Abstract
The deduced amino acid sequences of the flagellins of Pseudomonas syringae pv. tabaci and P. syringae pv. glycinea are identical; however, their abilities to induce a hypersensitive reaction are clearly different. The reason for the difference seems to depend on the posttranslational modification of the flagellins. To investigate the role of this posttranslational modification in the interactions between plants and bacterial pathogens, we isolated genes that are potentially involved in the posttranslational modification of flagellin in P. syringae pv. glycinea (glycosylation island); then defective mutants with mutations in these genes were generated. There are three open reading frames in the glycosylation island, designated orf1, orf2, and orf3. orf1 and orf2 encode putative glycosyltransferases, and mutants with defects in these open reading frames, Δorf1 and Δorf2, secreted nonglycosylated and slightly glycosylated flagellins, respectively. Inoculation tests performed with these mutants and original nonhost tobacco leaves revealed that Δorf1 and Δorf2 could grow on tobacco leaves and caused symptom-like changes. In contrast, these mutants failed to cause symptoms on original host soybean leaves. These data indicate that putative glycosyltransferases encoded in the flagellin glycosylation island are strongly involved in recognition by plants and could be the specific determinants of compatibility between phytopathogenic bacteria and plant species.
Flagellin is an essential component of the flagellum filament of bacteria. In recent years, there has been increasing evidence that flagellin is posttranslationally glycosylated (4, 20). However, workers have just started to elucidate the molecular basis of this modification. Thus, little is known about its biological significance.
The isolates of the phytopathogenic bacterium Pseudomonas syringae can be classified into more than 50 pathovars on the basis of virulence for host plant species. In a previous study, P. syringae flagellin was found to elicit hypersensitive reactions (HR) in nonhost plants (27). Several studies to characterize elicitor flagellins have been performed with Pseudomonas avenae (Acidovorax avenae) (5) and P. syringae pv. tabaci (7). These flagellins have been reported to induce plant defense responses in cultured cells of rice (5), tomato, and Arabidopsis thaliana (7). A 22-amino-acid peptide (flg22) of a conserved domain near the N terminus based on the sequence of Pseudomonas aeruginosa flagellin has been reported to induce plant defense responses but not hypersensitive cell death (7). Thus, flg22 is known as a general elicitor of an innate immune response in plants (10). However, flagellins from P. avenae (5) and flagellins from P. syringae pv. glycinea and P. syringae pv. tomato (27) are known to induce plant cell death in nonhost rice and tobacco plants, respectively. These results indicate that the flagellin proteins have roles both as nonspecific elicitors of general defense responses and as specific elicitors of HR cell death in defined plant-bacterium interactions. Furthermore, a flagellin-defective mutant (ΔfliC) of P. syringae pv. tabaci lost both the ability to cause HR in nonhost tomato plants (24) and virulence for host tobacco plants (13). These results suggest that flagella are indispensable for establishing both incompatible and completely compatible relationships.
It has recently been reported that the deduced amino acid sequences of the flagellin proteins of P. syringae pv. tabaci and P. syringae pv. glycinea are identical; however, the flagellin protein of P. syringae pv. glycinea caused cell death in nonhost tobacco cells, whereas that of P. syringae pv. tabaci did not (27). Actually, the flagellin proteins of these two pathovars were shown to be glycosylated (28). Furthermore, a study performed with a ΔfliC mutant of P. syringae pv. tabaci and mutants with complements of fliC genes of different pathovars showed that any flagellin modified in a P. syringae pv. tabaci-specific manner did not induce HR death of tobacco cells (28). These results suggest that posttranslational modification of the flagellin determines the ability to induce HR. Recently, a genomic island that is necessary for flagellin glycosylation in P. aeruginosa was identified directly upstream of the fliC gene (1). This report provided a clue to the identity of the gene(s) involved in the posttranslational modification of the flagellin protein of P. syringae. It has also been found that part of a sequence homologous to the glycosylation island of P. aeruginosa is present upstream of fliC in P. syringae pv. tabaci (24).
Here, we describe genetic and functional analyses of the genes in the glycosylation island involved in the posttranslational modification of flagellin in P. syringae pv. glycinea. In particular, we found that the putative glycosyltransferases determined the ability to induce HR in nonhost tobacco plants and also affected the virulence for host soybean plants. To our knowledge, this is the first report that shows that glycosyltransferases of bacterial flagellin influence pathogenicity for eukaryotes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. P. syringae pv. glycinea (race 4) was grown principally on King's B (KB) medium (15) at 27°C. For the inoculation test, bacteria were cultured in Luria-Bertani (LB) medium with 10 mM MgCl2 at 25°C. For purification of flagellin proteins, bacteria that had been cultured overnight were incubated in minimal medium containing 10 mM mannitol and fructose as carbon sources for 24 h at 23°C (12). Escherichia coli strains were grown on LB medium at 37°C.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH5a | F−λ− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) supE44 thi-1 gyrA relA1 | Takara, Kyoto, Japan |
| S17-1 | thi pro hsdR hsdM+recA [chr::RP4-2-Tc::Mu-Km::Tn7] | 22 |
| P. syringae pv. glycinea strains | ||
| race 4 | Wild type, Nalr | A. Collmer |
| race 4-d1 | race 4 Δorf1 | This study |
| race 4-d2 | race 4 Δorf2 | This study |
| race 4-d3 | race 4 Δorf3 | This study |
| Plasmids | ||
| pUC18 | 2.7-kb cloning vector, Apr | Takara, Kyoto, Japan |
| pUCSac1 | 1.8-kb SacI-SacI fragment in pUC18, Apr | This study |
| pUCSac2 | 13.4-kb SacI-SacI fragment in pUC18, Apr | This study |
| pUCSal1 | 1.5-kb SalI-SalI fragment in pUC18, Apr | This study |
| pCR-blunt II-TOPO | 3.5-kb cloning vector for PCR blunt end product, Kmr | Invitorgen |
| pK18mobsacB | Small mobilizable vector, Kmr, sucrose sensitive (sacB) | 22 |
| pM1 | 2.18-kb chimeric PCR product deleting orf1 cloned into pK18mobsacB at EcoRI site, Kmr | This study |
| pM2 | 1.79-kb chimeric PCR product deleting orf2 cloned into pK18mobsacB at EcoRI site, Kmr | This study |
| pM3 | 1.69-kb chimeric PCR product deleting orf3 cloned into pK18mobsacB at EcoRI site, Kmr | This study |
Apr, ampicillin resistance; Kmr, kanamycin resistance, Nalr, nalidixic acid resistance.
The following antibiotics were added to the growth media when appropriate: ampicillin (50 μg/ml), kanamycin (25 μg/ml), and nalidixic acid (30μg/ml).
Plant material and inoculation procedures.
Tobacco (Nicotiana tabacum cv. Xanthi NC) and soybean (Glycine max cv. Shirofumi) plants were grown in a growth chamber at 25°C and 30 to 40% relative humidity with a 12-h photoperiod. To carry out plant inoculation, P. syringae pv. glycinea was suspended in 10 mM MgSO4-0.02% Silwet L77 (OSI Specialties Inc., Danbury, Conn.) at a density of 2 × 108 CFU ml−1. Five-week-old tobacco plants or three-week-old soybean plants were spray inoculated with an airbrush until both the abaxial and adaxial leaf surfaces were uniformly wet. For the investigation of bacterial growth on tobacco leaves, 10 μl of bacteria (2 × 105 CFU) was put on needle-pricked leaves, and the bacterial populations were measured. At different times, leaf disks (diameter, 0.5 cm) were punched out from inoculated areas and homogenized in 10 mM MgSO4; then serial dilutions of the homogenate were plated on KB agar plates. For the investigation of bacterial growth on soybean leaves, bacteria (2 × 108 CFU ml−1) were spray inoculated as described above. Before the bacterial populations were measured, the surfaces of inoculated soybean leaves were sterilized with 15% hydrogen peroxide.
Isolation of glycosylation island.
A genomic DNA library of P. syringae pv. glycinea was constructed by using a lambda FIX II vector kit (Stratagene, La Jolla, Calif.). Because the glycosylation island in P. aeruginosa has been reported to be linked to a flagellin gene (fliC) (1), a clone containing the fliC gene was isolated by using the fliC gene as a probe.
Generation of mutant strains.
P. syringae pv. glycinea strains with each open reading frame (ORF) in the glycosylation island deleted were generated based on homologous recombination by using the method described previously (24). Approximately 1-kb DNA fragments located on each side of the orf1, orf2, and orf3 genes were amplified by PCR with primers P1UF (5′-TACGACTCACTATAGGGCGT-3′) and P1UR (5′-CGGGATCCGGAAGTTACCGGTCAAGTAA-3′) for the upstream region of orf1; primers P1DF (5′-CGGGATCCTGTTTAATGCGCTCCAGT-3′) and P1DR (5′-GGAGTAGTGTGCATAGGCTT-3′) for the downstream region of orf1; primers P2UF (5′-TCAGCTCAATGAAGTGCAGG-3′) and P2UR (5′-CGGGATCCTGGAGCGCATTAAACAGGT-3′) for the upstream region of orf2; primers P2DF (5′-CGGGATCCTCCGCCAATAATCGCAAA-3′) and P2DR (5′-CTGCATCGCCAAACAACATG-3′) for the downstream region of orf2; primers P3UF (5′-CCGAATTATCGACGTGGCTT-3′) and P3UR (5′-CGGGATCCGATGAGTAAACAGGTCAT-3′) for the upstream region of orf3; and primers P3DF (5′-CGGGATCCGTCATGTTGGTTTGGTTTC-3′) and P3DR (5′-TATCTTGTACGCGACCCAGT-3′) for the downstream region of orf3 (underlining indicates artificial BamHI sites) (Fig. 1B). Each amplified DNA fragment for upstream and downstream regions was ligated at the BamHI site, and the resulting fragments were amplified by PCR by using the pair of primers PIUF and PIDR for deletion of orf1, P2UF and P2DR for deletion of orf2, and P3UF and P3DR for deletion of orf3. The PCR products were inserted into pCR-blunt II-TOPO vectors (Invitrogen, Carlsbad, Calif.), and we confirmed the expected deletion of each ORF and the absence of nucleotide substitutions in the amplified regions. The constructs were digested with EcoRI for generation of the orf1 and orf2 mutants or with HindIII for generation of the orf3 mutant and then introduced into the mobilizable cloning vector pK18mobsacB (5.7 kb, Kmr) (22). The resulting plasmids, designated pM1, pM2, and pM3, were electrotransformed into E. coli S17-1 and then transferred by conjugation from E. coli S17-1 to P. syringae pv. glycinea. After conjugation, the plasmids were excised on a KB agar plate containing 10% sucrose, and specific deletions were confirmed by PCR by using the pairs of primers described above. The resulting bacteria were designated race 4-d1, race 4-d2, and race 4-d3 for the Δorf1, Δorf2, and Δorf3 mutants, respectively.
FIG. 1.
Flagellum gene structure of P. syringae pv. glycinea (A) and generation of the Δorf1, Δorf2, and Δorf3 mutants (B). Restriction sites in the flagellum gene cluster and fragments subcloned into the plasmid vector are indicated by horizontal bars. Pentagons labeled with gene designations represent ORFs found in the genomic clone. The glycosylation islands composed of three ORFs are indicated by black (orf1), gray (orf2), and striped (orf3) pentagons. To generate mutants with deletions in orf1, orf2, and orf3, two sets of DNA fragments were amplified with the appropriate primers and ligated with artificial BamHI sites as described in Materials and Methods. Sc, SacI; Sl, SalI; H, HindIII; B, artificial BamHI.
Motility testing.
The motility of each mutant was compared with that of the wild-type strain on semisolid (0.3%) MMMF agar plates as previously described (24).
Growth comparisons.
The optical density at 600 nm of each bacterial culture was adjusted to 0.1, and the cultures were grown in LB medium with 10 mM MgCl2 with shaking at 25°C. The optical density at 600 nm was determined after 3, 6, 9, and 24 h.
Purification of flagellin proteins.
Flagellin proteins were purified as previously described (27), with a minor modification. Bacteria that had been cultured overnight were collected by centrifugation at 5,000 × g for 10 min. The bacterial pellet was resuspended in phosphate buffer (50 mM sodium phosphate, pH 7.0), and flagella were sheared off from the cells by vortexing for 1 min. Cells and cell debris were removed by centrifugation at 10,000 × g for 30 min. The resulting supernatant was filtered through a 0.45-μm-pore-size filter and centrifuged at 100,000 × g for 30 min. The resulting pellet was suspended in water and used as purified flagellin.
SDS-PAGE analysis and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (16). For detection of flagellin proteins, the bacterial pellet was solubilized in a sample buffer and then subjected to 12% acrylamide gel electrophoresis. After electrophoresis, proteins were transferred to a polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England) for immunological detection as previously described (27).
Detection of glycoproteins.
For detection of glycoproteins, the SDS-PAGE gel was stained by using a GelCode glycoprotein staining kit (Pierce, Rockford, Ill.) as previously described (28).
NBT reduction assay.
O2− was detected by the nitroblue tetrazolium (NBT) reduction assay as described previously (28), with a slight modification. Cells of the wild-type strain or each mutant bacterium, which had been grown in LB medium with 10 mM MgCl2, were resuspended in 10 mM MgSO4 at a concentration of 1 × 109 CFU ml−1, and they were used as a bacterial suspension. Two-milliliter aliquots of a reaction mixture containing 50 mM Tris-HCl (pH 7.6), 2.5 mg of NBT (Wako Pure Chemical Inc., Osaka, Japan) ml−1, and 0.1 volume of the bacterial suspension were poured into six-well plates. Tobacco leaf disks were floated on the reaction mixture with the abaxial leaf surface down and incubated at 25°C with gentle agitation. During this incubation, O2− generation was monitored by determining the concentration of blue formazan converted from NBT at 560 nm.
Sequencing.
For DNA sequencing, DNA fragments of the flagellum gene and the glycosylation island were subcloned into plasmids as shown in Fig. 1A. DNA sequencing was performed with a DNA sequencer (ABI PRISM TM3100; PE Applied Biosystems, Chiba, Japan) by using a BIG Dye terminator cycle sequencing kit. Amino acid sequence similarities were investigated by using the Blast search protocol and Genetyx-Mac (Software Development, Tokyo, Japan).
Nucleotide sequence accession number.
The nucleotide sequence of the flagellum gene cluster with the glycosylation island has been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession number AB106910.
RESULTS
Structural analysis of putative glycosylation island.
To obtain the genomic sequence of the region surrounding a fliC gene of P. syringae pv. glycinea, we screened a genomic clone from a DNA library. One phage clone containing an fliC gene was isolated by using the PCR product of the fliC gene (27) as a DNA probe, and then the nucleotide sequence of a 15.3-kb region of the phage clone was determined. The sequence data revealed three ORFs, designated orf1, orf2, and orf3, between the flagellum structural genes flgL and fliC. These three ORFs are thought to be transcribed in the same direction as the fliC gene (Fig. 1). The spacer regions between flgL and orf1, between orf1 and orf2, and between orf2 and orf3 are 88, 141, and 283 bp long, respectively. We searched for promoter sequences in the spacer regions by using Genetyx-Mac and found that each of these ORFs has its own promoter.
The annotation of these ORFs is summarized in Table 2. The predicted numbers of amino acids encoded and molecular masses are 1,191 amino acids and 134 kDa for orf1, 968 amino acids and 107 kDa for orf2, and 308 amino acids and 33 kDa for orf3, respectively. orf1 and orf2 are homologous to each other, and there is 27.9% amino acid identity between the ca. 660-amino-acid regions encoded by the central region of orf1 and the C terminus of orf2. Recently, the whole genome sequence of P. syringae pv. tomato strain DC3000 was determined (http://www.ncbi.nlm.nih.gov/genomes/framik.cgi?db = genome&gi = 279). The genetic region between flgL and fliC in DC3000 contains three corresponding ORFs, orf1, orf2, and orf3. Each ORF in DC3000 exhibited the highest level of homology to the corresponding ORF in P. syringae pv. glycinea (the sequences encoded by orf1, orf2, and orf3 had 92.9, 91.6, and 98.7% homology, respectively, at the amino acid level). We also found a sequence homologous to orf3 of P. syringae pv. tomato DC3000 and P. syringae pv. glycinea in the upstream region of fliC of P. syringae pv. tabaci (24), suggesting that in P. syringae these ORFs are conserved at the same location in the genome.
TABLE 2.
Similarities of P. syringae pv. glycinea ORFs to proteins in databases
| Gene | Size of product (amino acids) | Homologue
|
|||
|---|---|---|---|---|---|
| Protein and function | Bacterium | % Amino acid homology | Accession no. (reference) | ||
| orf1 | 1,191 | Putative glycosyltransferase | P. syringae pv. tomato | 92.9 | AE016862-221 (unpublished data) |
| OrfN, putative glycosyltransferase | P. aeruginosa | 31.7 | AF332547 (1) | ||
| RfbC, O-antigen biosynthesis | Myxococcus xanthus | 30.1 | U36795 (11) | ||
| Y4gl, biosynthetic protein for rhamnose-rich lipopolysaccharide | Rhizobium sp. strain NGR234 | 30.3 | AE000074 (8) | ||
| orf2 | 968 | Putative glycosyltransferase | P. syringae pv. tomato | 91.6 | AE016862-222 (unpublished data) |
| OrfN, putative glycosyltransferase | P. aeruginosa | 37.8 | AF332547 (1) | ||
| RfbC, O-antigen biosynthesis | Myxococcus xanthus | 29.9 | U36795 (11) | ||
| Y4gl, biosynthetic protein for rhamnose-rich lipopolysaccharide | Rhizobium sp. strain NGR234 | 28.3 | AE000074 (8) | ||
| orf3 | 308 | Putative 3-oxoacyl-(acyl carrier protein) synthase III (partial sequence) | P. syringae pv. tabaci | 100 | AB061230-1 (24) |
| Putative 3-oxoacyl-(acyl carrier protein) synthase III | P. syringae pv. tomato | 98.7 | AE016862-223 (unpublished data) | ||
| Putative 3-oxoacyl-(acyl carrier protein) synthase III | P. putida KT2440 | 82.7 | AE016790-134 (19) | ||
| OrfC, unknown | P. aeruginosa | 29.2 | AF332547 (1) | ||
orf1 and orf2 also showed significant homology to orfN, which was found in the glycosylation island of P. aeruginosa strain PAK; at the amino acid level there was 31.7% homology in an 860-amino-acid region and there was 37.8% homology in a 577-amino-acid region, respectively (1). The proteins encoded by orf1 and orf2 also exhibited homology to RfbC, an O-antigen biosynthesis protein of Myxococcus xanthus (30% homology) (11), and y4gl, a biosynthetic protein for rhamnose-rich lipopolysaccharide in the symbiotic plasmid of Rhizobium sp. strain NGR234 (28 to 30% homology) (8). These levels of homology suggest that orf1 and orf2 encode glycosyltransferase proteins. The orf1 and orf2 products also are similar to the putative protein RbfC of Xanthomonas campestris pv. campestris strain ATCC 33913 (37.3 and 43.5% homology, respectively) (6; data not shown) and a hypothetical protein (PA1091) of P. aeruginosa strain PAO1 (42.5 and 34.1% homology, respectively) (26; data not shown). The functions of these hypothetical proteins of X. campestris pv. campestris and P. aeruginosa are not clear yet. On the other hand, the orf3 product showed significant homology to putative 3-oxoacyl-(acyl carrier protein) synthase III of Pseudomonas putida strain KT2440 (82.7% homology) (19) and the product of orfC in the glycosylation island of P. aeruginosa strain PAO1 (29.2% homology) (1).
Generation of Δorf1, Δorf2, and Δorf3 mutants of P. syringae pv. glycinea.
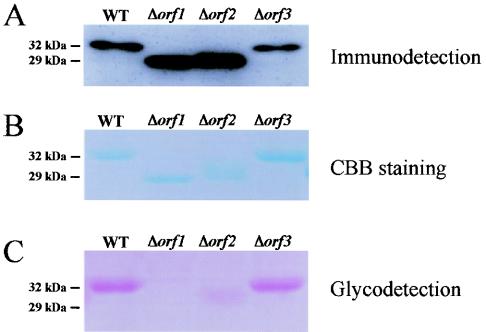
To examine whether the genes that exhibited homology to the genes encoding putative glycosyltransferases are responsible for flagellin glycosylation in P. syringae pv. glycinea, we generated a defective mutant with a mutation in each ORF based on the homologous recombination method. To analyze the effect of the mutation in each ORF on the molecular masses of the flagellin proteins, we performed a Western blot analysis using whole proteins of the resulting mutants with anti-flagellin antibody (Fig. 2A). Western blot analysis showed that the flagellin protein of the Δorf1 mutant migrated at a position that was approximately 3 kDa lower than the position of the protein of the wild-type strain. These mobility data revealed that there was a ca. 29-kDa protein, which is consistent with the predicted sizes of the protein deduced from the sequence of the fliC gene and deglycosylated flagellin as previously reported (28). The molecular mass of the flagellin protein in the Δorf2 mutant was also less than the molecular mass of the flagellin protein in the wild-type strain, but it was a little more than the molecular mass of the flagellin protein in the Δorf1 mutant. The effect of deletion of orf3 was not obvious in the mobility of flagellin, as determined by the Western blot analysis. We also analyzed the molecular masses of purified flagellins from wild-type and mutant strains of P. syringae pv. glycinea by SDS-PAGE. As shown in Fig. 2B, the mobilities of purified flagellins were very consistent with the results obtained by Western blot analysis.
FIG. 2.
Glycosylation of flagellins from the wild type (WT) and the Δorf1, Δorf2, and Δorf3 mutants of P. syringae pv. glycinea. (A) Western blot analysis of the flagellin protein in the total proteins. (B) Coomassie brilliant blue (CBB) staining of the purified flagellins. (C) Staining of the glycosyl moiety in purified flagellin.
The glycosylation status of purified flagellin proteins from each mutant was examined by using a GelCode glycoprotein staining kit (Pierce). As shown in Fig. 2C, glycosylation was not detected in flagellin from the Δorf1 mutant, and only a small amount of glycosylation was detected in flagellin from the Δorf2 mutant. There was no difference in the intensities of glycodetection for flagellins from the wild-type strain and the Δorf3 mutant, suggesting that orf3 has little, if any, responsibility for glycosylation of flagellin and the mobility shift in molecular mass.
Furthermore, deletion of each ORF had no effect on the motility on a soft agar plate, as tested by a swarming assay. It also had no effect on the doubling time (data not shown).
Inoculation of mutant bacteria onto tobacco and soybean leaves.
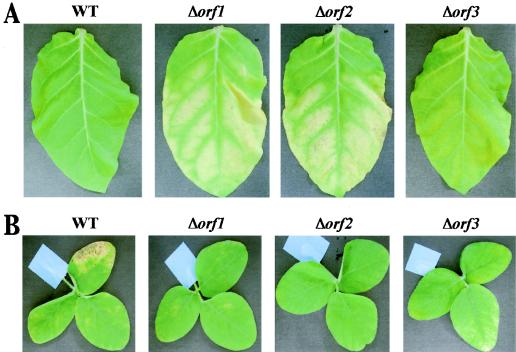
To evaluate the effect of a defect in the genes in the glycosylation island on host and nonhost plants, we inoculated each bacterium onto tobacco (nonhost) and soybean (host) leaves (Fig. 3 and 4). For the symptom assay, plants were spray inoculated as described in Materials and Methods. Inoculation of the nonhost tobacco leaves with the Δorf1 and Δorf2 mutants resulted in lesion-like changes 10 days after inoculation, whereas inoculation with the wild-type strain did not have this effect (Fig. 3A). Furthermore, inoculation of the Δorf3 mutant resulted in a phenotype of tobacco leaves intermediate between the phenotype of wild type-inoculated leaves and the phenotype of Δorf2 mutant-inoculated leaves. The symptoms caused by the three mutants differed in degree and speed. Tobacco leaves inoculated with the Δorf1 mutant exhibited the most drastic changes compared to the leaves inoculated with the wild-type strain. In contrast, when host soybean leaves were inoculated with each strain of P. syringae pv. glycinea, the wild-type strain caused symptoms 13 days after inoculation. However, the Δorf1 and Δorf2 mutants failed to cause prominent symptoms, and the Δorf3 mutant caused only weak symptoms (Fig. 3B).
FIG. 3.
Inoculation tests with nonhost tobacco leaves (A) and host soybean leaves (B). Leaves were inoculated with each bacterium by spraying. Tobacco and soybean leaves were photographed 10 and 13 days after inoculation, respectively. The tests were repeated two or three times. The photographs show typical results. WT, wild type.
FIG. 4.
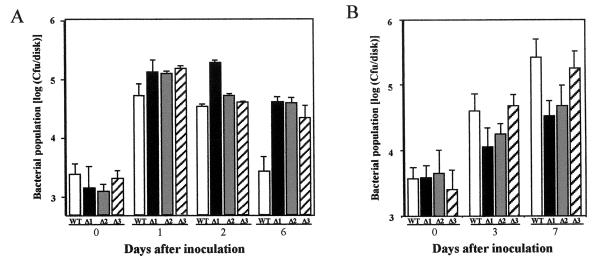
Bacterial growth in tobacco leaves (A) and soybean leaves (B) inoculated with P. syringae pv. glycinea wild type (WT), the Δorf1 mutant (Δ1), the Δorf2 mutant (Δ2), or the Δorf3 mutant (Δ3). The bacterial populations in the leaves were measured 0, 1, 2, and 6 days after inoculation onto tobacco leaves and 0, 3, and 7 days after inoculation onto soybean leaves. The bars indicate means and the error bars indicate standard errors for four independent experiments.
To examine whether the disease symptoms shown in Fig. 3 were accompanied by bacterial propagation, the bacterial growth in each inoculated leaf was analyzed (Fig. 4). In tobacco leaves, all bacteria could transiently propagate within 1 day; however, by 2 days after inoculation, the population of wild-type P. syringae pv. glycinea had decreased remarkably, while the populations of the three mutants remained high (Fig. 4A). On the other hand, we observed propagation of wild-type P. syringae pv. glycinea in soybean leaves, whereas the populations of the Δorf1 and Δorf2 mutants were suppressed compared to the wild-type population. However, suppression of growth of the Δorf3 mutant was not obvious (Fig. 4B). Thus, the reduced virulence of these mutants that we observed (Fig. 3B) on the host soybean plants was reflected in the bacterial growth (Fig. 4B).
Effects of mutant bacteria on oxidative burst in nonhost tobacco leaves.
Oxidative burst is known to be a typical early defense response of nonhost plants when they are inoculated with avirulent pathogens. A previous study based on an NBT reduction assay showed that P. syringae pv. glycinea and P. syringae pv. tomato caused prominent oxidative bursts when they were inoculated onto nonhost tobacco leaves, whereas P. syringae pv. tabaci, a bacterium with virulence for tobacco, did not (28). To evaluate the effect of deleting each ORF on the ability to cause oxidative burst in tobacco, O2− generation was monitored by the NBT reduction assay. As shown in Fig. 5, the levels of O2− generation caused by the mutants, especially the Δorf1 and Δorf2 mutants, were lower than that caused by the wild type.
FIG. 5.
NBT reduction assay on the surfaces of tobacco leaves inoculated with P. syringae pv. glycinea wild type (WT), the Δorf1 mutant (Δ1), the Δorf2 mutant (Δ2), or the Δorf3 mutant (Δ3). Formation of blue formazan was measured 10, 50, and 90 min after inoculation. The bars indicate the means and the error bars indicate the standard errors for four independent experiments. OD 560, optical density at 560 nm.
DISCUSSION
Glycosylation of flagellin is found in some pathogenic bacteria, such as P. aeruginosa (1), Campylobacter jejuni (30), Campylobacter coli (17), and Helicobacter pylori (14), but its influence on pathogenicity is poorly understood, especially in phytopathogenic bacteria. In phytopathogenic bacteria, the ability of flagellin to elicit a plant defense response was reported previously for several pathovars of P. syringae (7, 27, 28) and P. avenae (5). Che et al. (5) and Taguchi et al. (28) detected glycosylation in flagellin proteins in P. avenae and P. syringae, respectively. Taguchi et al. also suggested the importance of posttranslational modification of flagellin proteins of P. syringae in flagellin-induced hypersensitive death of tobacco cells (27, 28). In this study, we identified the genes involved in flagellin glycosylation and examined their influence on plant-microbe interactions.
Three ORFs, orf1, orf2, and orf3, located between the flgL and fliC genes, were termed the glycosylation island. The putative proteins encoded by orf1 and orf2 exhibited 31.7 and 37.8% similarity, respectively, to OrfN of the glycosylation island in P. aeruginosa strain PAK (1). Interestingly, amino acid sequences encoded by orf1 and orf2 exhibited 27.9% identity to each other. Deletion of orf1 and orf2 resulted in a reduction in the molecular mass as determined by SDS-PAGE analysis (Fig. 2A and B), indicating that both gene products are necessary for flagellin glycosylation. The deletion of orf1 completely eliminated the ability to glycosylate flagellin proteins, whereas some glycosyl residues remained in the flagellin of the Δorf2 mutant (Fig. 2C). In the biosynthetic pathway, several glycosyltransferases are thought to be required to form one mature glycoprotein. In the system which we examined, two glycosyltransferases are thought to function in a certain order. Our results indicate that the orf1 product seemed initially to transfer some saccharide(s) to flagellin proteins, and this was followed by modification by orf2. The Δorf1 and Δorf2 mutants acquired pathogenicity to originally nonhost tobacco plants (Fig. 3A and 4A). In other words, the Δorf1 and Δorf2 mutants have reduced abilities to induce an oxidative burst in tobacco (Fig. 5), which allows parasitic propagation of bacteria and expansion of the disease symptoms. Thus, glysosyl moieties of flagellins from incompatible pathogenic bacteria seem to possess the ability to induce a plant defense response. In the Δorf3 mutant, the molecular mass of flagellin was not obviously changed, as determined by SDS-PAGE analysis. However, an inoculation test with nonhost tobacco leaves revealed that the growth of the Δorf3 mutant was greater than that of the wild type (Fig. 4A). Furthermore, the level of oxidative burst caused by the Δorf3 mutant was slightly lower than that caused by the wild type. Thus, orf3 is also likely to contribute to formation of an epitope for the HR-inducing elicitor. Orf3 showed significant homology to 3-oxoacyl-(acyl carrier protein) synthase III. The role of orf3 in flagellin modification is obscure at present, but considering that acylation of lipooligosaccharides has been reported to be involved in the sensitivity of nontypeable Haemophilus influenzae to human β-defensins (25), acylation of flagellin seems to have a considerable role in innate immunity.
Because the Δorf1 and Δorf2 mutants failed to invade the original host soybean plants, addition or variation of the glycosyl moiety may reflect an evolutionary adaptation of flagellin to avoid recognition by the host defense surveillance system. In many cases, a carbohydrate modification rather than amino acid epitopes appears to represent an immune-dominant determinant (4). A previous study showed that flagellin glycosylation only in the manner found in P. syringae pv. tabaci eliminated HR death of tobacco cells (28). Thus, to establish a compatible relationship, flagellin proteins need to be glycosylated in the original pathogenic bacterial manner. In the case of P. syringae pv. glycinea, the glycosyl moiety of flagellin might interfere with efficient recognition by host soybean cells, leading to HR death of cells, and this may be the reason that P. syringae pv. glycinea has the ability to invade soybean plants. Further investigation of this is necessary.
Why do flagellin proteins need to be glycosylated? Although the results of the swarming assay showed that all mutants retain mobility, we speculate that the glycosylation of flagellin might increase the hydrophilicity of flagella. When incompatible relationships are considered, plants might have evolved by acquisition of the ability to recognize flagellin, especially its glycosyl moieties, and to initiate the defense responses. In general, race-cultivar specificity in plant-pathogen interactions is explained by the ligand-receptor model based on the gene-for-gene theory of plant disease resistance (23, 29). In this model, an avirulence protein encoded by a bacterial avirulence (avr) gene is considered a ligand that is recognized by a corresponding receptor encoded by a plant resistance (R) gene. Once a plant recognizes avirulent bacteria, the HR, involving rapid plant cell death at the point of invasion, occurs. Although the products recognized by the tobacco plant are supposedly not putative glycosyltransferases themselves, orf1 and orf2 should be defined as avr-like genes in the sense that these genes are thought to endow P. syringae pv. glycinea with avirulence for tobacco. An interesting example of an indirect avirulence gene is avrD of P. syringae pv. tomato (18). avrD specifies production of a low-molecular-weight compound, syringolide, that triggers plant defense responses via the condensation of d-xylulose and a β-ketoalkanoic acid. Recently, Boller and colleagues found that the FLS2 gene, encoding a leucine-rich repeat receptor-like kinase, is involved in recognition of flg22 and leads to activation of defense responses (2, 3, 9). flg22 has not been reported to induce HR cell death (10). On the other hand, flagellin proteins purified from each pathovar have been found to elicit HR in nonhost plants (27). Thus, the flagellin molecule is thought to possess at least two epitopes that trigger plant defense responses; one is a region that corresponds to flg22 and induces general defense responses without cell death, and the other is a peptide region outside flg22 and/or the glycosylation motif in flagellin that induces HR cell death. Further investigation is required to determine the role of flagellin glycosylation in plant-pathogen interactions.
The glycosyl structures and their amino acid linkages that are responsible for the observed changes in virulence and avirulence remain to be determined. The lengths of the sugar chains in pathogenic bacterial proteins so far are considered shorter (mono- to trisaccharide substituents) than those of eukaryotes (4). If this idea is applicable to our study, about 10 sugar chains would be attached to the flagellin peptide region by a 2- to 3-kDa shift in the SDS-PAGE assay (Fig. 2). We identified only two genes for putative glycosyltransferases; thus, the structure of carbohydrates linked to flagellin might not be not very complicated. Otherwise, these glycosyltransferases might be multicatalytic enzymes, because some of the bacterial glycosyltransferases involved in polysaccharide synthesis have been reported to be bifunctional (21, 31), and the predicted molecular masses of both orf1 and orf2 are relatively large (134 kDa for orf1 and 107 kDa for orf2). However, we cannot exclude the possibility that there may be other glycosyltransferases that are responsible for flagellin glycosylation.
Recently, a sequence that is highly homologous to the glycosylation island in P. syringae pv. tabaci was found in the upstream region of the fliC gene (24; F. Taguchi, K. Takeuchi, H. Kaku, E. Katoh, K. Murata, T. Kawasaki, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose, unpublished data), which suggests that glycosyltransferases are present. It is conceivable that these regions of the two pathovars determine the difference in the flagellins characterized as ligands for plant cells. We are now investigating the role of the glycosylation of flagellin in the specificity of pathovars for plant species by swapping the glycosylation islands in P. syringae pv. glycinea and P. syringae pv. tabaci. The molecular mechanisms of plant recognition might be clarified by utilizing the mutant strains that have been produced.
Acknowledgments
We thank A. Collmer (Cornell University, Ithaca, N.Y.) for providing P. syringae pv. glycinea and Chihiro Yasuda for technical assistance with the inoculation experiments. We are also grateful to T. Mukaihara (RIBS, Okayama, Japan) and Y. Kimura (Okayama University, Okayama, Japan) for valuable discussions concerning the generation of mutant bacteria and glycosylation studies, respectively.
This work was supported in part by Grant-in-Aids for Scientific Research on Priority Area (A) 12052215 and 12052219 and for Scientific Research (S) 15108001 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Arora, S. K., M. Bangera, S. Lory, and R. Ramphal. 2001. A genomic island in Pseudomonas aeruginosa carries the determinants of flagellin glycosylation. Proc. Natl. Acad. Sci. USA 98:9342-9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai, T., G. Tena, J. Plotnikova, M. R. Willmann, W. L. Chiu, L. Gomez-Gomez, T. Boller, F. M. Ausubel, and J. Sheen. 2002. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415:977-983. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, Z., L. Gomez-Gomez, T. Boller, and G. Felix. 2001. Sensitivity of different ecotypes and mutants of Arabidopsis thaliana towards the bacterial elicitor flagellin correlates with the presence of receptor binding sites. J. Biol. Chem. 276:45669-45676. [DOI] [PubMed] [Google Scholar]
- 4.Benz, I., and M. A. Schmidt. 2002. Never say never again: protein glycosylation in pathogenic bacteria. Mol. Microbiol. 45:267-276. [DOI] [PubMed] [Google Scholar]
- 5.Che, F. S., Y. Nakajima, N. Tanaka, M. Iwano, T. Yoshida, S. Takayama, I. Kadota, and A. Isogai. 2000. Flagellin from an incompatible strain of Pseudomonas avenae induces a resistance response in cultured rice cells. J. Biol. Chem. 275:32347-32356. [DOI] [PubMed] [Google Scholar]
- 6.da Silva, A. C., J. A. Ferro, F. C. Reinach, C. S. Farah, L. R. Furlan, R. B. Quaggio, C. B. Monteiro-Vitorello, M. A. Van Sluys, N. F. Almeida, L. M. Alves, A. M. do Amaral, M. C. Bertolini, L. E. Camargo, G. Camarotte, F. Cannavan, J. Cardozo, F. Chambergo, L. P. Ciapina, R. M. Cicarelli, L. L. Coutinho, J. R. Cursino-Santos, H. El-Dorry, J. B. Faria, A. J. Ferreira, R. C. Ferreira, M. I. Ferro, E. F. Formighieri, M. C. Franco, C. C. Greggio, A. Gruber, A. M. Katsuyama, L. T. Kishi, R. P. Leite, E. G. Lemos, M. V. Lemos, E. C. Locali, M. A. Machado, A. M. Madeira, N. M. Martinez-Rossi, E. C. Martins, J. Meidanis, C. F. Menck, C. Y. Miyaki, D. H. Moon, L. M. Moreira, M. T. Novo, V. K. Okura, M. C. Oliveira, V. R. Oliveira, H. A. Pereira, A. Rossi, J. A. Sena, C. Silva, R. F. de Souza, L. A. Spinola, M. A. Takita, R. E. Tamura, E. C. Teixeira, R. I. Tezza, M. Trindade dos Santos, D. Truffi, S. M. Tsai, F. F. White, J. C. Setubal, and J. P. Kitajima. 2002. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459-463. [DOI] [PubMed] [Google Scholar]
- 7.Felix, G., J. D. Duran, S. Volko, and T. Boller. 1999. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18:265-276. [DOI] [PubMed] [Google Scholar]
- 8.Freiberg, C., R. Fellay, A. Bairoch, W. J. Broughton, A. Rosenthal, and X. Perret. 1997. Molecular basis of symbiosis between Rhizobium and legumes. Nature 387:394-401. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Gomez, L., Z. Bauer, and T. Boller. 2001. Both the extracellular leucine-rich repeat domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell 13:1155-1163. [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Gomez, L., and T. Boller. 2002. Flagellin perception: a paradigm for innate immunity. Trends Plant Sci. 7:251-256. [DOI] [PubMed] [Google Scholar]
- 11.Guo, D., M. G. Bowden, R. Pershad, and H. B. Kaplan. 1996. The Myxococcus xanthus rfbABC operon encodes an ATP-binding cassette transporter homolog required for O-antigen biosynthesis and multicellular development. J. Bacteriol. 178:1631-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huynh, T., D. Dahlbeck, and B. Staskawicz. 1989. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science 245:1374-1377. [DOI] [PubMed] [Google Scholar]
- 13.Ichinose, Y., R. Shimizu, Y. Ikeda, F. Taguchi, M. Marutani, T. Mukaihara, Y. Inagaki, K. Toyoda, and T. Shiraishi. 2003. Need for flagella for complete virulence of Pseudomonas syringae pv. tabaci: genetical analysis with flagella defective mutants, ΔfliC and ΔfliD, in host tobacco plants. J. Gen. Plant Pathol. 69:244-249.
- 14.Josenhans, C., L. Vossebein, S. Friedrich, and S. Suerbaum. 2002. The neuA/flmD gene cluster of Helicobacter pylori is involved in flagellar biosynthesis and flagellin glycosylation. FEMS Microbiol. Lett. 210:165-172. [DOI] [PubMed] [Google Scholar]
- 15.King, E. O., N. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyrocyanin and fluorescein. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Logan, S. M., J. F. Kelly, P. Thibault, C. P. Ewing, and P. Guerry. 2002. Structural heterogeneity of carbohydrate modifications affects serospecificity of Campylobacter flagellins. Mol. Microbiol. 46:587-597. [DOI] [PubMed] [Google Scholar]
- 18.Midland, S. L., N. T. Keen, and J. J. Sims. 1993. The structures of syringolides 1 and 2, novel C-glycosidic elicitors from Pseudomonas syringae pv. tomato. J. Org. Chem. 58:2940-2945. [Google Scholar]
- 19.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. Cris Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. Eisen, K. N. Timmis, A. Dusterhoft, B. Tummler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4:799-808. [DOI] [PubMed] [Google Scholar]
- 20.Power, P. M., and M. P. Jennings. 2003. The genetics of glycosylation in Gram-negative bacteria. FEMS Microbiol. Lett. 218:211-222. [DOI] [PubMed] [Google Scholar]
- 21.Rocchetta, H. L., L. L. Burrows, J. C. Pacan, and J. S. Lam. 1998. Three rhamnosyltransferases responsible for assembly of the A-band d-rhamnan polysaccharide in Pseudomonas aeruginosa: a fourth transferase, WbpL, is required for the initiation of both A-band and B-band lipopolysaccharide synthesis. Mol. Microbiol. 28:1103-1119. [DOI] [PubMed] [Google Scholar]
- 22.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 23.Scofield, S. R., C. M. Tobias, J. P. Rathjen, J. H. Chang, D. T. Lavelle, R. W. Michelmore, and B. J. Staskawicz. 1996. Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274:2063-2065. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu, R., F. Taguchi, M. Marutani, T. Mukaihara, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2003. The ΔfliD mutant of Pseudomonas syringae pv. tabaci, which secretes flagellin monomers, induces a strong hypersensitive reaction (HR) in non-host tomato cells. Mol. Genet. Genomics 269:21-30. [DOI] [PubMed] [Google Scholar]
- 25.Starner, T. D., W. E. Swords, M. A. Apicella, and P. B. McCray, Jr. 2002. Susceptibility of nontypeable Haemophilus influenzae to human beta-defensins is influenced by lipooligosaccharide acylation. Infect. Immun. 70:5287-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K.-S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi, F., R. Shimizu, R. Nakajima, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2003. Differential effects of flagellins from Pseudomonas syringae pvs. tabaci, tomato and glycinea on plant defense response. Plant Physiol. Biochem. 41:165-174. [Google Scholar]
- 28.Taguchi, F., R. Shimizu, Y. Ikeda, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2003. Post-translational modification of flagellin determines the specific induction of HR. Plant Cell Physiol. 44:342-349. [DOI] [PubMed] [Google Scholar]
- 29.Tang, X. Y., R. D. Frederick, J. M. Zhou, D. A. Halterman, Y. L. Jia, and G. B. Martin. 1996. Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 274:2060-2063. [DOI] [PubMed] [Google Scholar]
- 30.Thibault, P., S. M. Logan, J. F. Kelly, J.-R. Brisson, C. P. Ewing, T. J. Trust, and P. Guerry. 2001. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J. Biol. Chem. 276:34862-34870. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, L., J. Radziejewska-Lebrecht, D. Krajewska-Pietrasik, P. Toivanen, and M. Skurnik. 1997. Molecular and chemical characterization of the lipopolysaccharide O-antigen and its role in the virulence of Yersinia enterocolitica serotype O:8. Mol. Microbiol. 23:63-76. [DOI] [PubMed] [Google Scholar]