Abstract
Multiple CD8+ suppressive T cell (Ts) subtypes are now recognized as essential regulators of the immune system that prevent autoimmunity through secretion of multiple cytokines and the subsequent inhibition of effector lymphocyte function. CD8+ Ts are an exciting area of study because of the possible therapeutic implications of inducing suppressive cells that are able to subdue or anergize autoimmune manifestations. Current research in systemic lupus erythematosus (SLE), a disease in which most effective therapies are widely immunosuppressive, is often focused on novel and highly targeted ways in which to treat this multiorgan disease. CD8+ Ts have been shown to be impaired in human and murine SLE. Our group and others have utilized tolerogenic peptides to induce and study CD8+ Ts in order to understand their function as well as investigate a possible new SLE therapy. This review will discuss the similarities and differences in CD8+ Ts subsets, the concept of tolerance as a therapy, and the current understanding of CD8+ Ts in mouse SLE models.
Keywords: lupus, CD8, suppressor, regulatory, tolerance
Introduction
Lymphocytes with the ability to suppress effector T cell function and autoantibody production by B cells - essentially cells having the potential to modulate the manifestations of many autoimmune diseases - have been the subject of intense interest for the past four decades. Many researchers in immunology regard the CD4+CD25+Foxp3+ regulatory T cell (Treg) as the quintessential suppressor based on the explosion of research on these cells in the past decade. However, other cell types are known to be crucial players in maintaining immune homeostasis, including CD8+, B, and other CD4+ cells [1-5]. In fact, research from 30-40 years ago initially implicated subpopulations of CD8+ T cells as the ‘original’ regulatory T cell [6-11], although, as is the case with many ahead-of-their-time discoveries, technological advances were necessary to fully appreciate these findings.
Regardless of lineage, all regulatory lymphocytes identified to date inhibit autoimmunity and induce self-tolerance by two general mechanisms. Regulatory cells can exert their suppressive function on autoimmune T cells, B cells and dendritic cells through either direct contact or cytokine secretion, often referred to as cell-extrinsic inhibition. Tolerance is also induced by cell-intrinsic mechanisms such as clonal deletion of autoreactive cells, T- or B-cell receptor editing of receptors that recognize self, and the initiation of anergic and apoptotic signals in the periphery after contact with self-antigens [4]. Of course, these two mechanisms are simplified and most likely are intertwined with each other, such as an ‘extrinsic’ extracellular signal activating ‘intrinsic’ anergic pathways. Nevertheless, it has long been recognized that in vivo induction of one or both tolerance mechanisms would be an ideal method of combating autoimmune disease and is the subject of intense research by many groups.
Patients with systemic lupus erythematosus (SLE) suffer from a multigenic, multisystem autoimmune disease characterized by autoantibody (pathogenic subsets are usually IgG) and immune complex production and deposition. Autoantibody production by plasma cells is augmented by helper T (Th) cells after Th activation provided by antigen presenting cells (APC) such as B cells, dendritic cells, monocytes, and/or macrophages. This cycle can be interrupted by regulatory cells of various types described above.
Data regarding the role of CD4+ Treg from SLE patients and mouse models of SLE in suppressing autoantibody production and T cell help are conflicting. Some studies show that CD4+ Treg are present in fewer numbers and are unable to suppress as well as Treg from healthy controls [3, 12, 13]. However, other studies have found that CD4+CD25hi cell numbers are not quantitatively reduced in SLE patients and they function normally [14, 15]. Furthermore, one study suggests that CD4+ Treg can function normally but are rendered ineffective by lupus APC via secretion of IFNα [16]. Yet other data support the idea that CD4+ Treg from SLE patients can suppress CD4+ effector cells from normal individuals but not from SLE patients, suggesting that the defect is in the helper/effector rather than the suppressor/responder cells [17]. The differences in these reports regarding patients with SLE may be accounted for by stage of disease at the time of study, activity of disease, the influence of therapies, the definition of surface and cytoplasmic markers of CD4+ Treg, and the heterogeneity of disease manifestations and mechanisms. Furthermore, there may be differences between natural CD4+ Treg which probably suppress effector T cells in an early immune response independent of the stimulating antigen, and induced CD4+ Treg which appear during disease or after tolerance induction, are antigen-restricted, and probably suppress T and B lymphocytes participating in ongoing autoimmune responses. Our lab [18] and others [19-21] have examined immunoglobulin peptide-induced CD4+ Treg in SLE mouse models; although these experiments are out of the scope of this paper, the reader is encouraged to read the following reviews about Treg in SLE [3, 22, 23].
With regard to CD8+ T cell regulators/suppressors, most studies to date suggest that in both human and murine lupus, CD8+ Ts are either low in numbers, defective in function, or both [24, 25]. As with studies of CD4+ Treg in patients, there is at least one contrasting report that CD8+CD28- T cells are not quantitatively reduced in patients compared to controls [26].
Overall, the work in CD4+ and CD8+ regulatory T cells implies that SLE and other autoimmune diseases could be driven in part by dysfunctional regulatory versus effector cell homeostasis, and rectification of this balance could lead to improved disease control. In this review, we will focus on the ‘older’ but less-characterized CD8+ non-cytotoxic suppressive/regulatory/inhibitory T cell populations (referred to from now on as Ts) and their importance to the induction of tolerance by multiple tolerogens in SLE-prone mouse models.
CD8+ Ts subtypes: how defined beyond CD8+?
One of the main hurdles facing immunologists studying regulatory T cells 30 years ago was the specific identification of suppressors by cell surface markers or specific cytoplasmic genes. Although technology has markedly improved the ways in which we phenotype cells, we still face some of the same problems in 2008 in that nobody has been able to identify one specific molecule that defines either CD8+ Ts or CD4+ Treg.
Initial reports characterized CD8+ Ts from humans as CD8+CD28-TCRαβ+ that expressed a limited TCRVβ repertoire, suppressed target cells via secretion of IFNγ and IL-6, and downregulated CD40, B7.1 and B7.2 on APCs [27-29]. These CD8+ Ts upregulate the Src family tyrosine kinase Lyn and interact directly with APC [30]. Scotto et al. illustrated that CD8+ Ts also express Foxp3 and that CD8+CD28-CD62L+ Ts are more effective suppressors than the same cells that do not express CD62L [31]. Secreted TGFβ also plays a role in initiating and maintaining Ts function [32, 33]. It is clear, however, that not all CD8+CD28- cells are Ts, and some Ts cells appear to be CD28+, as discussed below.
Recent reports have identified the β-chain of the IL-2 receptor (CD122) as being upregulated in a murine CD8+ Ts subset; CD8+CD122+ cells can suppress the proliferation of CD4+ and CD8+CD122- cells [34-36]. These cells appear to be a distinct CD8+ Ts group because they secrete IL-10 and block IFNγ production [37]. In contrast, data are mixed as to whether the IL-2Rα chain (CD25) is upregulated in human CD8+ Ts [31, 35, 38]. The IL-2Rγ chain (CD132) is known to be a component for many interleukin receptors, suggesting that its expression would not delineate regulatory T cells. Additionally, most activated T cells express the machinery to participate in IL-2 signaling, so these molecules might not be the ideal specific CD8+ Ts markers. Another group has identified the expression of a protein tyrosine phosphatase CD45 isoform (CD45RC) as being low in rat CD8+ Ts [39]. The HLA class Ib MHC molecule Qa-1 (HLA-E in humans), which is expressed briefly on activated T cells, has also been reported to be a target of induced antigen-specific CD8+ Ts in murine experimental allergic encephalomyelitis (EAE) (an interaction which suppresses disease) and a target in transplant rejection [40], but the role of Qa-1 in other CD8+ Ts subtypes and in murine or human SLE has not been addressed [41]. Importantly, these cells appear to be directly cytotoxic to activated T cells [42, 43], in contrast to the more traditional indirect suppression of CD8+ Ts through cytokine secretion.
In addition, transcripts of the traditional CD4+CD25+ Treg transcription factor Foxp3 is upregulated in some CD8+ Ts, including rat CD8+CD45RClo and mouse CD8+CD25+ Ts [39, 44]. Our group also observed Foxp3 upregulation in CD8+ Ts [45-47] as well as similar suppressive capacities of induced CD8+CD28-Foxp3+ and CD8+CD28+Foxp3+ cells in lupus-prone mice, suggesting that Foxp3 plays a larger role than does CD28 in CD8+ Ts from our tolerized SLE murine model [47]. Further complicating matters, Foxp3 does not appear to be expressed in CD8+CD122+ cells [34] and has been observed in both human and mouse non-lymphoid cells [48, 49], suggesting that the role of Foxp3 might be larger than solely defining and controlling regulatory T cells.
There are also CD8+ Ts subsets that have not been described outside of a few reports. These include CD8+CD75s+ cells from mice [50], CD8+ cells from healthy and ovarian cancer patients that are induced by plasmacytoid dendritic cells (pDC) and secrete IL-10 [51, 52], a human CD8+CD25+Foxp3+lymphocyte activation gene-3+ population that suppresses CD4+ T cells through CCL4 secretion [53], and a cytotoxic Tc1 population in rats that is CD8+CD45RChi [54], in contrast to the CD45RClo subset described above. Table 1 lists the different types of CD8+ Ts discussed above.
Table 1. List of CD8+ Ts discussed in this review.
References describing each Ts subset are given on the right.
| Phenotype | Cells studied | Suppression mechanism | References |
|---|---|---|---|
| CD8+CD28- (Foxp3+CD62L+) | MS, human transplant (heart, kidney. liver) | secretion of IFNγ, IL-6, TGFβ cell-cell contact induces ILT-3 and ILT-4) | 27-33, 57, 58 |
| CD8+CD122+ | murine Graves’ disease, normal mice | secretion of IL-10; blockade of IFNγ | 34-37 |
| CD8+CD25+ | mouse and human thymus | Possible secretion of TGFβ | 31, 35, 38 |
| CD8+CD45RCloFoxp3+ | normal rats, GVHD | cell-cell contact via CTLA4 and cell surface TGFβ | 39 |
| CD8+CD25+Foxp3+ | MHC class II-deficient mice | secretion of IFNγ, IL-10 | 42 |
| CD8+Foxp3+ (CD28+ and CD28-) | mouse SLE (SNF1, BWF1) | secretion of TGFβ | 43-45, 70, 71 |
| CD8+CD75s+ | normal mice | unknown | 48 |
| CD8+CD45RO+CCR7+ | human ovarian cancer | secretion of IL-10 | 49, 50 |
| CD8+CD25+Foxp3+LAG-3+ | Human granulomas | secretion of CCL4 | 51 |
| CD8+CD45RChi | rat induced SLE-like disease | secretion of IFNγ | 52 |
How do Ts suppress autoimmune lymphocytes?
Mechanisms of CD4+ Treg suppression are currently better understood than for CD8+ Ts. Natural CD4+ Treg in humans and mice are known to suppress primarily via direct cell-cell interactions, although it appears that Treg block multiple stages of autoreactive B and T cell functions through both direct contact and secretion of multiple cytokines. Mechanisms involved in Treg suppression include membrane-bound and secreted TGFβ, secretion of IL-10 and IL-35, induction of cytolytic molecules (Fas, granzymes, and perforin) in the effector cell, and upregulation of cAMP in the target cells by direct delivery of cAMP from Tregs to their target cell or the increase of adenosine in the immediate extracellular space through Treg expression of CD39 and CD73 [55].
CD8+ Ts also regulate autoimmune lymphocytes through direct and indirect methods, although their ability to suppress through secretion of inhibitory cytokines has been described in the majority of studies. Early work on CD8+ cells as a whole suggested that they secreted IFNγ, IL-4 and IL-10 [56]. Later experiments have shown that efficiency of CD8+ Ts suppression depends on the secretion of IFNγ, IL-10, and/or TGFβ the specific secreted cytokine probably depends on the Ts subtype [1, 25, 27, 37, 45, 51, 57]. These cytokines appear to be produced and secreted by CD8+ Ts directly. For CD8+ Ts to acquire this suppressive ability may be a complex process, as described by Gray et al. where, in the presence of IL-2, human CD8+ cells interact with NK cells that secrete TGFβ to convert CD8+ cells to Ts [33, 58].
Specific CD8+ Ts subsets can also suppress effector cell function through direct cell-cell contact, in a similar manner to CD4+Treg. Human CD8+CD25+ Ts appear to suppress CD4+ cells through direct interactions of CTLA-4 and cell surface TGFβ with CD4+ cells [35]. Human CD8+CD28-Foxp3+ cells directly interact with and induce anergy in APCs and endothelial cells by upregulating the ITIM-containing inhibitory receptors ILT-3 and -4 in a cytokine-independent manner [30, 59, 60]. The Suciu-Foca group showed that CD8+CD28- Ts from successful human heart transplant recipients induced increased levels of ILT-3 or ILT-4 in monocytes, but recipients that rejected a donor heart did not have increased ILT-3 or -4 [59], suggesting that these molecules play a large role in mediating CD8+ Ts-driven APC tolerance. In addition, the programmed cell death-1 (PD-1) gene can be upregulated on CD8+ Ts and suppresses CD4+ cells by interacting with its ligand ICOS/B7h in a murine transplant system [61]. In contrast, data from our group in a murine SLE system suggests that PD-1 levels are decreased on CD8+ Ts after tolerization; overall the role of PD-1 in Ts suppression is unclear (discussed in depth below) [46]. Although our understanding of CD8+ Ts suppression has grown considerably in the last few years, more study is needed to fully delineate the suppressive mechanisms of each specific Ts subset in murine and human autoimmunity.
Ts induction and tolerance
CD4+ Treg are often discussed by referring to ‘natural’ Treg (the small population of CD4+ T cells that exits in the thymus as CD4+CD25+Foxp3+) and ‘induced’ Treg (CD4+CD25- cells in the periphery that become CD25+Foxp3+ after being stimulated by specific cytokines or cell-cell contact). Although it is presumed that a population of ‘natural’ CD8+ Ts exists in the periphery after T cell development, these cells have not been characterized outside of cell surface marker expression. Induction of CD8+ Ts in vitro and in vivo is better understood, although not to the level of ‘induced’ Treg.
Generation of multiple CD8+ Ts subsets in vitro has been described in both humans and mice using IL-10, TGFβ, and IL-2, as discussed above [27, 62]. This conversion often involves co-culture of naïve CD8+ cells with Th cells and APCs, or pDC in the case of the human CD8+ Ts described above. Scotto et al. reported that a number of classical CD4+CD25+Treg transcripts (Foxp3, GITR, OX-40, 4-1BB, CTLA-4, and β7 integrin) are expressed at similar levels in CD8+CD28- Ts and CD4+ Treg that are induced ex vivo from healthy human patients [31]. The signaling mechanisms that take place during in vitro CD8+Ts production are not well described in the literature, however, and this will certainly be the focus of many future studies.
CD8+ Ts can also be induced in vivo by a number of methods, including vaccination and oral/i.v. tolerance. Vaccination or tolerization with T cells, DNA, and protein have been successfully utilized in expanding CD4+ Treg and CD8+ Ts concurrent with ablating disease in multiple murine autoimmune disease models, including SLE (discussed below), EAE (a mouse model for multiple sclerosis (MS)), and collagen-induced arthritis [1, 63-65]. Vaccination with DNA encoding the major MS autoantigen, myelin basic protein, is in clinical trials in humans [66].
Peptides and proteins administered orally, s.c., and i.v. have also been used recently to induce CD8+ Ts and subsequent tolerance in a number of animal models, including experimental autoimmune uveitis, EAE, diabetes, myasthenia gravis, MS, and arthritis [67-70]. More than a decade ago, data from two groups suggested that CD8+ T cells appeared to be playing a vital regulatory role in SLE-prone mouse models after anti-CD8 monoclonal antibody therapy [71, 72], and several groups, including ours, have verified these findings by utilizing peptides based on autoantigens in murine models of SLE to observe CD8+ Ts-mediated tolerance and amelioration of SLE disease manifestations, including delaying the occurrence of anti-DNA antibodies and nephritis [46, 47, 73, 74]. The rest of this review will focus on CD8+ Ts in these mouse models.
Ts mediate multiple tolerance mechanisms in SLE-prone mice
As stated above, some of the first clues to the involvement of CD8+ Ts in SLE were in first-generation male mice resulting from crossing New Zealand white mice with BXSB mice (NZW/BXSW F1). Male BXSB animals get a severe lupus-like syndrome that is linked to the Y-linked autoimmune accelerator (Yaa) region of the Y chromosome. Yaa is caused by the translocation of a number of X chromosome genes to the Y chromosome, and the subsequent amplification of one of these genes, Toll-like receptor 7, is one of the causative agents for driving SLE-like autoimmunity [75, 76]. Adachi et al. observed that treatment of NZW/BXSB F1 males with anti-CD4 antibodies ameliorated disease, but eliminating the CD8+ T cells with antibodies exacerbated disease [72]. These results suggested that a subpopulation of CD8+ cells acted as suppressors in this mouse model.
Our group and others have attempted to delineate the phenotypes and mechanisms behind regulatory T cell-mediated suppression in murine SLE by tolerizing animals with either histone-based peptides or peptides corresponding to heavy chain or complementarity determining region 1 sequences of anti-dsDNA antibodies. Earlier work done in our laboratory showed that as first-generation females from a cross between New Zealand black and New Zealand white mice (BWF1) age, CD4+ T cells and B cell populations expand dramatically. However, CD8+ cells expand far less and, compared to young mice, secrete less TGFβ and IL-2. These “aged” CD8+ T cells can no longer suppress anti-DNA antibody production [24]. These findings led our lab to further explore CD8+ Ts in SLE.
Tolerization or vaccination of BWF1 mice with an artificial peptide based on anti-DNA autoantibody heavy chain peptide induces CD8+ Ts
Our group developed a tolerogenic 15-amino acid peptide based on sequences from 439 peptides originating from heavy chains of four different BWF1 anti-dsDNA monoclonal IgG2a or IgG2b antibodies. Optimal stimulatory amino acids at each position were determined by computer algorithm, and the resulting peptide was called pConsensus (pCons) [77-79]. This peptide was also designed to contain MHC class II determinants that bind to I-Ed (BWF1 mice are I-Ed, I-Ez, I-Ad, I-Az). Williams et al. illustrated that, in addition to causing autoimmunity through binding dsDNA, anti-DNA antibodies themselves stimulate circulating T cells and augment SLE disease progression in humans [80], and our lab showed similar results with anti-DNA antibodies in BWF1 mice [81]. Our group hypothesized that inactivation of these Th cell responses through a tolerogenic treatment regimen could delay or prevent SLE onset. Initial experiments proved that this was the case; i.v. pCons treatment (1 mg) of SLE-prone female BWF1 mice significantly increased survival and delayed nephritis. There was also statistically significant delay in the appearance of multiple autoantibodies (against dsDNA, nucleosomes, and cardiolipin) [77].
After experiments that determined CD4+ Treg played a large role in pCons-mediated tolerance [18], our lab next examined whether CD8+ Ts were also involved. When the ability of ex vivo CD8+ T cell subsets to suppress anti-dsDNA was examined, both CD8+CD28+ and CD8+CD28- cell populations suppressed quite well [45, 46]. These results were in agreement with Kang et al. using histone-derived peptides as tolerogens, (discussed below) [73, 74], further suggesting that in multiple models of tolerized SLE-prone mice, the presence or absence of CD28 on the CD8+ Ts does not indicate suppressive capability.
Vaccination has also been utilized to induce tolerance, and our lab, in collaboration with Indiveri’s group, examined whether vaccinating BWF1 mice with BWF1-isolated B cells containing DNA encoding the pCons sequence would induce Ts and prevent SLE in a similar manner as i.v. pCons administration. Transfection of a DNA construct containing the pCons sequence in frame with part of a human Ig sequence (pIgCons) into BWF1 B cells followed by infusion of these cells into pre-nephritic mice led to reduced proteinuria and increased survival compared to gene transfer using either the human Ig sequence alone or a control peptide [65]. In addition, no human or murine Ig immune deposits were found in kidneys of pIgCons-vaccinated mice, and serum levels of murine IgG were significantly lower than in the control-vaccinated mice. Importantly, pIgCons gene transfer induced CD8+CD28- Ts that suppressed CD4+ proliferation in a TGFβ-dependent manner. Adoptive transfer of pIgpCons-induced CD8+ Ts led to decreased proteinuria in older BWF1 mice with hypergammaglobulinemia [65]. These results strongly suggest that amelioration of SLE through pCons-induced tolerization can be administered through both i.v. peptide injection or through B cell-mediated vaccination.
In BWF1 mice tolerized with pCons, Foxp3 expression was upregulated in CD8+ Ts. Furthermore, suppression of anti-dsDNA antibodies was abolished after transient transfection of small inhibitory RNA (siRNA) targeting Foxp3 [45, 47]. Although Foxp3 expression was significantly higher in the pCons-tolerized CD8+CD28- population versus CD8+CD28+, siRNA of Foxp3 abolished the suppressive capacities of both CD8+CD28-and CD8+CD28+ Ts. In addition, higher levels of Foxp3 induction persist for approximately one month in CD8+CD28- Ts, but the induction of Foxp3 transcript is more transient in CD8+CD28+ cells [47], suggesting that there might be subtle Foxp3 dosage effects in these two Ts subtypes and their mechanisms of suppression.
Additional experiments from our lab determined that efficacy of CD8+ Ts in pCons-tolerized BWF1 is dependent on TGFβ secretion. TGFβ transcript levels from in vivo pCons-tolerized CD8+ Ts were significantly elevated in both CD28+ and CD28- Ts populations for one week after pCons administration, and blocking soluble TGFβ with anti-TGFβ antibodies abolished the suppressive capacity of Ts in ex vivo culture systems [45, 47]. Stimulation of CD8+ Ts with TGFβ increases Foxp3 transcript levels approximately 15-fold, accompanied by an increase in intracellular Foxp3 protein levels as determined by flow cytometry [47].
To determine whether pCons-mediated CD8+ Ts actively suppress SLE in vivo, our lab conducted a series of adoptive transfer experiments where pCons-tolerized Ts (10 million) were injected i.v. into irradiated pre-nephritic BWF1 mice along with anti-DNA antibody-producing B cells from older, nephritic mice (1 million). Tolerized CD8+ Ts a) suppressed the appearance of anti-DNA antibodies in the serum, b) suppressed the appearance of proteinuria, and c) increased survival (100% of the animals given tolerized Ts survived 5 months after adoptive transfer versus 0% of the animals that received Ts from saline-treated animals) [45]. In addition, a small proportion of CD8+ Ts from tolerized animals expressed elevated granzyme B levels and were slightly cytotoxic towards B cells [46], suggesting that a subpopulation of Ts might contain the capacity to kill autoimmune lymphocytes.
Initial studies to elucidate a mechanistic basis behind pCons-mediated CD8+ Ts suppression of SLE implicated alterations in apoptosis. The antiapoptotic gene Bcl2 (upregulated 2.2-fold) was one of 60 transcripts significantly altered in tolerized CD8+ cells versus naïve CD8+ in a gene expression array [45], suggesting that CD8+ Ts in diseased BWF1 mice might go through dysregulated programmed cell death. Subsequent staining of CD8+ Ts outer membrane phosphatidylserine with annexin V and experiments inducing Fas-driven apoptosis with anti-Fas antibodies confirmed that pCons tolerization protects Ts from apoptosis [45, 46]. At this point, it is unclear whether pCons promotes anti-apoptotic pathways (Bcl2, COX-2, etc.), inhibits pro-apoptotic pathways (caspase activation, Fas-mediated death), or regulates both. It is possible that pCons functions to block CD8+ Ts apoptosis so that these cells can live longer and suppress with greater efficiency. We are currently addressing these questions using a variety of techniques.
Finally, our lab has started to examine the role of programmed death-1 (PD-1) in pCons-driven CD8+ Ts function. Signaling through PD-1, usually by binding PD-L1, leads to recruitment of the tyrosine phosphatases SHP-1 and SHP-2 to the PD-1 cytoplasmic tail, inhibition of positive (e.g., TCR) signaling pathways, and subsequent T cell anergy. These inhibitory signals only occur when PD-1 and the TCR are engaged concurrently and it is believed that PD-1 signals are vital for fine-tuning tolerance [82]. PD-1-/- mice suffer from nephritis and, although mechanisms are not presently clear, PD-1 is generally regarded as a protective molecule against autoimmunity [83]. Our group found that PD-1 transcript and protein levels were actually reduced in CD8+ Ts after tolerization, and effective suppression of anti-dsDNA antibodies temporally correlated with reduced PD-1 mRNA levels [46]. Although higher PD-1 levels correlate with less autoimmunity in some models, it appears that the opposite might the case in pCons-induced CD8+ Ts, and that negative signals mediated through PD-1 ligation might ultimately inhibit effective suppression. Because Foxp3 levels were increased in tolerized CD8+ Ts, our group examined the relationship between Foxp3 and PD-1 signals by blocking PD-1 signaling with antibodies against PD-1 and PD-L1. Indeed, the inhibition of PD-1 signals increased Foxp3 expression in naïve Ts. Strikingly, Foxp3 message decreased after blocking PD-1 signaling in pCons-tolerized Ts, suggesting that there is a basal level of PD-1 signaling required for Foxp3 expression and subsequent suppression of Th cell proliferation [46]. A simple analogy might be to compare PD-1 signaling in pCons-induced CD8+ Ts to the bus in the movie ‘Speed.’ Efficient Ts suppression is obtained only if the accelerator (in this case, PD-1) is constantly moderately engaged; if the accelerator is disengaged completely, the homeostatic signals are lost and the suppressive mission fails. When PD-1 is completely engaged, however, suppression is lost amid the anergic signals (or danger signals from driving too fast). Our lab is currently delineating the roles of PD-1 in regulatory T cell signaling.
Tolerization of BWF1 mice with an anti-DNA antibody hCDR1 peptide induces CD8+ cells and TGFβ while downregulating IFNγ and IL-10
The Mozes group has also examined regulatory T cells in BWF1 mice using a different tolerogenic epitope from an anti-dsDNA antibody. A tolerogenic sequence in the light chain complementarity determining region 1 (called hCDR1) region was administered and found to ameliorate disease [20, 84]. This group has published extensively on the ability of hCDR1 to induce functional CD4+ Treg in both BWF1 mice and Balb/c mice induced to develop SLE [19-21, 85]. hCDR1 treatment (50 μg) increased TGFβ levels and decreased IL-10 and IFNγ levels of cultured splenocytes, suppressed anti-DNA antibody production and Th cell proliferation, and suppressed lymphocyte apoptosis through Bcl-xL upregulation and caspase-3, -8, and JNK downregulation [19-21, 85, 86], suggesting that hCDR1 and pCons-induced tolerization probably decrease disease progression through similar mechanisms. However, the effect of hCDR1 on CD8+ Ts has not been studied save a report that hCDR1 tolerization of BWF1 mice causes an increase in total CD8+ cell numbers [21], again suggesting that hCDR1 and pCons tolerization might induce some of the same mechanisms. More studies are required to understand the full effect of hCDR1 on CD8+ regulatory T cells. Incidentally, this same group has studied CD8+CD28-Foxp3+ Ts in experimental autoimmune myasthenia gravis and found that these CD8+ Ts were necessary for proper CD4+ Treg function [68]. These results are quite provocative in that they suggest that CD8+ Ts and CD4+ Treg employ crosstalk mechanisms and are both necessary for maximal suppression in autoimmune diseases. Future studies on how these regulatory cells communicate with each other promises to be a major field of study.
Tolerization of NZBxSFR F1 mice with a nucleosomal histone peptide induces CD8+Ts
Histones are the major protein component of the chromosome-condensing nucleosome and act as epitopes for driving autoimmunity in SLE. Experimental systems have exploited administrations of immunogenic histone peptides to drive lupus-like disease in mice; however, Datta’s laboratory showed that high i.v. doses (300 μg) of these peptides induce B and Th tolerance in first-generation females resulting from crossing New Zealand black mice with SWR mice (SNF1) [87, 88]. SWR is a non-lupus-prone mouse strain that is susceptible to specific tumors late in life as well as to induced EAE. Attempting to direct their experiments towards translational applications, Kang et al. showed that biweekly s.c. administration of a very low dose (1 μg) of one specific 24 amino acid histone peptide, H471-94, was able to prolong survival and delay nephritis in SNF1 animals that began treatment at 12 weeks (i.e., healthy, pre-nephritic mice) [74]. Indeed, 60% of H471-94-treated animals were alive at 21 months whereas all saline-injected controls had died by 12 months. Three months of low dose tolerization with H471-94 led to an almost complete eradication of anti-nucleosome and anti–histone autoantibodies and a 50% decrease in anti-ds and –ss DNA antibodies [74].
Ex vivo experiments of H471-94-treated mice also illustrated that this tolerogen significantly reduced T cell IFNγ production [74]. To examine the role in H471-94-mediated suppression of autoimmunity, ex vivo experiments with CD4+ Treg or CD8+ Ts from H471-94-treated or untreated animals co-cultured with Th and B cells from older, untreated animals with lupus were performed. Both sets of regulatory T cells suppressed IFNγ production and autoantibody production against nucleosomes (and to a lesser extent, ds and ssDNA) [74]. In addition, no suppressive Ts differences were observed based on CD28 expression. These data suggest that induced SLE Ts might have a different phenotype than what is observed in transplant, EAE, and other disease models discussed earlier. In contrast to our results, Kang et al. found that Foxp3 expression was not upregulated in H471-94-induced CD8+ Ts. Autoantibody suppression by these CD8+ Ts was not dependent on IL-10 or cell-cell contact but was exquisitely dependent on TGFβ that was secreted by Ts in response to H471-94 tolerization. H471-94, although initially defined as having MHC class II epitopes, does indeed contain class I-binding motifs and can signal through Ts, similar to pCons [45, 74].
Kang et al. also proved CD8+ Ts suppress in vivo by isolating CD8+ T cells from tolerized SNF1 mice and adoptively transferring them (1 million cells) into pre-nephritic mice that were then treated one day later with another histone peptide that causes rapid onset of lupus nephritis. Recipients of the CD8+ Ts had significantly lower autoantibody titers extending two months after transfer [74]. Approximately 50% of CD8+ Ts recipient SNF1 mice died after 3.5 months, compared with 25% of CD4+ Treg recipients, suggesting that the heterogenous CD8+ population that was transferred might contain cytotoxic and/or pathogenic cells. The presence of cytotoxic CD8+ Ts in SNF1 was similar to the studies from our lab in BWF1 mice, although our lab did not observe the increased mortality after CD8+ adoptive transfer. This could certainly be due to strain differences between SWR and NZW, although both models share a NZB parent. Regardless, these results suggest that until a more specific phenotype for SLE CD8+ Ts cells is defined, we must be cautious as to how a mixed population of CD8+ cells will manifest in a biological system.
Further experiments performed by Kang et al. showed that adaptive transfer of pDC, but not B cells, from H471-94-treated SNF1 mice prolonged survival and delayed nephritis [73]. Subsequent data illustrated that pDC induce CD8+ Ts, and to a lower extent Treg, that are responsible for attenuating autoantibody production and disease progression. Interestingly, H471-94 treatment alone does not expand CD8+ Ts numbers in an ex vivo system, in contrast to the proliferation observed in CD4+ Treg [73], suggesting that CD8+ Ts are either not very amenable to proliferation in the SNF1 system or additional unknown factors missing in the co-culture system are present in vivo. These elegant experiments have greatly aided the SLE field in providing us with answers to how CD8+ Ts are both induced and carry out their suppressive function in SLE-prone mice.
Conclusions
After 35 years, suppressive CD8+ cells are finally receiving proper credit for their crucial roles in maintaining immune homeostasis. Many groups, working in a number of human and rodent disease models, have isolated different CD8+ Ts phenotypes that share functional commonalities, such as the ability to suppress indirectly through TGFβ secretion and the suppression of autoimmunity. CD8+ Ts in murine SLE models are less understood than Ts in other diseases, although our group and others agree that these cells can a) be induced by tolerization and vaccination regimens utilizing small peptides or DNA encoding the tolerogen, respectively, b) suppress regardless of CD28 expression, c) delay autoantibody production and inhibit Th cell proliferation, and d) mediate suppression in large part to the secretion of TGFβ. It is clear that expression of Foxp3 by these cells and secretion of TGFβ are important to “permit” their suppressive capacities. In addition, membrane expression of and signaling mediated through PD-1 has to be tightly regulated for them to function. Their relative resistance to apoptosis, compared to CD8+ T cells that have not been subjected to tolerance, may be critical to allow them to survive long enough to exert a clinically useful suppression of autoimmunity. For a summary of CD8+ Ts induced by tolerogenic peptides, see Figure 1. Combination tolerogenic therapy of autoantibody-based peptide tolerogens with histone peptides might have a synergistic effect, as anti-DNA antibodies were not as well suppressed by H471-94 as anti-nucleosome and -histone antibodies. Future experiments in SLE-prone mice will certainly lead to a deeper understanding of the mechanisms by which CD8+ Ts are able to keep autoimmunity in check and could lead to novel therapeutic options involving the induction of CD8+ and CD4+ regulatory T cells.
Figure 1. Cartoon of Ts induction and function in SLE-prone mice.
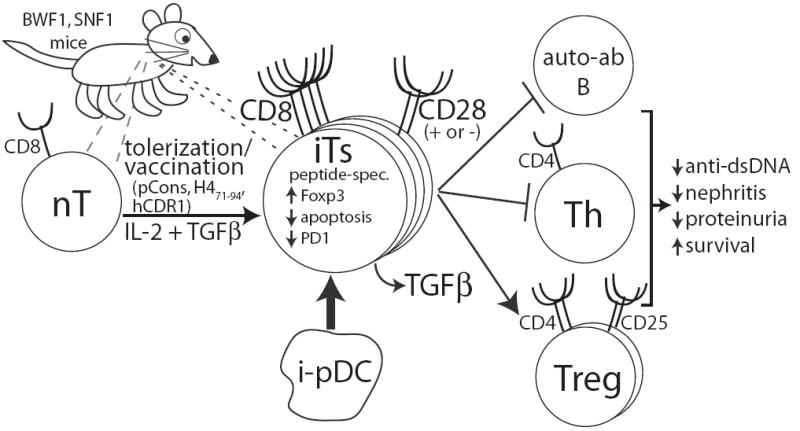
After tolerization or vaccination with anti-DNA antibody/histone peptides or in vitro stimulation with IL-2 and TGFβ, a population of naïve CD8+ T cells (nT) expands and acquires suppressive characteristics (Ts). A small number of peripheral Ts (‘natural’ Ts) also exist (right dashed lines). Tolerization also induces pDC (i-pDC) that can promote Ts development (thick arrow). The suppressive capacity of these cells does not appear to depend on CD28 expression but appears to be driven, at least in part, by Foxp3. These cells are peptide-specific, secrete TGFβ, and decrease apoptosis and PD-1 expression. In addition, Ts suppress B cells making autoantibodies (anti-DNA, anti-histone, anti-nucleosome) and CD4+ Th cells while activating and expanding the CD4+CD25+ Treg population. This leads to decreases in nephritis and proteinuria and increased survival of the SLE-prone animal.
Abbreviations
- APC
antigen presenting cell
- BWF1
first generation female mice from New Zealand black/New Zealand white cross
- cAMP
cyclic adenosine monophosphate
- CTLA-4
cytotoxic T lymphocyte antigen-4
- dsDNA
double stranded deoxyribonucleic acid
- EAE
experimental allergic encephalomyelitis
- H471-94
histone 4 peptide (amino acids 71-94) used for tolerizing SLE-prone mice
- hCDR1
human complementarity-determining region-1 peptide used for tolerizing SLE-prone mice
- HLA
human leukocyte antigen
- IFN
interferon
- IL
interleukin
- IL-2R
interleukin-2 receptor
- ILT
immunoglobulin-like transcript
- ITIM
immunoreceptor tyrosine inhibitory motif
- MHC
major histocompatibility complex
- MS
multiple sclerosis
- NK
natural killer
- pCons
artificial 15-amino acid peptide based on heavy chain sequences of anti-dsDNA antibodies used to tolerize SLE-prone mice
- PD-1
programmed death-1
- pDC
plasmacytoid dendritic cell
- siRNA
small inhibitory ribonucleic acid
- SLE
systemic lupus erythematosus
- SNF1
first-generation female mice resulting from New Zealand black/SWR cross
- ssDNA
single stranded deoxyribonucleic acid
- TCR
T cell receptor
- TGFβ
transforming growth factor beta
- Th
helper T cell
- Treg
CD4+CD25+ regulatory T cell
- Ts
CD8+ suppressive T cell
- Yaa
Y-linked autoimmune accelerator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tang XL, Smith TR, Kumar V. Specific control of immunity by regulatory CD8 T cells. Cell Mol Immunol. 2005;2:11. [PubMed] [Google Scholar]
- 2.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 3.La Cava A. T-regulatory cells in systemic lupus erythematosus. Lupus. 2008;17:421. doi: 10.1177/0961203308090028. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Costantino CM, Baecher-Allan CM, Hafler DA. Human regulatory T cells and autoimmunity. Eur J Immunol. 2008;38:921. doi: 10.1002/eji.200738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker PJ, Stashak PW, Amsbaugh DF, Prescott B, Barth RF. Evidence for the existence of two functionally distinct types of cells which regulate the antibody response to type 3 pneumococcal polysaccharide. J Immunol. 1970;105:1581. [PubMed] [Google Scholar]
- 7.Barth RF, Singla O, Liu C. Suppressor T cells and host resistance to tye 111 pneumococcus after treatment with antilymphocyte serum. Infect Immun. 1975;12:1307. doi: 10.1128/iai.12.6.1307-1312.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723. [PMC free article] [PubMed] [Google Scholar]
- 9.Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976;143:1391. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantor H, Hugenberger J, McVay-Boudreau L, Eardley DD, Kemp J, Shen FW, et al. Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. J Exp Med. 1978;148:871. doi: 10.1084/jem.148.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eardley DD, Hugenberger J, McVay-Boudreau L, Shen FW, Gershon RK, Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978;147:1106. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 13.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 14.Yates J, Whittington A, Mitchell P, Lechler RI, Lightstone L, Lombardi G. Natural regulatory T cells: number and function are normal in the majority of patients with lupus nephritis. Clin Exp Immunol. 2008;153:44. doi: 10.1111/j.1365-2249.2008.03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Zhang X, Tang FL, Zhu LP, Liu Y, Lipsky PE. Clinical significance of increased CD4+CD25-Foxp3+ T cells in patients with new-onset systemic lupus erythematosus. Ann Rheum Dis. 2008;67:1037. doi: 10.1136/ard.2007.083543. [DOI] [PubMed] [Google Scholar]
- 16.Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S. Dysfunctional CD4+,CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis Rheum. 2008;58:801. doi: 10.1002/art.23268. [DOI] [PubMed] [Google Scholar]
- 17.Vargas-Rojas MI, Crispin JC, Richaud-Patin Y, Alcocer-Varela J. Quantitative and qualitative normal regulatory T cells are not capable of inducing suppression in SLE patients due to T-cell resistance. Lupus. 2008;17:289. doi: 10.1177/0961203307088307. [DOI] [PubMed] [Google Scholar]
- 18.La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black × New Zealand White)F1 mice suppress in vitro production of antibodies to DNA. J Immunol. 2004;173:3542. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- 19.Sharabi A, Luger D, Ben-David H, Dayan M, Zinger H, Mozes E. The role of apoptosis in the ameliorating effects of a CDR1-based peptide on lupus manifestations in a mouse model. J Immunol. 2007;179:4979. doi: 10.4049/jimmunol.179.8.4979. [DOI] [PubMed] [Google Scholar]
- 20.Sharabi A, Zinger H, Zborowsky M, Sthoeger ZM, Mozes E. A peptide based on the complementarity-determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF-beta. Proc Natl Acad Sci U S A. 2006;103:8810. doi: 10.1073/pnas.0603201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharabi A, Azulai H, Sthoeger ZM, Mozes E. Clinical amelioration of murine lupus by a peptide based on the complementarity determining region-1 of an autoantibody and by cyclophosphamide: similarities and differences in the mechanisms of action. Immunology. 2007;121:248. doi: 10.1111/j.1365-2567.2007.02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mudd PA, Teague BN, Farris AD. Regulatory T cells and systemic lupus erythematosus. Scand J Immunol. 2006;64:211. doi: 10.1111/j.1365-3083.2006.01808.x. [DOI] [PubMed] [Google Scholar]
- 23.Kang HK, Datta SK. Regulatory T cells in lupus. Int Rev Immunol. 2006;25:5. doi: 10.1080/08830180500544480. [DOI] [PubMed] [Google Scholar]
- 24.Karpouzas GA, La Cava A, Ebling FM, Singh RR, Hahn BH. Differences between CD8+ T cells in lupus-prone (NZB × NZW) F1 mice and healthy (BALB/c × NZW) F1 mice may influence autoimmunity in the lupus model. Eur J Immunol. 2004;34:2489. doi: 10.1002/eji.200424978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, et al. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol. 2001;166:6452. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- 26.Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, Baranda L, Layseca-Espinosa E, Abud-Mendoza C, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Balashov KE, Khoury SJ, Hafler DA, Weiner HL. Inhibition of T cell responses by activated human CD8+ T cells is mediated by interferon-gamma and is defective in chronic progressive multiple sclerosis. J Clin Invest. 1995;95:2711. doi: 10.1172/JCI117973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28- T cells. Int Immunol. 1998;10:775. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 29.Pennesi G, Liu Z, Ciubotariu R, Jiang S, Colovai A, Cortesini R, et al. TCR repertoire of suppressor CD8+CD28- T cell populations. Hum Immunol. 1999;60:291. doi: 10.1016/s0198-8859(98)00134-7. [DOI] [PubMed] [Google Scholar]
- 30.Suciu-Foca N, Cortesini R. Central role of ILT3 in the T suppressor cell cascade. Cell Immunol. 2007;248:59. doi: 10.1016/j.cellimm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Scotto L, Naiyer AJ, Galluzzo S, Rossi P, Manavalan JS, Kim-Schulze S, et al. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28- T suppressor cells. Hum Immunol. 2004;65:1297. doi: 10.1016/j.humimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Myllarniemi M, Frosen J, Calderon Ramirez LG, Buchdunger E, Lemstrom K, Hayry P. Selective tyrosine kinase inhibitor for the platelet-derived growth factor receptor in vitro inhibits smooth muscle cell proliferation after reinjury of arterial intima in vivo. Cardiovasc Drugs Ther. 1999;13:159. doi: 10.1023/a:1007700629728. [DOI] [PubMed] [Google Scholar]
- 33.Gray JD, Hirokawa M, Horwitz DA. The role of transforming growth factor beta in the generation of suppression: an interaction between CD8+ T and NK cells. J Exp Med. 1994;180:1937. doi: 10.1084/jem.180.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rifa’i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 36.Saitoh O, Abiru N, Nakahara M, Nagayama Y. CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves’ hyperthyroidism in a murine model. Endocrinology. 2007;148:6040. doi: 10.1210/en.2007-0300. [DOI] [PubMed] [Google Scholar]
- 37.Endharti AT, Rifa’i M, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 38.Maggi E, Cosmi L, Liotta F, Romagnani P, Romagnani S, Annunziato F. Thymic regulatory T cells. Autoimmun Rev. 2005;4:579. doi: 10.1016/j.autrev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Xystrakis E, Dejean AS, Bernard I, Druet P, Liblau R, Gonzalez-Dunia D, et al. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004;104:3294. doi: 10.1182/blood-2004-03-1214. [DOI] [PubMed] [Google Scholar]
- 40.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114:1218. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu L, Werneck MB, Cantor H. The immunoregulatory effects of Qa-1. Immunol Rev. 2006;212:51. doi: 10.1111/j.0105-2896.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 42.Tang X, Maricic I, Purohit N, Bakamjian B, Reed-Loisel LM, Beeston T, et al. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha+TCRalphabeta+ T cells. J Immunol. 2006;177:7645. doi: 10.4049/jimmunol.177.11.7645. [DOI] [PubMed] [Google Scholar]
- 43.Smith TR, Kumar V. Revival of CD8(+) Treg-mediated suppression. Trends Immunol. 2008;29:337. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Bienvenu B, Martin B, Auffray C, Cordier C, Becourt C, Lucas B. Peripheral CD8+CD25+ T lymphocytes from MHC class II-deficient mice exhibit regulatory activity. J Immunol. 2005;175:246. doi: 10.4049/jimmunol.175.1.246. [DOI] [PubMed] [Google Scholar]
- 45.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFbeta-secreting CD8+ T cell suppressors. J Immunol. 2005;175:7728. doi: 10.4049/jimmunol.175.11.7728. [DOI] [PubMed] [Google Scholar]
- 46.Singh RP, La Cava A, Hahn BH. pConsensus peptide induces tolerogenic CD8+ T cells in lupus-prone (NZB × NZW)F1 mice by differentially regulating Foxp3 and PD1 molecules. J Immunol. 2008;180:2069. doi: 10.4049/jimmunol.180.4.2069. [DOI] [PubMed] [Google Scholar]
- 47.Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol. 2007;178:7649. doi: 10.4049/jimmunol.178.12.7649. [DOI] [PubMed] [Google Scholar]
- 48.Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344. doi: 10.1158/0008-5472.CAN-06-3304. [DOI] [PubMed] [Google Scholar]
- 50.Zimring JC, Levery SB, Kniep B, Kapp LM, Fuller M, Kapp JA. CD75s is a marker of murine CD8(+) suppressor T cells. Int Immunol. 2003;15:1389. doi: 10.1093/intimm/dxg137. [DOI] [PubMed] [Google Scholar]
- 51.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 53.Joosten SA, van Meijgaarden KE, Savage ND, de Boer T, Triebel F, van der Wal A, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci U S A. 2007;104:8029. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Field AC, Bloch MF, Bellon B. Neonatal tolerance to a Th2-mediated autoimmune disease generates CD8+ Tc1 regulatory cells. J Autoimmun. 2003;21:201. doi: 10.1016/s0896-8411(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 55.Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression - a diverse arsenal for a moving target. Immunology. 2008;124:13. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kemeny DM. CD8+ T cells in atopic disease. Curr Opin Immunol. 1998;10:628. doi: 10.1016/s0952-7915(98)80080-0. [DOI] [PubMed] [Google Scholar]
- 57.Guillonneau C, Hill M, Hubert FX, Chiffoleau E, Herve C, Li XL, et al. CD40Ig treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:1096. doi: 10.1172/JCI28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gray JD, Hirokawa M, Ohtsuka K, Horwitz DA. Generation of an inhibitory circuit involving CD8+ T cells, IL-2, and NK cell-derived TGF-beta: contrasting effects of anti-CD2 and anti-CD3. J Immunol. 1998;160:2248. [PubMed] [Google Scholar]
- 59.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 60.Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, et al. Alloantigen specific CD8+CD28- FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16:1055. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 61.Izawa A, Yamaura K, Albin MJ, Jurewicz M, Tanaka K, Clarkson MR, et al. A novel alloantigen-specific CD8+PD1+ regulatory T cell induced by ICOS-B7h blockade in vivo. J Immunol. 2007;179:786. doi: 10.4049/jimmunol.179.2.786. [DOI] [PubMed] [Google Scholar]
- 62.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 63.Hellings N, Raus J, Stinissen P. T-cell vaccination in multiple sclerosis: update on clinical application and mode of action. Autoimmun Rev. 2004;3:267. doi: 10.1016/j.autrev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Ferrera F, La Cava A, Rizzi M, Hahn BH, Indiveri F, Filaci G. Gene vaccination for the induction of immune tolerance. Ann N Y Acad Sci. 2007;1110:99. doi: 10.1196/annals.1423.012. [DOI] [PubMed] [Google Scholar]
- 65.Ferrera F, Hahn BH, Rizzi M, Anderson M, Fitzgerald J, Millo E, et al. Protection against renal disease in (NZB × NZW)F(1) lupus-prone mice after somatic B cell gene vaccination with anti-DNA immunoglobulin consensus peptide. Arthritis Rheum. 2007;56:1945. doi: 10.1002/art.22700. [DOI] [PubMed] [Google Scholar]
- 66.Bar-Or A, Vollmer T, Antel J, Arnold DL, Bodner CA, Campagnolo D, et al. Induction of antigen-specific tolerance in multiple sclerosis after immunization with DNA encoding myelin basic protein in a randomized, placebo-controlled phase 1/2 trial. Arch Neurol. 2007;64:1407. doi: 10.1001/archneur.64.10.nct70002. [DOI] [PubMed] [Google Scholar]
- 67.Diedrichs-Mohring M, Thurau SR, Wildner G. Labrafil--a new adjuvant for peptide-specific oral tolerance in rat experimental autoimmune uveitis. Pharmacol Res. 2008;57:26. doi: 10.1016/j.phrs.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Ben-David H, Sharabi A, Dayan M, Sela M, Mozes E. The role of CD8+CD28 regulatory cells in suppressing myasthenia gravis-associated responses by a dual altered peptide ligand. Proc Natl Acad Sci U S A. 2007;104:17459. doi: 10.1073/pnas.0708577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176:7119. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 70.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruiz PJ, Zinger H, Mozes E. Effect of injection of anti-CD4 and anti-CD8 monoclonal antibodies on the development of experimental systemic lupus erythematosus in mice. Cell Immunol. 1996;167:30. doi: 10.1006/cimm.1996.0004. [DOI] [PubMed] [Google Scholar]
- 72.Adachi Y, Inaba M, Sugihara A, Koshiji M, Sugiura K, Amoh Y, et al. Effects of administration of monoclonal antibodies (anti-CD4 or anti-CD8) on the development of autoimmune diseases in (NZW × BXSB)F1 mice. Immunobiology. 1998;198:451. doi: 10.1016/s0171-2985(98)80052-1. [DOI] [PubMed] [Google Scholar]
- 73.Kang HK, Liu M, Datta SK. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J Immunol. 2007;178:7849. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- 74.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol. 2005;174:3247. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 75.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 76.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hahn BH, Singh RR, Wong WK, Tsao BP, Bulpitt K, Ebling FM. Treatment with a consensus peptide based on amino acid sequences in autoantibodies prevents T cell activation by autoantigens and delays disease onset in murine lupus. Arthritis Rheum. 2001;44:432. doi: 10.1002/1529-0131(200102)44:2<432::AID-ANR62>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 78.Ohnishi K, Ebling FM, Mitchell B, Singh RR, Hahn BH, Tsao BP. Comparison of pathogenic and non-pathogenic murine antibodies to DNA: antigen binding and structural characteristics. Int Immunol. 1994;6:817. doi: 10.1093/intimm/6.6.817. [DOI] [PubMed] [Google Scholar]
- 79.Tsao BP, Ebling FM, Roman C, Panosian-Sahakian N, Calame K, Hahn BH. Structural characteristics of the variable regions of immunoglobulin genes encoding a pathogenic autoantibody in murine lupus. J Clin Invest. 1990;85:530. doi: 10.1172/JCI114469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams WM, Staines NA, Muller S, Isenberg DA. Human T cell responses to autoantibody variable region peptides. Lupus. 1995;4:464. doi: 10.1177/096120339500400608. [DOI] [PubMed] [Google Scholar]
- 81.Ebling FM, Tsao BP, Singh RR, Sercarz E, Hahn BH. A peptide derived from an autoantibody can stimulate T cells in the (NZB × NZW)F1 mouse model of systemic lupus erythematosus. Arthritis Rheum. 1993;36:355. doi: 10.1002/art.1780360311. [DOI] [PubMed] [Google Scholar]
- 82.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 83.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eilat E, Dayan M, Zinger H, Mozes E. The mechanism by which a peptide based on complementarity-determining region-1 of a pathogenic anti-DNA auto-Ab ameliorates experimental systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2001;98:1148. doi: 10.1073/pnas.98.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharabi A, Haviv A, Zinger H, Dayan M, Mozes E. Amelioration of murine lupus by a peptide, based on the complementarity determining region-1 of an autoantibody as compared to dexamethasone: different effects on cytokines and apoptosis. Clin Immunol. 2006;119:146. doi: 10.1016/j.clim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 86.Rapoport MJ, Sharabi A, Aharoni D, Bloch O, Zinger H, Dayan M, et al. Amelioration of SLE-like manifestations in (NZB×NZW)F1 mice following treatment with a peptide based on the complementarity determining region 1 of an autoantibody is associated with a down-regulation of apoptosis and of the pro-apoptotic factor JNK kinase. Clin Immunol. 2005;117:262. doi: 10.1016/j.clim.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 87.Kaliyaperumal A, Michaels MA, Datta SK. Antigen-specific therapy of murine lupus nephritis using nucleosomal peptides: tolerance spreading impairs pathogenic function of autoimmune T and B cells. J Immunol. 1999;162:5775. [PubMed] [Google Scholar]
- 88.Lu L, Kaliyaperumal A, Boumpas DT, Datta SK. Major peptide autoepitopes for nucleosome-specific T cells of human lupus. J Clin Invest. 1999;104:345. doi: 10.1172/JCI6801. [DOI] [PMC free article] [PubMed] [Google Scholar]


