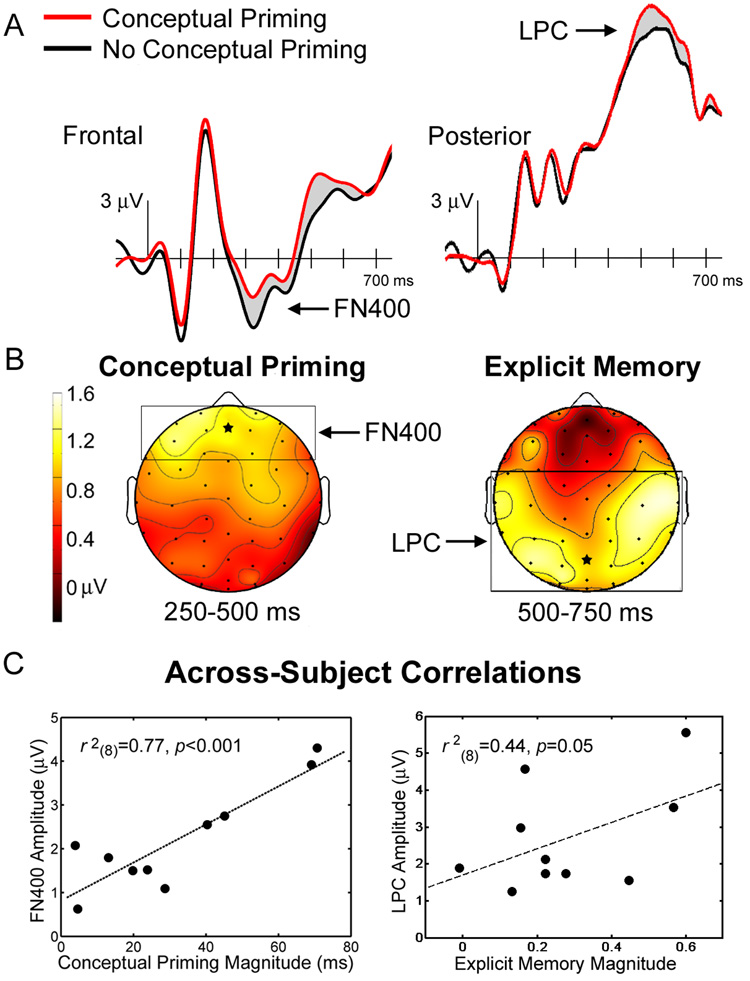
During the conceptual priming test, ERP differences between the set of faces primed with biographical information and the set of faces not primed with biographical information included FN400 potentials as well as late positive potentials at posterior locations (Fig. 2). Such late positive potentials (here termed LPC for Late Positive Complex) are frequently attributed to explicit memory retrieval (reviewed in Friedman & Johnson, 2000; Voss & Paller, 2008). Not only were FN400 potentials revealed through the conceptual priming contrast, FN400 potentials were also uniquely associated with conceptual priming via a correlational analysis. As shown in Fig. 2C (left), the magnitude of the FN400 difference was correlated across-subjects with the magnitude of conceptual priming indexed behaviorally, but it did not correlate significantly with the difference in explicit memory ratings between the two conditions. Conversely, the magnitude of the LPC difference between repeated faces with versus without conceptual priming was correlated with the corresponding difference in explicit memory ratings, as shown in Fig. 2C (right), and was not correlated with the magnitude of conceptual priming. The LPC difference in this ERP contrast was driven by responses to the celebrities with which subjects were unfamiliar, as these faces presumably elicited differential recollection as a function of conceptual information presented during the study phase. Furthermore, the LPC effect was most pronounced in an ERP contrast between the most and the least well known celebrities, as would be expected for an ERP correlate of explicit memory. Indeed, LPC differences were absent when priming was assessed with familiarity held constant (all highly familiar), whereas FN400 differences were associated with conceptual priming in the same comparison. The key characteristic of this experiment was that behavioral measures of both conceptual priming and explicit memory were obtained for the same sets of faces viewed during a priming test, as this resulted in the successful differentiation of neural correlates of conceptual implicit memory (FN400) and of explicit memory (LPC).
Figure 2. ERP correlates of conceptual implicit memory and explicit memory (Voss & Paller, 2006).
ERPs in A were recorded during a conceptual priming test. ERPs to famous faces that had also been presented with corresponding conceptual information in an earlier phase of the experiment (conceptual priming) differed from ERPs to famous faces previously presented without corresponding information (no conceptual priming), in the amplitude of FN400 potentials and LPC potentials, as indicated. In both cases ERPs were relatively more positive with conceptual priming. These ERP differences computed over two intervals are shown topographically in B (same format as in Fig. 1). The electrode locations for ERPs in A are marked with stars on the topographic maps. The magnitude of the FN400 difference was correlated across-subjects with the magnitude of conceptual priming indexed behaviorally, as shown in C (left), but it did not correlate significantly with the difference in explicit memory ratings between the two conditions. Conversely, the magnitude of the LPC difference between repeated faces with versus without conceptual priming was correlated with the corresponding difference in explicit memory ratings, as shown in C (right), and was not correlated with the magnitude of conceptual priming.
Two important conclusions can be made from these results. First, because FN400 potentials were correlated with conceptual priming, and because these potentials are commonly identified during recognition tests, our results attest to the likelihood that neural activity related to conceptual implicit memory is commonly produced in memory experiments designed to monitor explicit memory. Second, the hypothesis that the FN400 potential is a generic neural signature of familiarity must be called into question. When proper attention is given to the possible co-occurrence of familiarity memory and conceptual implicit memory, FN400 potentials have been linked with conceptual implicit memory.
In a follow-up experiment, we used similar methods to identify fMRI correlates of both conceptual priming and explicit memory for famous faces (Voss, Reber, Mesulam, Parrish, & Paller, 2007). Unlike our ERP experiment, we also employed a recognition test in order to specifically isolate episodic familiarity. We observed a double-dissociation between fMRI correlates of conceptual priming and familiarity, whereby conceptual priming was associated with fMRI response reductions in left prefrontal cortex whereas familiarity (both from a general explicit memory test, as in the aforementioned ERP study, and in an episodic familiarity memory test) was associated with response enhancements in right lateral parietal cortex. Previous studies have also linked conceptual priming to prefrontal response reductions (Buckner et al., 1998; Demb et al., 1995; Thompson-Schill, D'Esposito, & Kan, 1999; Wagner, Koutstaal, Maril, Schacter, & Buckner, 2000) and, separately, explicit memory to parietal response enhancements (reviewed in Wagner et al., 2005). Our study provided behavioral measures of both phenomena in order to disambiguate neuroimaging measures. Likewise, one prior study also succeeded in comparing fMRI correlates of conceptual priming and recognition memory (Donaldson, Petersen, & Buckner, 2001). Although familiarity was not specifically separated from other explicit memory phenomena (recollection), and neural measures of conceptual priming and explicit memory were obtained in two separate tests, the results from Donaldson and colleagues (2001) with visual word stimuli were generally consistent with the dissociation we observed for famous faces.
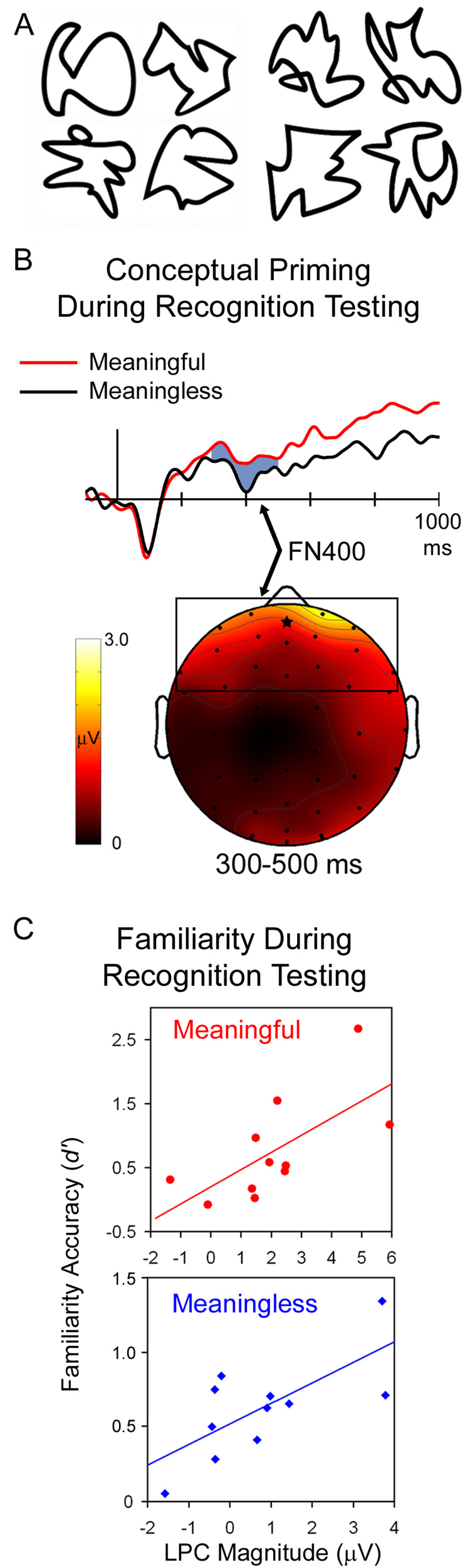
In our experiments with famous faces, we examined conceptual priming and explicit memory during priming tests (Voss & Paller, 2006; Voss et al., 2007). In contrast, neural correlates of familiarity have previously been studied almost exclusively during recognition tests. To allow further direct tests of the hypothesis that conceptual priming can operate incidentally during recognition testing, and that FN400 potentials are neural correlates of conceptual priming rather than familiarity under these circumstances, we developed additional procedures for disentangling familiarity and conceptual priming (Voss & Paller, 2007). In this regard, it was particularly advantageous to study memory for abstract stimuli called squiggles (Fig. 3A), following prior studies by Groh-Bordin and colleagues (2006). These stimuli are not at all meaningful in comparison to faces. Nonetheless, the perceived meaning that they do evince varies greatly from person to person; a particular abstract shape might be perceived as related to a meaningful object by some subjects, whereas others will attribute no meaning at all to the same shape. We thus refer to “meaningful squiggles” as those stimuli idiosyncratically given a high meaningfulness rating (i.e., a different set of squiggles for each subject). We observed reliable conceptual priming for meaningful squiggles, in that meaningfulness ratings were made more quickly the second time. In contrast, “meaningless squiggles,” which were those given low meaningfulness ratings, did not appear to support conceptual priming, as repetition did not produce faster responding.
Figure 3. Disentangling ERP correlates of conceptual implicit memory and familiarity during a recognition test (Voss & Paller, 2007).
Behavioral measures of conceptual priming were exhibited for squiggles that were meaningful, but not those that were meaningless (sample squiggles shown in A; meaningful and meaningless conditions defined based on subjective ratings made by each subject). To identify ERP correlates of conceptual priming during recognition testing, contrasts were made between meaningful and meaningless squiggles that were equated in explicit memory strength. ERPs for both conditions appear in B for the frontal midline electrode indicated by a star on the topographic plot. The FN400 difference between meaningful and meaningless items attributed to conceptual priming is shown topographically in B. In contrast, the magnitude of LPC potentials correlated across-subjects with the accuracy of familiarity-based recognition (d' for “know” responses), as shown in C. These correlations were significant for both meaningful and meaningless items. Thus, FN400 potentials varied as a function of meaningfulness ratings and corresponding ability to support conceptual priming, whereas LPC potentials were associated with familiarity-based recognition irrespective of meaningfulness.
Importantly, subjects can explicitly recognize a repeated squiggle whether it is meaningful or meaningless. We were thus able to analyze ERPs to squiggles that yielded familiarity-based recognition during recognition testing and to categorize trials separately according to meaningfulness. In other words, we selected sets of squiggles matched in explicit memory strength that varied systematically in their ability to support conceptual priming (robust conceptual priming for meaningful squiggles; no conceptual priming for meaningless squiggles). ERP contrasts between these two categories thus yielded neural correlates of conceptual priming resembling FN400 potentials (Fig. 3B). LPC potentials were associated with familiarity ratings during recognition irrespective of meaningfulness level (Fig. 3C).
We obtained converging evidence that FN400 potentials reflect conceptual priming for squiggles in a follow-up experiment (Voss, Schendan, & Paller, unpublished data). We administered the conceptual priming test used in our previous experiments (Voss & Paller, 2007) for the same squiggle stimuli that were also segregated into meaningful and meaningless categories. Again, behavioral evidence for conceptual priming was present only for the meaningful items, and the magnitude of conceptual priming was directly correlated with the magnitude of FN400 potentials during the conceptual priming test. We also observed perceptual implicit memory for squiggles using a task requiring discriminating between squiggles with a loop versus without a loop (as in Voss & Paller, 2007), but perceptual implicit memory was not associated with FN400 potentials.
Results from our studies of conceptual priming and familiarity suggest that each process is supported by distinct neurocognitive processing operations performed by distinct brain networks (Voss & Paller, 2006, , 2007; Voss et al., 2007). Under certain testing conditions, then, familiarity and conceptual priming appear to be functionally distinct. If familiarity experiences in our studies resulted from conceptual priming, one would expect neural markers of the two to at least be partly overlapping. For famous faces, ERP and fMRI correlates of each memory phenomenon were dissociated during the performance of a single conceptual priming task (i.e., in the absence of confounding task differences). Similarly, ERP correlates of each were also dissociated using minimalist visual shapes. These results converge with the finding that these memory processes can be dissociated in amnesia (Levy et al., 2004), and pose serious difficulties for any theory proposing a tight and consistent coupling between familiarity and conceptual priming. Furthermore, these results were obtained using two types of stimuli (faces and squiggles), and in diverse memory testing conditions—it is of interest to determine whether these results generalize to other experimental situations. Thus, whether a tighter coupling between familiarity and conceptual priming obtains with other stimuli or testing conditions should be explored in future studies.
Another way to scrutinize the hypothesized functional relationship between conceptual priming and familiarity would be to use functional connectivity analyses to assess interactions between their neural correlates. In addition, it would be informative to determine if the neural events of memory encoding that predict later conceptual priming are distinct from those responsible for the formation of an explicit memory.
In sum, much progress has been made in identifying component processes of human memory capabilities and characterizing corresponding neural substrates of memory. However, there is much more to be learned so as to demystify the cognitive, biological, and phenomenological facets of memory. The chief methodological consideration stressed by the studies we have reviewed is that appropriate behavioral measures of memory must be included in any neuroimaging experiment in order to unambiguously map neurophysiological events onto hypothesized cognitive processes. Given the likely prevalence of implicit memory processes during explicit memory testing, it is not reasonable to investigate neural correlates of explicit memory without also taking implicit memory into account. Additionally, it would be ideal to also include manipulations that can dissociate implicit and explicit memory. Recent studies have made significant headway in clarifying the neurocognitive processing events responsible for the critical distinctions between implicit and explicit memory. In addition to elucidating differences between conscious and nonconscious memory expressions, a deeper understanding of this issue will allow us to characterize how these distinct forms of memory interact in a variety of situations to drive memory performance.
Acknowledgements
Research support was provided by grant 0518800 from the National Science Foundation and grants R01-NS34639 and P30-AG13854 from the National Institutes of Health. J.L.V. was supported by National Institutes of Health grant T32-AG20506. Small portions of this article appear in Voss and Paller (2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Information can also be held in awareness for an extended period of time, while rehearsed and/or manipulated (for a summary of research on primary memory or working memory, see Passingham & Sakai, 2004; Ranganath & D'Esposito, 2005). Our emphasis here is on memory phenomena occurring when information that was initially encoded is later brought back to mind after a delay, which is what William James (1890, pp648) termed secondary memory.
References
- Bentin S, Moscovitch M, Heth I. Memory with and without awareness: Performance and electrophysiological evidence of savings. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:1270–1283. doi: 10.1037//0278-7393.18.6.1270. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, et al. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nature Reviews Neuroscience. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Curran T, Tepe KL, Piatt C. ERP explorations of dual processes in recognition memory. In: Zimmer HD, Mecklinger A, Lindenberger U, editors. Handbook of binding and memory: Perspectives from cognitive neuroscience. Oxford: Oxford University Press; 2006. pp. 467–492. [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL. Dissociating memory retrieval processes using fMRI: Evidence that priming does not support recognition memory. Neuron. 2001;31:1047–1059. doi: 10.1016/s0896-6273(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R., Jr Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microscopy Research and Technique. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE. Cognitive neuroscience of human memory. Annual Review of Psychology. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Groh-Bordin C, Zimmer HD, Ecker UK. Has the butcher on the bus dyed his hair? When color changes modulate ERP correlates of familiarity and recollection. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.04.215. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- James W. The principles of psychology. New York: H. Holt and company; 1890. [Google Scholar]
- Keane MM, Orlando F, Verfaellie M. Increasing the salience of fluency cues reduces the recognition memory impairment in amnesia. Neuropsychologia. 2006;44:834–839. doi: 10.1016/j.neuropsychologia.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleider HM, Goldinger SD. Illusions of face memory: Clarity breeds familiarity. Journal of Memory and Language. 2004;50:196–211. doi: 10.1016/j.jml.2003.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten C, Kluender R. Psycholinguistics electrified II (1994–2005) In: Gernsbacher MA, Traxler M, editors. Handbook of Psycholinguistics. 2nd edition. New York: Elsevier Press; 2006. pp. 659–724. [Google Scholar]
- Levy DA, Stark CE, Squire LR. Intact conceptual priming in the absence of declarative memory. Psychological Science. 2004;15:680–686. doi: 10.1111/j.0956-7976.2004.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Mayes AR, Roberts N. Theories of episodic memory. Philosophical Transactions of the Royal Society of London. 2001;356:1395–1408. doi: 10.1098/rstb.2001.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklinger A. Electrophysiological measures of familiarity memory. Clinical EEG and Neuroscience. 2006;37:292–299. doi: 10.1177/155005940603700406. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia. Electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Paller KA. Recall and stem-completion priming have different electrophysiological correlates and are modified differentially by directed forgetting. Journal of Experimental Psychology Learning Memory and Cognition. 1990;16:1021–1032. doi: 10.1037//0278-7393.16.6.1021. [DOI] [PubMed] [Google Scholar]
- Paller KA. Neurocognitive foundations of human memory. In: Medin DL, editor. The Psychology of Learning and Motivation. Vol. 40. San Diego, CA: Academic Press; 2001. pp. 121–145. [Google Scholar]
- Paller KA. Cross-cortical consolidation as the core defect in amnesia: Prospects for hypothesis-testing with neuropsychology and neuroimaging. In: Squire LR, Schacter DL, editors. Neuropsychology of Memory. Vol. 3. 2002. pp. 73–87. [Google Scholar]
- Paller KA, Bozic VS, Ranganath C, Grabowecky M, Yamada S. Brain waves following remembered faces index conscious recollection. Cognitive Brain Research. 1999;7:519–531. doi: 10.1016/s0926-6410(98)00050-0. [DOI] [PubMed] [Google Scholar]
- Paller KA, Hutson CA, Miller BB, Boehm SG. Neural manifestations of memory with and without awareness. Neuron. 2003;38:507–516. doi: 10.1016/s0896-6273(03)00198-3. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M. Brain potentials during memory retrieval provide neurophysiological support for the distinction between conscious recollection and priming. Journal of Cognitive Neuroscience. 1992;4:375–391. doi: 10.1162/jocn.1992.4.4.375. [DOI] [PubMed] [Google Scholar]
- Paller KA, Voss JL, Boehm SG. Validating neural correlates of familiarity. Trends in Cognitive Sciences. 2007;11:243–250. doi: 10.1016/j.tics.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Passingham D, Sakai K. The prefrontal cortex and working memory: Physiology and brain imaging. Current Opinion in Neurobiology. 2004;14:163–168. doi: 10.1016/j.conb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Rajaram S, Geraci L. Conceptual fluency selectively influences knowing. Journal of Experimental Psychology Learning Memory and Cognition. 2000;26:1070–1074. doi: 10.1037//0278-7393.26.4.1070. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M. Directing the mind's eye: Prefrontal, inferior and medial temporal mechanisms for visual working memory. Current Opinion in Neurobiology. 2005;15:175–182. doi: 10.1016/j.conb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Roediger HL., 3rd Implicit memory. Retention without remembering. The American Psychologist. 1990;45:1043–1056. doi: 10.1037//0003-066x.45.9.1043. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Implicit memory: History and current status. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Tulving E. Memory Systems 1994. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Current Opinion in Neurobiology. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schott B, Henson RN, Richardson-Klavehn A, Becker C, Thoma V, Heinze HJ, et al. Redefining implicit and explicit memory: The functional neuroanatomy of priming, remembering, and control of retrieval. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1257–1262. doi: 10.1073/pnas.0409070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott B, Richardson-Klavehn A, Heinze HJ, Duzel E. Perceptual priming versus explicit memory: Dissociable neural correlates at encoding. Journal of Cognitive Neuroscience. 2002;14:578–592. doi: 10.1162/08989290260045828. [DOI] [PubMed] [Google Scholar]
- Schott B, Richardson-Klavehn A, Henson RN, Becker C, Heinze H, Duzel J. Neuroanatomical dissociation of encoding processes related to priming and explicit memory. The Journal of Neuroscience. 2006;26:792–800. doi: 10.1523/JNEUROSCI.2402-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–552. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Tunney RJ, Fernie G. Repetition priming affects guessing not familiarity. Behavioral and Brain Functions. 2007;3:40. doi: 10.1186/1744-9081-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C, Senkfor AJ. Memory for words and novel visual patterns: Repetition, recognition, and encoding effects in the event-related brain potential. Psychophysiology. 1996;33:491–506. doi: 10.1111/j.1469-8986.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Cermak LS. Perceptual fluency as a cue for recognition judgments in amnesia. Neuropsychology. 1999;13:198–205. doi: 10.1037//0894-4105.13.2.198. [DOI] [PubMed] [Google Scholar]
- Voss JL, Baym CL, Paller KA. Accurate forced-choice recognition without awareness of memory retrieval. Learning & Memory. 2008;15:454–459. doi: 10.1101/lm.971208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Fluent conceptual processing and explicit memory for faces are electrophysiologically distinct. The Journal of Neuroscience. 2006;26:926–933. doi: 10.1523/JNEUROSCI.3931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Neural correlates of conceptual implicit memory and their contamination of putative neural correlates of explicit memory. Learning & Memory. 2007;14:259–267. doi: 10.1101/lm.529807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Neural substrates of remembering: Electroencephalographic studies. In: Byrne J, editor. Learning and memory: A comprehensive reference. Vol. 3. Oxford: Elsevier; 2008. pp. 79–97. [Google Scholar]
- Voss JL, Reber PJ, Mesulam MM, Parrish TB, Paller KA. Familiarity and conceptual priming engage distinct cortical networks. Cerebral Cortex. 2007;18:1712–1719. doi: 10.1093/cercor/bhm200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL. Task-specific repetition priming in left inferior prefrontal cortex. Cerebral Cortex. 2000;10:1176–1184. doi: 10.1093/cercor/10.12.1176. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Whittlesea BW, Williams LD. Why do strangers feel familiar, but friends don't? A discrepancy-attribution account of feelings of familiarity. Acta Psychologia. 1998;98:141–165. doi: 10.1016/s0001-6918(97)00040-1. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Berman AR, Holcomb PJ, Daffner KR, Budson AE. Patients with mild Alzheimer's disease attribute conceptual fluency to prior experience. Neuropsychologia. 2005;43:1662–1672. doi: 10.1016/j.neuropsychologia.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Taylor JR, Karayanidis F, Henson RN. Event-related potentials associated with masked priming of test cues reveal multiple potential contributions to recognition memory. Journal of Cognitive Neuroscience. 2008;20:1114–1129. doi: 10.1162/jocn.2008.20076. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]