Abstract
The last several years have seen a significant increase in the number of reports describing the use of cationic polymers to design new materials and nanoscale assemblies that promote the surface-mediated delivery of DNA to cells and tissues. In general, these approaches fall into one of two broad categories: (i) methods based upon the physical adsorption of preformed, colloidal aggregates of cationic polymer and DNA (polyplexes) to surfaces, and (ii) methods for the layer-by-layer adsorption of DNA and cationic polymers on surfaces to fabricate multilayered thin films that provide control over the release of DNA into solution or to cells. In this Opinion, we discuss several recent examples of each of these approaches and provide commentary on ways in which the physical and chemical behaviours of cationic polymers have played key roles and present future opportunities to develop new methods for localized and surface-mediated cell transfection in vitro and in vivo.
Keywords: Polyelectrolytes, DNA, Cell Transfection, Surfaces, Thin Films, Nanoscale Assemblies
1. Introduction
The potential impact of methods that can be used to release DNA from surfaces – and that can thereby be used to promote the surface-mediated delivery of DNA to cells – is enormous. Potential applications of such methods range from advances in basic biomedical research and the engineering of complex tissues to, ultimately, the promise of localized gene-based therapies.
The development of materials-based approaches to the delivery of DNA to cells (cell transfection) is an inherently interdisciplinary endeavour, and an area of research that continues to advance rapidly. It is also an area of research in which the chemical and physical properties of polyelectrolytes – and cationic polymers in particular – have played critical roles. In this Opinion, we highlight recent literature and describe current progress toward the use of cationic polymers to design macromolecular assemblies that provide control over the release of DNA from surfaces and promote surface-mediated cell transfection.
Polymer-based approaches to the delivery of DNA have advanced considerably over the last decade. Much of this past work has focused on the design of cationic polymers that interact with DNA to form soluble interpolyelectrolyte complexes that can be administered readily to cells or tissues [1–3]. In addition to these solution-based approaches to delivery, several groups have sought to develop polymer-based methods that can be used to sustain or prolong the release of DNA. A considerable amount of work has been focused, for example, on the bulk encapsulation and subsequent sustained release of DNA from degradable polymer matrices [3, 4]. This work has contributed to the development of functional polymer scaffolds for tissue engineering as well as the development of important new methods for the localized delivery of DNA from the surfaces of implantable devices (e.g., by coating DNA-containing thin films onto the surfaces of indwelling devices [5]).
It is not our intention to provide here a discussion of recent progress in each of the general areas described above –interested readers will find additional detailed information describing these and other polymer-based approaches to the delivery of DNA in several comprehensive reviews [1–4]. Instead, we focus on recent reports that make use of the chemical properties and physical behaviours of cationic polymers to (i) assemble, organize, and present DNA at surfaces, (ii) provide control over the release or availability of DNA at surfaces, and (iii) promote the internalization of DNA by cells in ways that provide opportunities to enhance levels of surface-mediated cell transfection.
Recently, there has been a significant increase in the number of reports describing the use of cationic polymers to design new materials and assemblies that promote surface-mediated cell transfection. In general, these approaches fall into one of two broad categories: (i) methods based upon the adsorption of preformed polymer/DNA complexes to surfaces, and (ii) methods for the layer-by-layer adsorption of DNA and cationic polymers on surfaces to fabricate multilayered thin films that provide control over the release of DNA. Below, we highlight several recent examples of each of these approaches and provide commentary on ways in which cationic polymers have played important roles and provide future opportunities to optimize the performance of new materials for surface-mediated cell transfection.
2. Cationic Polymers for DNA Delivery: A Primer
It is difficult to overstate the fundamental importance of polyelectrolytes to the development of new methods for the delivery of DNA. It is important to recognize at the outset, of course, that DNA is, itself, a polyelectrolyte [6] – the negatively charged sugar phosphate backbone of DNA influences, among other things: (i) the conformation and dynamics of DNA in solution, (ii) the nature of its chemical and physical interactions with both small and large molecules, and (iii) the manner in which it adsorbs at surfaces and interfaces.
Owing to the above considerations, many approaches to the delivery of DNA have focused on the design of positively charged polymers. As noted above (and described in further detail below), cationic polymers can interact with DNA in solution through electrostatic interactions to form aggregates or assemblies with sizes, charges, and other properties that can promote the internalization and processing of DNA by cells [1–3]. The nature of the charge-based interactions between DNA and cationic polymers – and the extents to which these interactions can (or cannot) be controlled – thus lies at the heart of many conventional polymer-based approaches to DNA delivery.
A brief discussion of the primary cell-based obstacles to successful DNA delivery is useful here, and will provide a basis for our discussion of surface-mediated approaches to cell transfection, below. The overarching goal of any cell transfection procedure is, in general, to transfer a DNA construct into a cell in a manner that results in the successful expression of a protein encoded by the DNA. Because DNA is both large and negatively charged, it is generally not internalized efficiently by cells. To transfer DNA into cells efficiently, DNA must often be combined with, or accompanied by, a ‘transfection agent’ capable of promoting entry into a cell and addressing other cell-based obstacles to the processing of DNA [1–3]. In general, such an agent must be able to: (i) complex with DNA to form aggregates small enough to be internalized by cells (e.g., ~100 nm to 200 nm), (ii) promote the internalization of DNA by cells (e.g., by an active transport process such as endocytosis), (iii) prevent the intracellular degradation of DNA, and, finally, (iv) release the DNA payload at an appropriate time and place such that it is available for further processing [1–3].
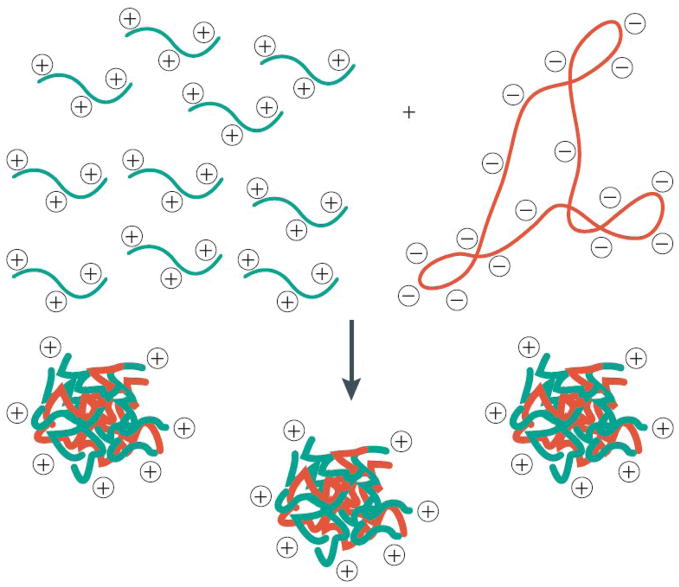
Cationic polymers – and polyamines in particular – are used widely to deliver DNA because they interact spontaneously with DNA in ways that can be used to address several of the cell-based barriers outlined above. For example, the simple mixing of a cationic polymer such as poly(lysine) (a polyamine that is almost completely protonated at physiological pH) with DNA in solution generally leads to the formation of soluble polymer/DNA interpolyelectrolyte complexes (called ‘polyplexes’; Figure 1) [2]. The formation of polyplexes is spontaneous and entropically driven, and, provided that certain conditions are met, leads to colloidal assemblies with dimensions on the order of 100 nm to 200 nm. Moreover, depending on the relative amounts of cationic polymer and DNA used, polyplexes having positive zeta potentials can be formed [2]. In general, positively charged particles are internalized more readily by cells by non-specific endocytosis than particles that are negatively charged or charge neutral. Thus, in practice, the use of cationic polymers to form nanoscale polyplexes provides a simple and straightforward approach with which to address the first two important barriers to transfection noted above (i.e., by providing a means to both condense DNA and promote internalization by cells) [1–3].
Figure 1.
Schematic illustration of nanoscale ‘polyplexes’ formed by the mixing of aqueous solutions of cationic polymer and negatively charged DNA. The formation of polyplexes is spontaneous and entropically driven, and leads to the formation of colloidal aggregates with dimensions on the order of 100 to 200 nm. Depending on the relative amounts of cationic polymer and DNA used, polyplexes having positive zeta potentials can be formed. Adapted with permission from reference [3].
Upon internalization by endocytosis, polyplexes are sequestered in intracellular vesicles called endosomes and, over time, are trafficked to other intracellular vesicles called lysosomes [2, 3]. The microenvironment inside endosomes is generally acidic, and lysosomes are both acidic and contain enzymes that can degrade DNA. The overall success of any polymer-mediated transfection process thus depends critically on the ability of the polymer to either avoid these vesicles or mediate the escape of DNA from them and transport DNA unharmed into the cytoplasm. Behr and coworkers demonstrated in 1995 that levels of cell transfection could be increased dramatically by formulating polyplexes using poly(ethylene imine) (PEI), a hyperbranched polyamine that responds to changes in pH in the range from pH 7 to pH ~5 (i.e., the range of pH encountered inside cell endosomes and lysosomes) [7]. The mechanism(s) through which PEI promotes enhanced cell transfection remain to be fully elucidated, but several lines of evidence support the view that PEI mediates the escape of DNA from endosomes and lysosomes more effectively than polyamines such as poly(lysine) [2, 7]. This early work has motivated the design of a broad range of new cationic polymers with structures and functions capable of protecting and/or directing the intracellular trafficking of DNA [1–3].
We close this section by returning briefly to consider the last of the important barriers to transfection noted above – the requirement that a transfection agent ultimately release (or dissociate from) DNA. While the spontaneous and entropically driven condensation of DNA into polyplexes provides a straightforward approach to promoting cellular entry, the self-disassembly of polyplexes can be generally regarded as entropically unfavourable, or at least relatively inefficient, in physiological media [8]. In general, the development of methods to promote the dissociation of DNA from cationic polymers could lead to methods that promote more efficient transfection. Several groups have begun to synthesize new cationic polymers and to develop new principles that should facilitate this process. We return to these ideas again in the discussion below.
3. From Solution-Based Delivery to Surface-Mediated Approaches to Cell Transfection
The polyplexes described above are typically formed in solution and, as a result, provide a convenient platform for the solution-based delivery of DNA to cells. Here, we consider briefly one additional barrier to transfection that was not discussed above – the requirement that polyplexes be transported physically from solution to the surfaces of cells prior to entry. It seems clear, for example, that a cell cannot internalize a polyplex until the polyplex is brought into contact with the cell membrane. It follows that approaches that increase the rates or extents to which polyplexes are transported to cell surfaces should also provide opportunities to improve levels of transfection. Several past reports have validated this concept experimentally. For example, Luo and Saltzman demonstrated that the adsorption of DNA onto dense silica nanoparticles – which can sediment from solution onto cells more effectively than polyplexes or lipid-based aggregates that are less dense – can promote enhanced cell transfection in vitro [9].
The notion that cell transfection can be enhanced using methods that increase the concentration of DNA at cell surfaces has also provided motivation for the development of surface-mediated and substrate-mediated methods of delivery. Substrate-mediated approaches, which generally seek to transfect cells that are physically attached to or growing on the surface of a substrate, can promote cell transfection by localizing and presenting surface-bound DNA directly to cells. Likewise, methods for the adsorption and subsequent release of DNA from surfaces can be used to elevate concentrations of DNA at cell/surface interfaces and spatially localize the delivery of DNA to cells or tissues (e.g., located in the vicinity of an implant site).
In the sections below, we describe recent approaches to surface-mediated or substrate-mediated delivery of DNA that incorporate cationic polymers as key structural or functional elements. We begin by considering approaches based on the physical adsorption or chemical conjugation of preformed polyplexes to solid surfaces.
4. Substrate-Mediated Transfection: Adsorption of Preformed Polyplexes to Surfaces
4.1 Non-Specific Adsorption of Polyplexes to Surfaces
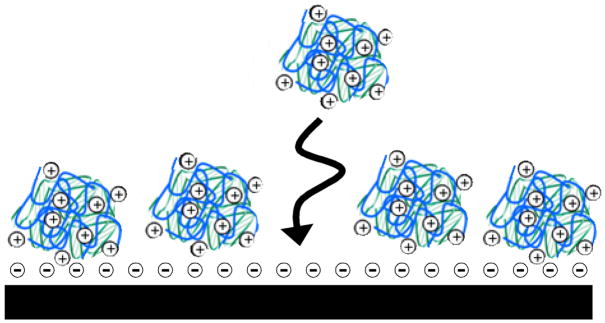
Bielinska et al. reported in 2000 that soluble polyplexes could be adsorbed to polymeric thin films and membranes in ways that promote the surface-mediated transfection of adherent cells [10•]. These investigators used polyplexes formed from cationic polyamidoamine dendrimers and plasmid DNA. Initial experiments demonstrated that these polyplexes could be adsorbed physically onto the surfaces of poly(lactic-co-glycolic acid) (PLGA) or collagen thin films (Figure 2), and that polyplex-functionalized films could promote the surface-mediated transfection of cells in vitro and in vivo [10•]. These results demonstrated that polyplexes could be adsorbed onto surfaces with retention of biological activity, and provided a framework for the development of other approaches to substrate-mediated cell transfection.
Figure 2.
Schematic illustration of pre-formed polymer/DNA polyplexes adsorbed from solution onto a negatively charged surface.
Before moving to a discussion of more recent applications of this approach, we pause to consider several additional aspects of this early study that serve to illustrate the important roles that the polyelectrolytes in these materials play with respect to developing and optimizing methods for surface-mediated cell transfection. For example, additional experiments by these investigators demonstrated that levels of surface-mediated transfection were dependent on both (i) the ratio of polymer to DNA used to prepare the polyplexes and (ii) the amount of the polyplexes adsorbed to the polymer thin films [10•].
As described in Section 2 above, the relative ratio of cationic polymer to DNA used to fabricate a polyplex can influence the surface charges, or zeta potentials, of the resulting colloidal aggregates [1–3]. In the context of cell transfection, cells often internalize polyplexes with positive charges more readily than polyplexes that are negatively charged or charge neutral. However, in the context of the adsorption of polyplexes to surfaces, the signs and magnitudes of the zeta potentials of polyplexes could clearly also influence the strength with which polyplexes adsorb (or do not adsorb) to a particular surface (e.g., through electrostatic interactions). It seems clear that strategies seeking to promote efficient substrate-mediated transfection must ultimately strike an appropriate balance between the factors governing the adsorption and desorption of polyplexes while also maintaining the properties necessary to address cell-based barriers to entry and expression.
Bielinska et al. reported that dendrimer/DNA complexes adsorbed to collagen thin films dissociated rapidly upon incubation in aqueous media [10•]. However, when films were prepared by incorporating an anionic lipid agent (intended to increase the anionic surface charge of the films) polyplexes were retained and released over longer periods. These films could be used to promote efficient substrate-mediated transfection (e.g., from 50% to 75% of cells transfected, depending on the dendrimer/DNA charge ratios used). These results demonstrate the importance of both polyplex properties and substrate design to the development of methods of substrate-mediated transfection based on the non-specific adsorption of polyplexes to surfaces.
Shea and coworkers extended the basic concepts described above to investigate approaches to substrate-mediated cell transfection using polyplexes formed from DNA and PEI [11•, 12]. As described above under Section 2, PEI is a functional polyamine that is capable of addressing important intracellular barriers to transfection and it is, in general, an effective gene delivery agent when administered to cells using conventional solution-based methods [2]. Approaches to substrate-mediated delivery that incorporate polyplexes formed using PEI (or other polyamines designed to address intracellular barriers to the processing of DNA) could thus lead to more effective methods for substrate-mediated transfection.
Bengali et al. investigated the adsorption of polyplexes formed from plasmid DNA and PEI to materials used commonly to culture cells in vitro or to fabricate degradable biomedical implants [11•]. The primary focus of this investigation was to characterize the influence of changes in polyplex properties (e.g., DNA/PEI ratio, size, zeta potential) and substrate preparation to (i) modulate polyplex binding affinity and (ii) optimize levels of substrate-mediated transfection in vitro. Polyplexes adsorbed to samples of tissue culture polystyrene that were pretreated by incubation in protein-containing serum mediated levels of cell transfection up to 1500 times greater than levels mediated be adsorption to unmodified substrates [11•]. The nature of the physico-chemical interactions between the polyplexes and serum-coated surfaces was not characterized explicitly in this study. However, these results provide additional support for the view that substrate properties play important roles in mediating polyplex adsorption and cell transfection and suggest that it will be possible to engineer further the properties of these surfaces (or, alternatively, the properties of the polyplexes) to more effectively balance the forces governing polyplex adsorption and the internalization of DNA by cells. Subsequent work by this group demonstrated that this approach could be extended to the immobilization of polyplexes on the surfaces of thin films and three-dimensional porous matrices fabricated from PLGA, a biodegradable polymer commonly used to fabricate biomedical implants and mechanical cell supports for tissue engineering [11•, 12].
The work above provides proof-of-concept and illustrates one way in which methods for the substrate-mediated administration of polyplexes could prove useful for the design of functional polymer scaffolds for applications such as tissue engineering, for which it is desirable to maintain local control over the delivery of growth factors or other agents to cells and developing tissues [13]. Further, because these methods can be used to adsorb DNA to the surfaces of scaffolds after the scaffold is fabricated, this approach could provide an alternative to conventional methods for the encapsulation of DNA within polymer scaffolds (such as solvent casting or gas foaming processes [13]) that expose DNA to chemicals or mechanical challenges that can damage DNA or perturb the structures and activities of polyplexes. Saul et al. recently used a combination of these two approaches (i.e., polyplex adsorption and the bulk encapsulation of polyplexes) to achieve controlled and long-term release and expression of DNA from porous scaffolds fabricated from the natural polymer fibrin [14]. In general, this approach provides a potential basis for the design of scaffolds that could provide control over the release of two different DNA constructs at two different rates.
4.2 Surface-Tethered Polyplexes: Immobilization Through Chemospecific Supramolecular Interactions
Segura et al. have demonstrated in a series of recent reports an approach to modulating or tuning binding interactions between polyplexes and solid surfaces using biotin-functionalized cationic polymers [15•–17]. Biotin is a biological ligand that binds with very high affinity and specificity to the tetrameric protein avidin. The underlying concept here is thus that polyplexes designed to display biotin functionality on their surfaces can be immobilized or ‘tethered’ in a specific manner to surfaces that are functionalized, coated, or patterned with avidin.
In an initial report, these investigators demonstrated that polyplexes formed using plasmid DNA and biotin-functionalized poly(lysine) could be immobilized on surfaces functionalized with neutravidin (a non-glycosylated derivative of avidin) [15•]. In general, this method of tethering could be used to control the amount of DNA immobilized through manipulation of the molecular weight of the biotinylated polymer, the degree of biotinylation of the polymer, or by using mixtures of biotinylated and non-biotinylated polymer to tune the average number of ‘tethers’ presented on the surfaces of the polyplexes. Differences in surface density were observed to correlate to differences in levels of cell transfection when cells were grown on polyplex-tethered surfaces in vitro [15•].
Segura et al. have also demonstrated that this biotin-based tethering approach can be extended to the immobilization of polyplexes formed using plasmid DNA and biotin-functionalized PEI [16]. Levels of surface-mediated gene expression using immobilized DNA/PEI complexes were twofold higher than levels mediated by immobilized DNA/poly(lysine) complexes. This increase in transfection was attributed, at least in part, to the ability of PEI to influence and promote the intracellular trafficking of DNA. The ability to immobilize polyplexes using specific polyplex/substrate interactions also provides opportunities to pattern the locations of polyplexes and provides an approach to exerting spatial control over cell transfection [17].
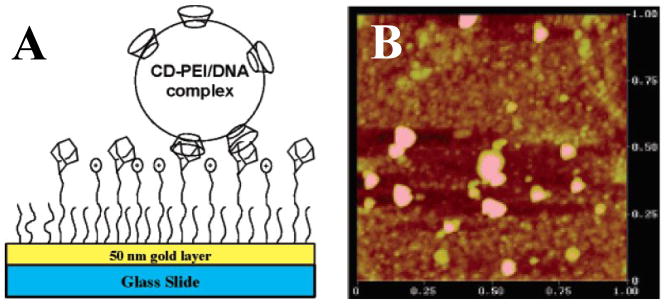
Park et al. recently reported another approach to the chemospecific immobilization of polyplexes on surfaces that takes advantage of the ability of carbohydrate-based cyclodextrin molecules to form inclusion complexes (or host-guest complexes) with hydrophobic organic compounds [18]. These investigators used PEI functionalized with β-cyclodextrin (a cyclic molecule composed of seven glucose units) to form DNA/PEI polyplexes. Subsequent experiments demonstrated that these cyclodextrin-functionalized polyplexes could bind specifically to gold surfaces modified to present bulky, hydrophobic adamantane groups (Figure 3A) [18]. Because β-cyclodextrin is well known to bind to and act as a molecular ‘host’ for adamantane in aqueous environments [19], the specificity of the binding in these experiments could be understood in terms of multivalent host-guest interactions between cyclodextrin-functionalized polyplexes and the adamantane-functionalized surfaces.
Figure 3.
A) Schematic illustration of a cyclodextrin-functionalized polyplex immobilized on an adamantane-functionalized surface by inclusion complex formation. B) AFM image of a polyplex-functionalized surface. Adapted with permission from reference [18].
Characterization of polyplex-decorated surfaces using atomic force microscopy revealed the presence of nanoparticulate structures ranging in size from 50 to 100 nm (Figure 3B) [18]. In general, more analytical work will be required to characterize the nanometer-scale and molecular-level structures of polyplexes adsorbed to surfaces through either specific or non-specific interactions. However, the results of this recent study suggest that polyplexes can adsorb through supramolecular host-guest interactions without substantial, large-scale disruption of polymer/DNA interactions.
The ability of this latter approach to promote cell transfection has not yet been demonstrated. However, several past studies have demonstrated that polyplexes formed from cyclodextrin-modified PEI [20] and other cyclodextrin-containing cationic polymers [21] can transfect cells effectively when administered using conventional methods (e.g., from solution or by intravenous injection). This general approach thus appears to be well suited for the development of methods for the surface-mediated delivery of DNA to cells and tissues.
5. Surface-Mediated Delivery of DNA Using Multilayered Polyelectrolyte Thin Films
We now turn our attention to a fundamentally different polyelectrolyte-based approach to the immobilization and controlled release of DNA from surfaces – an approach based on methods for the alternating, layer-by-layer adsorption of DNA and cationic polymers on surfaces. Similar to the approaches described above, these methods seek to capitalize upon multivalent electrostatic interactions between cationic polymers with DNA. However, rather than relying on the assembly and adsorption of pre-formed polymer/DNA polyplexes, these methods lead to the formation of multilayered, polyelectrolyte-based, DNA-containing thin films on surfaces. Below, we provide a brief introduction to layer-by-layer methods of assembly and a discussion of several recent examples that serve to illustrate the potential of this approach in the context of surface-mediated DNA delivery.
5.1 Layer-by-Layer Assembly of Multilayered Polyelectrolyte Thin Films
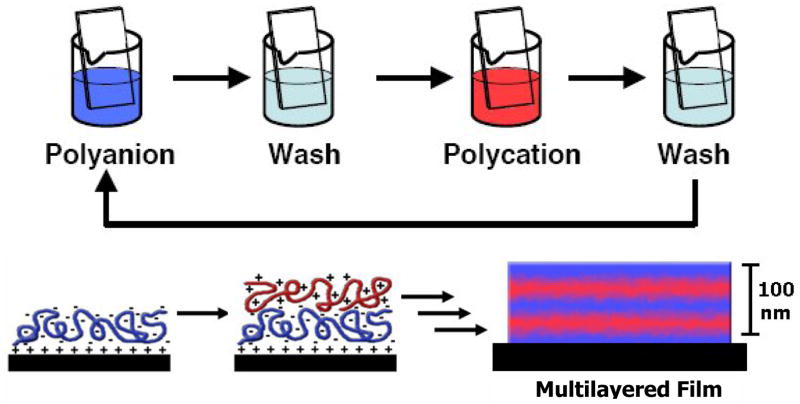
Methods for the alternating, layer-by-layer adsorption of oppositely charged polymers on surfaces provide a practical approach to the bottom-up assembly of thin polymer films [22–24••]. These methods take advantage of attractive electrostatic forces between oppositely charged polymers and are, in general, technically straightforward to implement: the iterative dipping of an object into two different solutions of oppositely charged polyelectrolytes yields multilayered films composed of alternating layers of cationic and anionic polymers (Figure 4). The thicknesses of the resulting films can range from tens or hundreds of nanometers to several micrometers depending upon the number of layers deposited and other experimental conditions (such as the pH or ionic strength of polyelectrolyte solutions, etc.) used during fabrication.
Figure 4.
The layer-by-layer deposition of oppositely charged polyelectrolytes on surfaces is a well-established method for the fabrication of thin multilayered films. Fabrication proceeds by iterative dipping of a substrate into dilute aqueous solutions of cationic and anionic polymers. Electrostatic interactions guide the assembly of multilayered polyelectrolyte assemblies. Adapted with permission from reference [28•].
This general approach offers precise control over the compositions and thicknesses of thin polyelectrolyte-based films. The methods described above are also entirely aqueous-based, and can thus be used to fabricate films using a broad range of naturally occurring and biologically important polyelectrolytes, including DNA, often without loss of biological function [24••]. Additional details related to the fabrication, properties, and potential applications of multilayered polyelectrolyte assemblies can be found in several recent reviews [22–25].
In the context of the development of methods for the surface-mediated delivery of DNA, layer-by-layer assembly offers several potential practical advantages relative to methods based on the adsorption of pre-formed polymer/DNA complexes [26–28•]. First, because DNA can be incorporated directly within these films as an anionic layer, this approach permits straightforward control over the loading of DNA at a surface by control over the number of layers of polymer and DNA deposited. Second, these methods can be used to fabricate films containing multiple different layers of multiple different types of DNA; this approach thus offers potential opportunities to control the surface-mediated release of multiple different DNA constructs in ways that would likely be difficult to achieve using methods based simply on the adsorption of polyplexes. Finally, we note that these multilayered films are inherently multicomponent and contain one layer of cationic polymer for every layer of anionic DNA deposited during fabrication (see Figure 4). The inherent juxtaposition of the DNA in these films with alternating layers of cationic polymers creates additional opportunities to design films that promote the internalization and trafficking of DNA by cells. We return to each of these points again in the discussion below.
5.2 Fabrication of DNA-Containing Films Using Degradable Cationic Polymers
The first example of the incorporation of DNA into a multilayered polyelectrolyte thin film was reported by Lvov et al. in 1993, who demonstrated that it was possible to fabricate films using DNA and synthetic cationic polymers such as poly(allylamine) [29•]. The resulting films were not evaluated in a biological context, but this seminal report did provide a framework for applications of this basic approach in the contexts of controlled release and cell transfection. Below, we highlight recent reports describing the fabrication of films from DNA and cationic polymers with backbone or side-chain functionality that can be cleaved under physiologically relevant conditions (e.g., by chemical hydrolysis, by enzymatic hydrolysis, or by exposure to reductive conditions) to promote film erosion and the release of DNA into solution or to cells.
Zhang et al. demonstrated in 2004 that it is possible to fabricate DNA-containing multilayered films that erode gradually in physiologically relevant media – and release transcriptionally active DNA from surfaces – by fabricating films using plasmid DNA and hydrolytically degradable polymer 1 [30••]. Polymer 1 can be rendered cationic in aqueous media (by protonation) and it is hydrolytically degradable (by virtue of the ester functionality in the polymer backbone). The basic concept underlying this approach is thus that this polymer can both (i) enable layer-by-layer assembly through electrostatic interactions with DNA, and (ii) provide a means of promoting the disassembly of a film (and the release of DNA) by polymer degradation. Films ~100 nm thick fabricated from polymer 1 and plasmid DNA eroded and released DNA over a period of ~30 hours when incubated in phosphate-buffered saline (PBS) at 37 °C [30••].
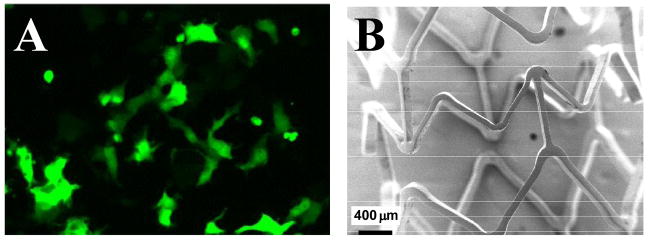
Jewell et al. expanded upon the observations above to demonstrate that films fabricated from polymer 1 and plasmid DNA could be used to promote surface-mediated cell transfection in vitro [31••]. For example, when quartz slides coated with films  fabricated using polymer 1 and plasmid DNA encoding enhanced green fluorescent protein (EGFP) were placed in contact with COS-7 cells, expression of EGFP was observed after 48 hours (Figure 5A). Transfection could be localized to cells growing directly under film-coated portions of the quartz slides, demonstrating a basis for achieving spatial control over the transfection of sub-populations of cells using this approach [31••]. These investigators also demonstrated recently that this approach can also be used to fabricate ultrathin films that release transcriptionally active DNA from the surfaces of intravascular stents (Figure 5B) [32]. With further development, these methods could prove useful for the localized release of DNA from the surfaces of a broad range of other implantable materials.
fabricated using polymer 1 and plasmid DNA encoding enhanced green fluorescent protein (EGFP) were placed in contact with COS-7 cells, expression of EGFP was observed after 48 hours (Figure 5A). Transfection could be localized to cells growing directly under film-coated portions of the quartz slides, demonstrating a basis for achieving spatial control over the transfection of sub-populations of cells using this approach [31••]. These investigators also demonstrated recently that this approach can also be used to fabricate ultrathin films that release transcriptionally active DNA from the surfaces of intravascular stents (Figure 5B) [32]. With further development, these methods could prove useful for the localized release of DNA from the surfaces of a broad range of other implantable materials.
Figure 5.
A) Fluorescence microscopy image showing surface-mediated transfection of COS-7 cells promoted by contact with glass slides coated with multilayered films fabricated from plasmid DNA and polymer 1. B) Scanning electron microscopy image of a stainless steel intravascular stent coated with a multilayered film fabricated from polymer 1 and plasmid DNA. Adapted with permission from references [31••] and [32], respectively.
Approaches that permit tunable control over the rates at which DNA is released from multilayered films could ultimately lead to enhanced gene-based therapies. Zhang et al. demonstrated recently that it is possible to manipulate the rates at which multilayered films erode and release DNA (or other model anionic polymers) over periods ranging from several days to several weeks by using analogs of polymer 1 that are more hydrophobic [33] or that contain side-chains with functionality that increases or decreases polymer charge density or other properties [34].
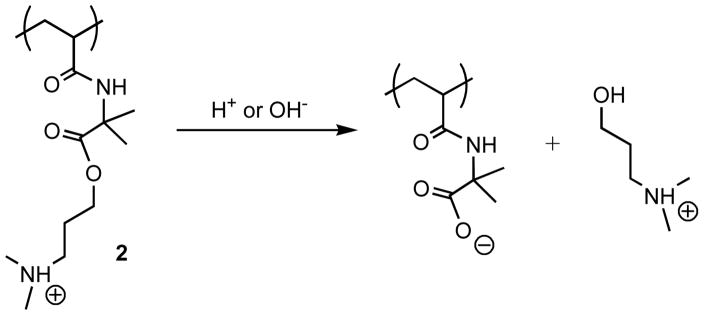
Zhang et al. also demonstrated recently a new approach to achieving the prolonged release of DNA from multilayered films based on the fabrication of films using ‘charge-shifting’ cationic polymer 2 [35•]. The backbone of polymer 2 is not degradable, but the amine functionality in the side chain of the polymer is attached to the backbone through a degradable ester linkage. Hydrolysis of the side-chains of polymer 2 results in removal of cationic charge, the introduction of negative charge, and, as a result, a time-dependent ‘shift’ in the net charge of the polymer (Figure 6). Zhang et al. demonstrated that this time-dependent shift can be used to disrupt DNA-containing films under physiologically relevant conditions, and that this approach can be used to release plasmid DNA from surfaces for up to 90 days [35•].
Figure 6.
Structure of a side-chain functionalized ‘charge-shifting’ cationic polymer. Side chain hydrolysis results in a gradual reduction, or shift, of the net charge of the polymer. Adapted with permission from reference [35•].
The erosion of films fabricated from degradable polymers such as polymer 1 is likely facilitated by dramatic reductions in polymer molecular weight that occur upon polymer backbone hydrolysis. The introduction of ‘charge-shifting’ polymers such as polymer 2 represents an interesting alternative to the use of degradable polyamines, because the backbone of polymer 2 does not degrade (and the molecular weight of the polymer does not change substantially upon side chain hydrolysis). The ability to tune the rates and extents to which side chain hydrolysis occurs (for example, by modifying the number of cleavable side chains or the chemistry of the degradable linker) could thus offer new opportunities to design films that permit tunable control over the release of DNA, or other agents, for prolonged periods.
Several groups have demonstrated approaches to the fabrication of erodible multilayered films based upon the incorporation of enzymatically degradable polyelectrolytes. In a series of recent reports, Ren et al. described an approach involving films fabricated from DNA and poly(L-lysine) (PLL) [36, 37]. Films formed from PLL and salmon sperm DNA were stable in PBS buffer, but eroded over ~35 hours upon exposure of the films to the enzyme α-chymotrypsin [36]. The films described in this initial study were not evaluated in the context of cell transfection. However, subsequent reports by these researchers demonstrated that release rates could be tuned by controlling film properties such as crosslinking density [37].
Several other recent reports have demonstrated a basis for the triggered destruction of multilayered films under reductive conditions in ways that could prove useful for the surface-mediated delivery of DNA [38–40]. As one example, Blacklock et al. have described the assembly of multilayered films using plasmid DNA and a high molecular weight cationic polypeptide containing disulfide linkages in the backbone of the polymer [39]. These DNA-containing films were observed to be stable in non-reducing media. However, in the presence of the chemical reducing agent dithiothreitol, the films eroded and released DNA over a period of ~24 hours. Chen et al. recently described a similar approach based on the incorporation of a reductively degradable cationic poly(amidoamine) into DNA-containing films [40]. The films described in these last two studies have not yet been evaluated in the context of cell transfection. However, methods of film disruption based on thiol-disulfide redox chemistry could prove useful for the triggered release of DNA from films exposed to or implanted in the vicinity of chemically reducing physiological environments.
5.3 Leveraging the Properties of Multilayered Films to Release Multiple DNA Constructs
As described above, one potential advantage of methods for the layer-by-layer assembly of multicomponent polymer thin films is the stepwise nature of the assembly process. These methods provide potential opportunities to control with precision the relative locations of one or more types of DNA in multiple different layers of a multilayered film. These methods thus provide, at least in principle, new approaches to the design of thin films and coatings that permit sophisticated levels of control over the release and surface-mediated expression of multiple different genes. A report by Jessel et al. recently demonstrated a basis for achieving such control [41••].
Jessel et al. demonstrated that placing two different plasmid DNA constructs at different depths in films fabricated from PLL and poly(glutamic acid) (PGA) yields films that provide control over the timing with which each plasmid is expressed in cells growing on the surfaces of these enzymatically degradable materials [41••]. In an initial experiment, a single layer of plasmid DNA and a cationic cyclodextrin agent were imbedded deep within a multilayered PLL/PGA assembly. These films were able to support the attachment, growth, and transfection of several different cell types. In subsequent experiments, two different layers of two different plasmid DNA constructs – each encoding a different gene product – were imbedded within the films. For these experiments, each plasmid was deposited at a different depth within the film and separated from each other by the deposition of intermediate PLL/PGA layers. When cells were grown on these films, control over the timing of the expression of each plasmid construct in cells was demonstrated (e.g. expression of the plasmid located in the lower layers of the films was delayed by ~4 hours relative to the expression of the plasmid located in the topmost portion of the films) [41••]. Reversing the order in which the two plasmids were incorporated into the films was reported to reverse the order in which the two genes were expressed in cells. Zhang et al. recently demonstrated that it is also possible to fabricate films that provide control over both the order and the timing with which two plasmids are released into solution by fabricating films using a side-chain functionalized degradable polyamine [34].
While the delay in the expression of the two genes in the example described above is relatively short, it should prove possible to further manipulate the timing with which multiple agents are released or expressed by varying the number of intermediate layers between the agents or the structures of the polymers that are used to construct these layers. Wood et al. have demonstrated, for example, that the chemical crosslinking of intermediate layers in films fabricated using polymer 1 can provide control over film erosion and the release of two different polysaccharide species [42]. This general approach could also, in principle, be applied to the fabrication of films that provide control over the timing and the order with which two different types of DNA are released to or expressed by cells.
5.4 Investigating the Function of Cationic Film Components
We close this section by returning to consider one additional potential advantage of layer-by-layer assembly in the context of DNA delivery. As described above, the multilayered films highlighted here are multicomponent and contain equal numbers of layers of DNA and cationic polymer. The possibility that the cationic polymers in these materials could contribute – or could ultimately be engineered to contribute – to the internalization or intracellular trafficking of DNA by cells is a particularly attractive and potentially powerful aspect of this approach.
In general, much more analytical work will be required to determine the extent to which the cationic polymers in these materials remain bound to DNA during or after erosion, or whether any such polymer/DNA aggregates might have properties (such as sizes and charges) similar to those of the ‘pre-formed’ polyplexes described above in Sections 2 and 4. However, several of the reports discussed above provide preliminary physical evidence based on light scattering experiments [32, 35•], zeta potential analysis [35•], and electron microscopy [36], as well as biological evidence arising from cell transfection experiments [31, 32], suggesting that (i) DNA is released in a form that is at least partially bound to cationic polymer and (ii) that the polymer could aid in promoting surface-mediated cell transfection.
Finally, it bears noting that, to date, the focus of the work described above has been directed primarily toward the introduction of new chemical functionality and other design elements necessary to promote film erosion and control the release of DNA in physiological media. In general, this work has been conducted using cationic polymers that were either not designed for, or at least not optimized for, gene delivery applications. This initial work thus serves to set the stage and provide a framework for the next level of development, which should include an emphasis on the design and incorporation of cationic polymers (and other species) designed to address the specific intracellular barriers to cell transfection described in Section 2.
An important illustration of the potential of this approach is provided by Jessel et al. who, in the example described above, demonstrated that the incorporation of a cationic cyclodextrin-based transfection agent into DNA-containing films promoted significant levels of cell transfection (whereas films into which this agent was not incorporated did not) [41••]. Meyer et al. have also fabricated multilayered films using preformed PEI/DNA polyplexes and demonstrated that the resulting thin film materials can mediate transgene expression in cells in vitro [43].
The past several years have seen an explosion in the development of functional and multifunctional cationic polymers developed specifically for gene delivery using conventional, solution-based methods. The time is now right to capitalize upon and exploit the availability of these new polymers for the design of thin multilayered films that promote efficient surface-mediated gene delivery.
6. Summary and Outlook
Cationic polymers have played central roles in the development of methods for the transfer of DNA into cells. The importance of cationic materials in the context of cell transfection flows directly from the ability of these positively charged polyelectrolytes to interact with DNA through electrostatic interactions and form assemblies that can address cell-based barriers to DNA delivery. A significant amount of the past work in this area has focused on the design of cationic polymers that form soluble, colloidal complexes, and on the administration of these complexes to cells or tissues from solution. In this Opinion, we have discussed recent trends and emerging themes related to the use of cationic polymers to develop methods for surface-mediated cell transfection.
Both of the approaches described above leverage the physico-chemical behaviors of cationic polymers to design surfaces capable of releasing DNA and promoting transfection. It is important to keep in mind, however, that these approaches are new, and important questions and obstacles remain. In general, for example, levels of surface-mediated transfection that can be achieved using the methods above are lower than those that can be achieved using solution-based methods. Approaches based on the adsorption of polyplexes can leverage past work to optimize the properties of polyplexes for solution-based delivery. Will such formulations ultimately be found to be optimal for surface mediated transfection? Do the compositions and structures of polyplexes formed in solution change upon adsorption to a surface in ways that influence their activities? Approaches based on the layer-by-layer assembly of DNA-containing thin films take advantage of new methods of assembly that have arisen largely in the materials community, but biophysical questions remain. To what extent do the polyamines in these materials contribute to promoting the internalization or intracellular trafficking of DNA? Can the incorporation of new polymers developed by the gene delivery community be leveraged to influence these processes further?
More research will be required to answer questions such as those posed above and to develop effective new methods for surface-mediated transfection in biomedical and clinical contexts. In this sense, the work discussed above merely serves to set the stage and provide a framework for the next levels of development. In the broader context of what may ultimately prove possible, the work described here has, very likely, only begun to scratch the surface.
Acknowledgments
Research from the authors’ own laboratory was supported by the National Institutes of Health and the Arnold and Mabel Beckman Foundation. D. M. L. is a Research Fellow of the Alfred P. Sloan Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References and Recommended Readings
- 1.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 2.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 3.Putnam D. Polymers for gene delivery across length scales. Nat Mater. 2006;5:439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 4.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol Ther. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Klugherz BD, Jones PL, Cui X, Chen W, Meneveau NF, DeFelice S, Connolly J, Wilensky RL, Levy RJ. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat Biotechnol. 2000;18:1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- 6.FrankKamenetskii MD. Biophysics of the DNA molecule. Phys Rep. 1997;288:13–60. [Google Scholar]
- 7.Boussif O, Lezoualch F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in-Vivo - Polyethylenimine. P Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67:598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Luo D, Saltzman WM. Enhancement of transfection by physical concentration of DNA at the cell surface. Nat Biotechnol. 2000;18:893–895. doi: 10.1038/78523. [DOI] [PubMed] [Google Scholar]
- 10•.Bielinska AU, Yen A, Wu HL, Zahos KM, Sun R, Weiner ND, Baker JR, Roessler BJ. Application of membrane-based dendrimer/DNA complexes for solid phase transfection in vitro and in vivo. Biomaterials. 2000;21:877–887. doi: 10.1016/s0142-9612(99)00229-x. An early paper describing the adsorption of preformed colloidal complexes formed from DNA and a synthetic polyamine to the surfaces of natural and synthetic materials to develop methods for surface-mediated transfection in vitro and in vivo. [DOI] [PubMed] [Google Scholar]
- 11•.Bengali Z, Pannier AK, Segura T, Anderson BC, Jang JH, Mustoe TA, Shea LD. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnol Bioeng. 2005;90:290–302. doi: 10.1002/bit.20393. This paper describes a systematic investigation of the influence of the sizes, charges, and other properties of polyplexes formed using poly(ethylene imine) on polyplex binding affinity and cell transfection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Mol Ther. 2005;12:475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 14.Saul JM, Linnes MP, Ratner BD, Giachelli CM, Pun SH. Delivery of non-viral gene carriers from sphere-templated fibrin scaffolds for sustained transgene expression. Biomaterials. 2007;28:4705–4716. doi: 10.1016/j.biomaterials.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 15•.Segura T, Shea LD. Surface-tethered DNA complexes for enhanced gene delivery. Bioconjugate Chem. 2002;13:621–629. doi: 10.1021/bc015575f. An early paper describing an approach to the adsorption of polyplexes to surfaces using specific and high-affinity biotin-streptavidin interactions, as opposed to non-specific electrostatic or hydrophobic interactions. [DOI] [PubMed] [Google Scholar]
- 16.Segura T, Volk MJ, Shea LD. Substrate-mediated DNA delivery: role of the cationic polymer structure and extent of modification. J Control Release. 2003;93:69–84. doi: 10.1016/j.jconrel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Segura T, Chung PH, Shea LD. DNA delivery from hyaluronic acid-collagen hydrogels via a substrate-mediated approach. Biomaterials. 2005;26:1575–1584. doi: 10.1016/j.biomaterials.2004.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park IK, Von Recum HA, Jiang SY, Pun SH. Supramolecular assembly of cyclodextrin-based nanoparticles on solid surfaces for gene delivery. Langmuir. 2006;22:8478–8484. doi: 10.1021/la061757s. [DOI] [PubMed] [Google Scholar]
- 19.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: Past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 20.Pun SH, Bellocq NC, Liu AJ, Jensen G, Machemer T, Quijano E, Schluep T, Wen SF, Engler H, Heidel J, Davis ME. Cyclodextrin-modified polyethylenimine polymers for gene delivery. Bioconjugate Chem. 2004;15:831–840. doi: 10.1021/bc049891g. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez H, Hwang SJ, Davis ME. New class of polymers for the delivery of macromolecular therapeutics. Bioconjugate Chem. 1999;10:1068–1074. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 22.Bertrand P, Jonas A, Laschewsky A, Legras R. Ultrathin polymer coatings by complexation of polyelectrolytes at interfaces: suitable materials, structure and properties. Macromol Rapid Comm. 2000;21:319–348. [Google Scholar]
- 23.Hammond PT. Form and function in multilayer assembly: New applications at the nanoscale. Adv Mater. 2004;16:1271–1293. [Google Scholar]
- 24••.Tang ZY, Wang Y, Podsiadlo P, Kotov NA. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv Mater. 2006;18:3203–3224. A comprehensive review of current applications of layer-by-layer assembly in biomedically-oriented areas. This paper provides numerous leading references and details several examples of the incorporation of peptides, proteins, DNA, and other biologically active species into multilayered polyelectrolyte films and outlines potential applications of bioactive films. [Google Scholar]
- 25.Johnston APR, Cortez C, Angelatos AS, Caruso F. Layer-by-layer engineered capsules and their applications. Curr Opin Colloid In. 2006;11:203–209. [Google Scholar]
- 26.Lynn DM. Layers of opportunity: nanostructured polymer assemblies for the delivery of macromolecular therapeutics. Soft Matter. 2006;2:269–273. doi: 10.1039/b517860f. [DOI] [PubMed] [Google Scholar]
- 27.Lynn DM. Peeling Back the Layers: Controlled Erosion and Triggered Disassembly of Multilayered Polyelectrolyte Thin Films. Adv Mater. 2007:4118–4130. [Google Scholar]
- 28•.Jewell CM, Lynn DM. Multilayered Polyelectrolyte Assemblies as Platforms for the Delivery of DNA and Other Nucleic Acid-Based Therapeutics. Adv Drug Deliver Rev. 2008 doi: 10.1016/j.addr.2008.02.010. In press. A comprehensive review on the application of layer-by-layer assembly to fabricate thin films and colloidal particles using DNA and other nucleic acid materials and applications of these materials in the context of DNA delivery and cell transfection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Lvov Y, Decher G, Sukhorukov G. Assembly of Thin-Films by Means of Successive Deposition of Alternate Layers of DNA and Poly(Allylamine) Macromolecules. 1993;26:5396–5399. This paper was the first to demonstrate the fabrication of multilayered polyelectrolyte thin films using DNA as an anionic film component. This work was not conducted in a biological context, but this seminal contribution provided a template for the other examples discussed in this article. [Google Scholar]
- 30••.Zhang J, Chua LS, Lynn DM. Multilayered thin films that sustain the release of functional DNA under physiological conditions. Langmuir. 2004;20:8015–8021. doi: 10.1021/la048888i. This paper describes the fabrication of erodible multilayered polyelectrolyte thin films using DNA and a hydrolytically degradable polyamine. This report was the first to describe layer-by-layer films fabricated using transcriptionally active plasmid DNA as an anionic film component and the subsequent sustained release of DNA in physiologically relevant media. [DOI] [PubMed] [Google Scholar]
- 31••.Jewell CM, Zhang J, Fredin NJ, Lynn DM. Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J Control Release. 2005;106:214–223. doi: 10.1016/j.jconrel.2005.04.014. This paper was the first to demonstrate that multilayered thin films fabricated from plasmid DNA and degradable polyamines could promote surface-mediated cell transfection when placed in contact with cells in vitro. [DOI] [PubMed] [Google Scholar]
- 32.Jewell CM, Zhang J, Fredin NJ, Wolff MR, Hacker TA, Lynn DM. Release of Plasmid DNA from Intravascular Stents Coated with Ultrathin Multilayered Polyelectrolyte Films. Biomacromolecules. 2006;7:2483–2491. doi: 10.1021/bm0604808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Fredin NJ, Janz JF, Sun B, Lynn DM. Structure/property relationships in erodible multilayered films: influence of polycation structure on erosion profiles and the release of anionic polyelectrolytes. Langmuir. 2006;22:239–245. doi: 10.1021/la052360b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JT, Montanez SI, Jewell CM, Lynn DM. Multilayered films fabricated from plasmid DNA and a side-chain functionalized poly(beta-amino ester): Surface-type erosion and sequential release of multiple plasmid constructs-from surfaces. Langmuir. 2007;23:11139–11146. doi: 10.1021/la702021s. [DOI] [PubMed] [Google Scholar]
- 35•.Zhang J, Lynn DM. Ultrathin Multilayered Films Assembled from ‘Charge-Shifting’ Cationic Polymers: Extended, Long-Term Release of Plasmid DNA from Surfaces. Adv Mater. 2007;19:4218–4223. This paper introduces a new mechanism for the controlled disruption of DNA-containing multilayered films in physiologically relevant media. The concept makes use of non-degradable, charge-shifting cationic polymers and, in the example described in this paper, provides a means of achieving the release of transcriptionally active DNA from surfaces for several months. [Google Scholar]
- 36.Ren KF, Ji J, Shen JC. Construction and enzymatic degradation of multilayered poly-L-lysine/DNA films. Biomaterials. 2006;27:1152–1159. doi: 10.1016/j.biomaterials.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Ren KF, Ji J, Shen JC. Tunable DNA release from cross-linked ultrathin DNA/PLL multilayered films. Bioconjugate Chem. 2006;17:77–83. doi: 10.1021/bc050264g. [DOI] [PubMed] [Google Scholar]
- 38.Zelikin AN, Li Q, Caruso F. Degradable polyelectrolyte capsules filled with oligonucleotide sequences. Angew Chem Int Edit. 2006;45:7743–7745. doi: 10.1002/anie.200602779. [DOI] [PubMed] [Google Scholar]
- 39.Blacklock J, Handa H, Soundara Manickam D, Mao G, Mukhopadhyay A, Oupicky D. Disassembly of layer-by-layer films of plasmid DNA and reducible TAT polypeptide. Biomaterials. 2007;28:117–124. doi: 10.1016/j.biomaterials.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Huang SW, Lin WH, Zhuo RX. Tunable film degradation and sustained release of plasmid DNA from cleavable polycation/plasmid DNA multilayers under reductive conditions. Small. 2007;3:636–643. doi: 10.1002/smll.200600301. [DOI] [PubMed] [Google Scholar]
- 41••.Jessel N, Oulad-Abdeighani M, Meyer F, Lavalle P, Haikel Y, Schaaf P, Voegel JC. Multiple and time-scheduled in situ DNA delivery mediated by beta-cyclodextrin embedded in a polyelectrolyte multilayer P. Natl Acad Sci USA. 2006;103:8618–8621. doi: 10.1073/pnas.0508246103. This paper describes an approach to the layer-by-layer assembly of DNA-containing multilayered thin films that leverages the stepwise nature of the fabrication process to deposit two different plasmid DNA constructs at different depths within films fabricated using enzymatically degradable polyelectrolytes. This approach provides temporal control over the relative order in which two different genes are expressed by cells growing on the surfaces of the films. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood KC, Chuang HF, Batten RD, Lynn DM, Hammond PT. Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films P. Natl Acad Sci USA. 2006;103:10207–10212. doi: 10.1073/pnas.0602884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer F, Ball V, Schaaf P, Voegel JC, Ogier J. Polyplex-embedding in polyelectrolyte multilayers for gene delivery. BBA-Biomembranes. 2006;1758:419–422. doi: 10.1016/j.bbamem.2005.11.015. [DOI] [PubMed] [Google Scholar]