Abstract
PcrA is an essential helicase in gram-positive bacteria, and a gene encoding this helicase has been identified in all such organisms whose genomes have been sequenced so far. The precise role of PcrA that makes it essential for cell growth is not known; however, PcrA does not appear to be necessary for chromosome replication. The pcrA gene was identified in the genome of Bacillus anthracis on the basis of its sequence homology to the corresponding genes of Bacillus subtilis and Staphylococcus aureus, with which it shares 76 and 72% similarity, respectively. The pcrA gene of B. anthracis was isolated by PCR amplification and cloning into Escherichia coli. The PcrA protein was overexpressed with a His6 fusion at its amino-terminal end. The purified His-PcrA protein showed ATPase activity that was stimulated in the presence of single-stranded (ss) DNA (ssDNA). Interestingly, PcrA showed robust 3′→5′ as well as 5′→3′ helicase activities, with substrates containing a duplex region and a 3′ or 5′ ss poly(dT) tail. PcrA also efficiently unwound oligonucleotides containing a duplex region and a 5′ or 3′ ss tail with the potential to form a secondary structure. DNA binding experiments showed that PcrA bound much more efficiently to oligonucleotides containing a duplex region and a 5′ or 3′ ss tail with a potential to form a secondary structure than to those with ssDNAs or duplex DNAs with ss poly(dT) tails. Our results suggest that specialized DNA structures and/or sequences represent natural substrates of PcrA in biochemical processes that are essential for the growth and survival of gram-positive organisms, including B. anthracis.
Bacillus anthracis is a gram-positive, spore-forming bacteria that is the etiological agent of anthrax in humans (reviewed in references 13, 20, and 27). B. anthracis is a potential biological weapon, and an in-depth understanding of the cellular processes that are important for its growth and survival is critical to combat bioterror agents created on the basis of this and related organisms. DNA helicases are required for critical cellular processes such as DNA replication, transcription, recombination, and repair (3, 9, 12, 22-24). Most bacterial species contain several DNA helicases. The DnaB helicase of gram-negative bacteria is necessary for cell survival and is known to be involved in the theta-type replication of the chromosome as well as of several plasmids (6, 9, 26, 33). Gram-positive organisms such as Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, and B. anthracis contain a homolog of the replicative DnaB helicase of Escherichia coli termed DnaC (http://www.tigr.org). It has been shown that DnaC is required for chromosome replication in B. subtilis and S. aureus (5, 28, 29), and it is highly likely that this is also the case for other gram-positive organisms, including B. anthracis.
In addition to DnaC, gram-positive bacteria also contain another helicase, PcrA, which is essential for cell survival in S. aureus and B. subtilis (14, 28). PcrA belongs to superfamily I of DNA helicases that share seven conserved motifs (3, 12). Helicases of this family include the UvrD (helicase II) and Rep helicases of E. coli and UL5 of herpes simplex virus type 1 (3, 12, 24, 36). The chromosome of B. anthracis contains a sequence encoding a putative 747-amino-acid PcrA helicase that shares 62% identity and 76% similarity with the PcrA of B. subtilis and 58% identity and 72% similarity with the PcrA of S. aureus, as revealed by a BLAST search (1, 31) (http://www.tigr.org). The PcrA helicases of gram-positive bacteria also share approximately 40% homology with the UvrD and Rep helicases of E. coli that are involved in DNA repair and rolling-circle (RC) replication of single-stranded (ss) DNA (ssDNA) phages such as M13 and φX174, respectively (4, 8, 12, 22-25). Although likely, the essentiality of PcrA for the viability of B. anthracis and closely related organisms such as B. cereus and Bacillus thuringiensis has not yet been demonstrated.
In B. subtilis and S. aureus, PcrA has been shown to be required for both DNA repair and the replication of small drug resistance plasmids that replicate by an RC mechanism (4, 7, 10, 18, 19, 34). The PcrA helicases of two gram-positive organisms, Bacillus stearothermophilus and S. aureus, have been characterized, and the crystal structure of the B. stearothermophilus PcrA has been determined (7, 34-36, 38). PcrA acts as a monomer, in contrast to the more common replicative helicases which act as hexamers (3, 22-25, 34-36, 38). In this paper, we describe the isolation of the pcrA gene of B. anthracis and the purification and characterization of this helicase. The PcrA protein contained an ATPase activity that was stimulated by ssDNA. Interestingly, PcrA was equally active as a 5′→3′ helicase and a 3′→5′ helicase. Electrophoretic mobility-shift assays showed that the interaction of PcrA with duplex DNA substrates containing an ss region with potential to form a secondary structure was much stronger than that with ssDNA or with duplex substrates with a linear ss region. Our results are consistent with the possibility that PcrA plays a role in the resolution of blocked intermediates in various DNA transactions such as recombination and/or replication, making it an essential helicase required for the growth and viability of gram-positive organisms.
MATERIALS AND METHODS
Isolation of genomic DNA from B. anthracis.
Chromosomal DNA from the B. anthracis strain Sterne was isolated by incubating the cells in a cetyltrimethylammonium bromide solution at 65°C followed by chloroform extraction and isopropanol precipitation as previously described (2).
Cloning of the pcrA gene of B. anthracis.
Preliminary sequence data for the B. anthracis genome were obtained from the website of The Institute for Genomic Research (http://www.tigr.org). The genomic DNA of B. anthracis isolated by the above-described procedure was used as a template for the amplification of the pcrA gene (2.2 kb). The sequences of the primers used were 5′ CCGGATCCACAGATAGGTTATTAAATGGTTTAAACCCGCAACAAC 3′ for the forward primer and 5′ CCGGATCCCGTTTTTTTGCTATCTCTTTTGACATATCCTCATTCC 3′ for the reverse primer. The PCR primers contained BamHI linkers at their ends. The reaction mixtures contained 200 μM of each deoxynucleoside triphosphate, 50 ng of B. anthracis genomic DNA, 1 μM concentrations of each primer, and 5 U of Pfu polymerase (Stratagene, La Jolla, Calif.). The conditions of amplification were as follows: 94°C for 3 min; 94°C for 1 min, 60°C for 1 min, and 72°C for 6 min for 25 cycles; and 72°C for 10 min. The amplified product was gel purified and digested with BamHI. The pcrA gene was then fused in frame to the His6 epitope at the BamHI site of a pQE30 vector from Qiagen. This DNA was expected to encode a PcrA protein with His6 residues fused at its amino-terminal end. The ligation mixture was then introduced into E. coli M15 by electroporation, and the appropriate clones were isolated for protein overexpression.
Preparation of the His-PcrA protein.
The His-PcrA protein of B. anthracis was overexpressed by induction with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C for 2 h and purified by nickel affinity chromatography as described previously for the S. aureus PcrA helicase (7). The concentration of the His-PcrA preparation reached about 0.5 mg/ml in the peak fractions, and the purity was tested by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Coomassie brilliant blue.
ATPase assays.
The ATPase activity of PcrA was measured by hydrolysis of [α-32P]ATP, as described earlier (7). The products of the reaction were subjected to thin-layer chromatography followed by autoradiography, as previously described (7).
DNA helicase assays.
Double-stranded (ds) oligonucleotides containing a 5′ or 3′ ss region at one end were prepared by labeling one strand with 32P at the 5′ end with T4 polynucleotide kinase (32) and annealing the cold complementary strand at a threefold molar excess. Helicase reactions were performed at 37°C for 30 min in a buffer containing 20 mM Tris-HCl (pH 7.5), 100 mM KCl, 3 mM MgCl2, 3 mM ATP, 5 mM dithiothreitol, 10% glycerol, ∼1 ng of the DNA substrates, and the indicated amounts of the PcrA helicase. The reactions were stopped by the addition of SDS dye, and the products were analyzed by 10% native polyacrylamide gel electrophoresis (7). Gels were subsequently dried and exposed to Kodak X-ray films.
DNA binding assays.
The binding of the PcrA helicase to various DNA substrates was studied by electrophoretic mobility-shift assays. Approximately 1 ng of probes labeled at the 5′ end with 32P was incubated with the indicated amounts of PcrA in a reaction buffer consisting of 20 mM Tris-HCl (pH 8.0), 100 mM KCl, 1 mM EDTA, 100 ng of poly(dI-dC), 5 mM dithiothreitol, and 10% ethylene glycol. The reactions were incubated at room temperature for 15 min, and the DNA-protein complexes were resolved by electrophoresis on 6% native polyacrylamide gels. The gels were dried and subjected to autoradiography.
RESULTS
Overexpression and purification of the B. anthracis PcrA helicase.
The pcrA gene of B. anthracis was identified by a homology search of the B. anthracis genome sequence with the pcrA genes of S. aureus, B. stearothermophilus, and B. subtilis. While various PcrA helicases share approximately 60% identity in their amino acid sequences, they are more than 95% identical in the seven conserved motifs found in helicases belonging to superfamily I (Fig. 1). The pcrA gene of B. anthracis includes an open reading frame (ORF) of 747 amino acids. The pcrA ORF contained the ATG sequence at the first and third codons. The pcrA ORF was amplified by PCR such that it included amino acids 4 to 745 and was fused in frame (using the vector pQE30) to His6 residues at its amino-terminal end. The first three amino acids were not included to rule out the possibility of initiation from the ATG codons of pcrA instead of the ATG codon of the His6 epitope. The sequence of the cloned pcrA gene was confirmed by automated DNA sequencing. The His-PcrA fusion protein was purified by nickel affinity chromatography, and protease inhibitors were included throughout the purification procedures. SDS-PAGE analysis (Fig. 2) showed that the full-length protein was approximately 80 to 85% pure. The impurities essentially consisted of breakdown products of PcrA. In spite of the use of protease inhibitors during the purification procedures and differing conditions for IPTG induction of the cloned pcrA gene, the breakdown products persisted.
FIG. 1.
Alignment of PcrA of B. anthracis (Ba), B. stearothermophilus (Bst), B. subtilis (Bs), and S. aureus (Sa) in the seven conserved helicase motifs. Amino acids that differ from those of B. anthracis PcrA are shaded and boxed. Numbers correspond to the amino acid positions of the B. anthracis PcrA.
FIG. 2.
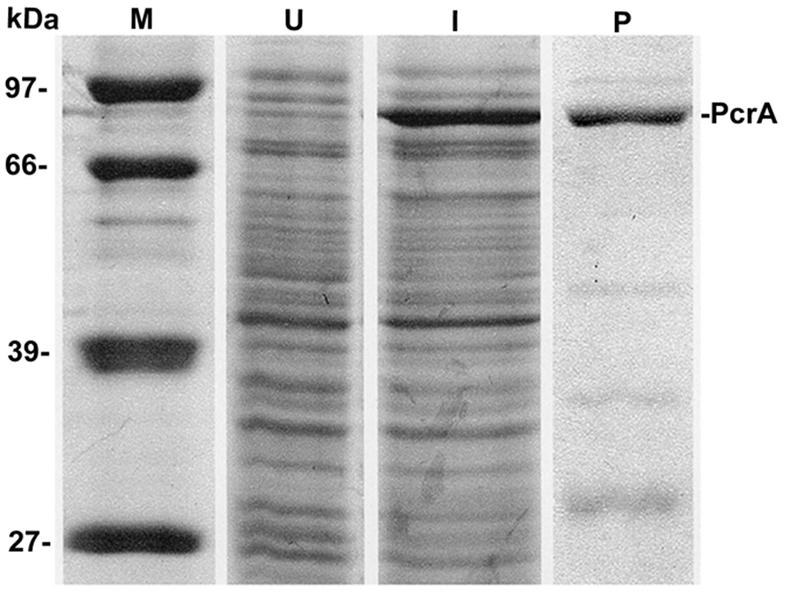
SDS-PAGE analysis of the purified B. anthracis PcrA protein. U, lysates from uninduced cells; I, lysates from IPTG-induced cells overexpressing the His-PcrA protein; P, PcrA protein purified by nickel affinity column chromatography; M, protein molecular-weight standards (in kilodaltons). Smaller fragments likely correspond to breakdown products of PcrA.
ATPase activity of the PcrA helicase.
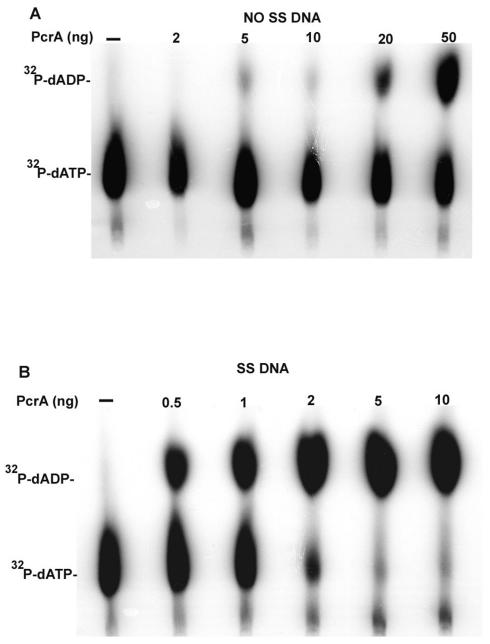
The ATPase activity of PcrA was tested by incubating different amounts of PcrA with α-32P-labeled dATP. Thin-layer chromatography showed that PcrA had a robust level of ATPase activity (Fig. 3A). Furthermore, its ATPase activity was stimulated in the presence of ssDNA (Fig. 3B).
FIG. 3.
ATPase activity of PcrA. (A) Products of [α-32P]dATP hydrolysis in the presence of increasing amounts of PcrA. (B) Stimulation of the dATPase activity of PcrA by a 53mer ss oligonucleotide (ssDNA). The products of [α-32P]dATP hydrolysis were analyzed by thin-layer chromatography.
Helicase activity of PcrA.
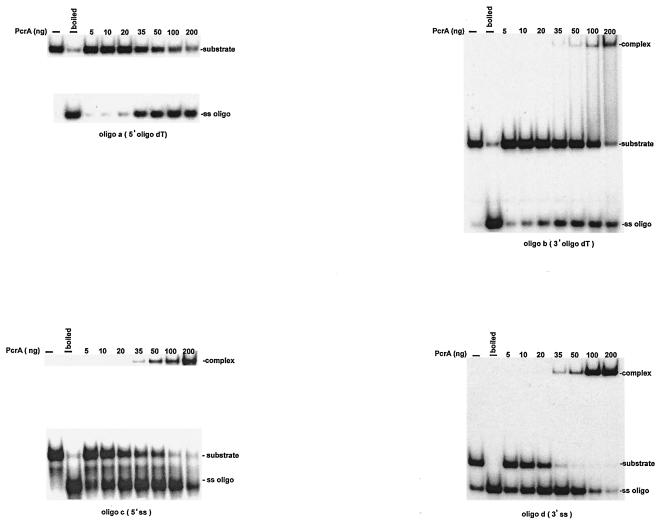
The helicase activity of PcrA was evaluated using partially ds oligonucleotides (Table 1). One set of probes consisted of an 18-bp duplex region with an oligo(dT)20 ss tail at the 5′ (oligonucleotide a) or 3′ (oligonucleotide b) end. Both oligonucleotides a and b were unwound by PcrA to similar extents (Fig. 4), suggesting that the B. anthracis PcrA helicase has both 3′→5′ and 5′→3′ helicase activities. To rule out the possibility that this dual activity was due to a contaminating enzyme in the PcrA preparations, protein lysates from the host E. coli M15 cells lacking the PcrA overexpressing plasmid were fractionated on a nickel affinity resin under conditions identical to those used for the purification of PcrA. Fractions corresponding to the sites at which PcrA eluted were then used in the helicase assays. No 3′→5′ or 5′→3′ helicase activity was detectable at concentrations at which the PcrA preparations showed robust helicase activity (data not shown). We wished to determine whether PcrA is able to unwind oligonucleotides in which the 5′ or 3′ ss region can assume a secondary structure. For this, we utilized oligonucleotide probes containing a 25-bp duplex region and a 28- or 29-nucleotide (nt) ss region at their 5′ or 3′ ends (oligonucleotides c and d) (Table 1). These probes represent the inverted repeat II region from the plasmid pT181 origin in which the sequences present in the 5′ or 3′ ss regions can assume a hairpin structure (16, 17). The underlined regions in oligonucleotides c and d (Table 1) contain 5′CCGG3′ sequences which (upon intrastrand base pairing) generate a cleavage site for the restriction endonuclease HpaII. Probes c and d were found to have been cleaved by HpaII, confirming that the 5′ or 3′ ss regions were present in a folded structure (data not shown). Oligonucleotides c and d are still expected to have a 6- or 7-nt unpaired sequence at their 5′ and 3′ ends, respectively (Table 1). Both oligonucleotide c and oligonucleotide d were efficiently unwound by PcrA (Fig. 4). These results showed that PcrA has both 3′→5′ and 5′→3′ helicase activities and that it can unwind DNA in which the ss region can acquire a secondary structure. At higher PcrA concentrations, an additional slow-migrating complex that presumably corresponds to a PcrA-DNA complex was observed with oligonucleotides b, c, and d (Fig. 5).
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequencea |
|---|---|
| a [5′ oligo(dT) overhang] | 5′ GCCTCGCTGCCGTCGCCA 3′ |
| 3′ CGGAGCGACGGCAGCGGTTTTTTTTTTTTTTTTTTTTT 5′ | |
| b [3′ oligo(dT) overhang] | 5′ GCCTCGCTGCCGTCGCCATTTTTTTTTTTTTTTTTTTT 3′ |
| 3′ CGGAGCGACGGCAGCGGT 5′ | |
| c (5′ overhang) | 5′ CATATGCACACAGTATGTGCGTCCA 3′ |
| 3′ GTATACGTGTGTCATACACGCAGGTTGGCCGATAATCTCATCGGCCAACCTAG 5′ | |
| d (3′ overhang) | 5′ GATCCATATGCACACAGTATGTGCGTCCAACCGGCTATTAGAGTAGCCGGTTGGATCC 3′ |
| 3′ CTAGGTATACGTGTGTCATACACGCAGGT 5′ |
Underlined sequences indicate complementary regions that can pair to generate a hairpin structure. Oligonucleotides c and d are expected to contain six or seven unpaired nucleotides at the 5′ or 3′ end, respectively.
FIG. 4.
Helicase activity of the PcrA protein. 32P-labeled substrates were incubated with the indicated amounts of PcrA, and the products were resolved by native polyacrylamide gel electrophoresis. The probes used are listed in Table 1 and correspond to duplex oligonucleotides (oligo) containing either a 5′ or 3′ ss region. Only one strand of the probe was labeled. “Complex” corresponds to a PcrA-DNA complex.
FIG. 5.
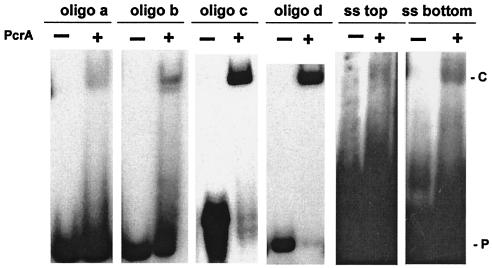
Binding of the PcrA helicase to various DNA substrates. The PcrA helicase (200 ng) was incubated with probes labeled at the 5′ end, and the DNA-protein complexes were resolved by electrophoresis on native 6% polyacrylamide gels. The probes used are indicated. ss top, top strand of oligonucleotide d; ss bottom, bottom strand of oligonucleotide c. P, free probe; C, PcrA-DNA complex.
DNA binding activity of the PcrA helicase.
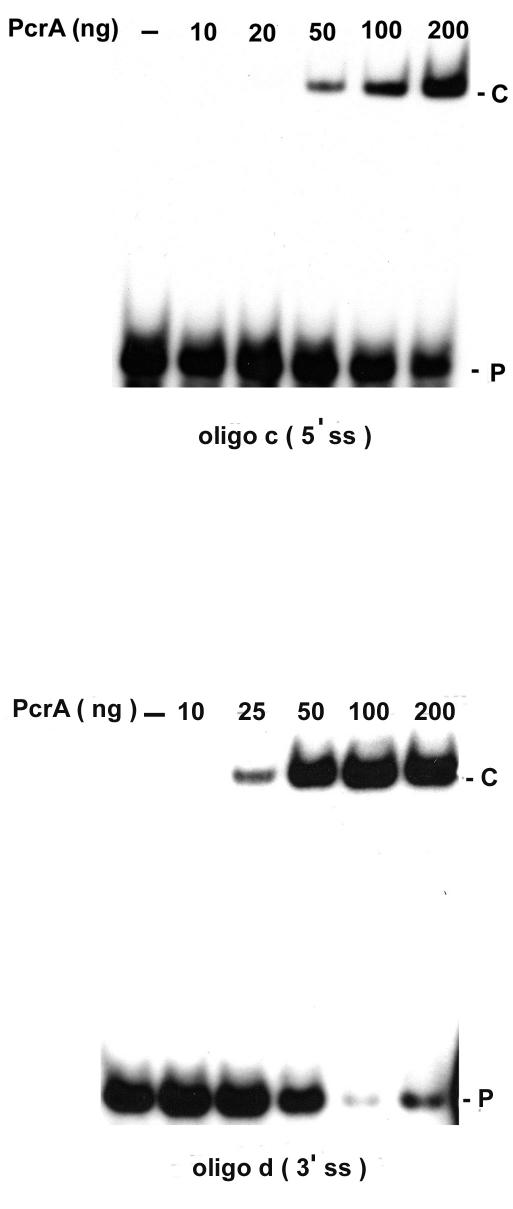
Experiments were carried out to determine whether PcrA can bind stably to the DNA substrates used in the helicase assays. PcrA bound weakly to oligonucleotide a and oligonucleotide b containing a duplex region along with 5′ or 3′ ss oligo(dT) tails (Fig. 5). Interestingly, PcrA showed much stronger binding to ds probes containing 5′ or 3′ ss tails that can potentially fold into hairpin structures (oligonucleotides c and d) (Fig. 5). Surprisingly, the interaction of PcrA with ssDNAs (representing the top and bottom strands of oligonucleotides d and c, respectively) with the potential to form secondary structures was very weak and detectable only upon overexposure of the gel (Fig. 5). These results suggested that PcrA binds most efficiently to DNAs containing ss-ds junctions along with folded ss regions. We further analyzed the binding of PcrA to oligonucleotides c and d representing similar substrates with either a 5′ or 3′ ss tail with the potential for secondary structure. PcrA bound efficiently to both of these probes in a dose-dependent manner, although binding to oligonucleotide d was more robust (Fig. 6). These data showed that dsDNA substrates containing a 5′ or 3′ ss region with a potential for secondary structure along with a short unpaired region at their ends were the preferred substrates for DNA binding by the PcrA helicase.
FIG. 6.
Dose-dependent binding of PcrA to duplex DNA substrates containing 5′ or 3′ ss regions (oligonucleotide [oligo] c or oligonucleotide d, respectively). 32P-labeled oligonucleotides were incubated with the indicated amounts of PcrA, and the products were resolved by polyacrylamide gel electrophoresis. P, free probe; C, PcrA-DNA complex.
DISCUSSION
PcrA helicase is conserved in most gram-positive bacteria and has been shown to be essential for the viability of S. aureus and B. subtilis (14, 15, 29, 30). Although yet to be demonstrated, it is highly likely that pcrA is also an essential gene in B. anthracis and related organisms such as B. cereus and B. thuringiensis. PcrA has been shown to be required for UV DNA repair and the replication of small drug resistance plasmids that replicate by an RC mechanism in gram-positive organisms (4, 7, 10, 18, 19, 34). RCR plasmids (such as pC194 and pE194) from S. aureus can be established in B. anthracis (20). Since these plasmids require PcrA for replication in their native host, it is likely that the heterologous PcrA helicase of B. anthracis can support their replication. Conditional knockouts of pcrA in B. subtilis have been used to show that chromosome replication is affected only slightly in such strains (29). It is unclear what function of PcrA is essential for cell viability given that it is not the major replicative helicase. Recently, suppressor mutants have been identified in B. subtilis that allow cell growth in the absence of the PcrA helicase (30). These suppressors are located in the recF, recO, and recR genes that are involved in promoting recA-dependent homologous recombination (30). These studies suggest that the resolution of blocked recombination structures generated by the RecFOR proteins that can interfere with DNA replication and/or resolution of stalled replication forks is a critical role for the PcrA helicase in gram-positive bacteria (30). In the absence of PcrA, these DNA intermediates may be toxic to the cell.
We have cloned and overexpressed the B. anthracis pcrA helicase gene in E. coli. The amino-terminal end of the PcrA helicase was fused to six histidine residues, and the fusion protein was purified by affinity chromatography. Previous studies with S. aureus PcrA had shown that His-PcrA was biologically active (7). The B. anthracis His-PcrA helicase was used to study its biochemical activities. Our experiments showed that PcrA had a robust ATPase activity that was stimulated in the presence of ssDNA (Fig. 3A and B). The ATPase activity of different helicases is generally stimulated in the presence of ssDNA. This is consistent with the observation that helicases use ss regions as entry points to translocate on the DNA and utilize the energy of ATP hydrolysis for their movement (3, 8, 11, 12).
Our results demonstrate that PcrA can efficiently unwind duplex DNA containing a 3′ or 5′ oligo(dT) ss tail (Fig. 4). PcrA also unwinds duplex substrates containing 5′ or 3′ ss regions that can potentially assume a folded structure (Fig. 4). Thus, B. anthracis PcrA has robust 3′→5′ as well as 5′→3′ helicase activities. The S. aureus PcrA also has both 3′→5′ and 5′→3′ helicase activities, while the PcrA of B. stearothermophilus has a robust 3′→5′ and a weak 5′→3′ helicase activity (7, 34). Generally, most helicases preferentially unwind DNA containing either a 3′ or 5′ ss region (22, 23). Therefore, the PcrA helicases of gram-positive bacteria appear to be unusual in that they are effective both as 3′→5′ and 5′→3′ helicases. Interestingly, both 3′→5′ and 5′→3′ helicases belonging to superfamily I share the seven conserved motifs shown in Fig. 1 (3, 12, 36). Our results suggest that the B. anthracis and other PcrA helicases function in multiple DNA transactions that require either of these two activities (see below).
Interestingly, our DNA binding studies demonstrate that PcrA interacts weakly with ssDNA and duplex DNAs containing ss oligo(dT) tails at the 5′ or 3′ end (Fig. 5). On the other hand, PcrA bound with high affinity to dsDNAs containing 5′ or 3′ ss tails with a potential to form a secondary structure (Fig. 5 and 6). Most DNA helicases are known to require ss regions for loading onto the DNA and therefore bind to ssDNAs efficiently (3, 9, 12, 22-25). The B. stearothermophilus PcrA binds with greater affinity to ssDNA than to dsDNA (34-36, 38). Our results showed that the interaction of B. anthracis PcrA with oligonucleotides c and d containing ssDNA-dsDNA junctions is much stronger than that with ssDNA representing the top or bottom strand of these oligonucleotides (Fig. 5). Since PcrA is essential for cell growth and viability, one or more of its biochemical activities must be required for an essential cellular process. Our observations with B. anthracis PcrA suggest that DNAs with certain structural or sequence features represent its natural substrates in vivo. Such structures might be generated during DNA recombination and replication processes (9, 22-24, 28).
Two recent reports have shown that the Srs2 helicase of S. cerevisiae, which has limited homology with PcrA, can inhibit DNA strand exchange mediated by the Rad51 protein (a homolog of RecA) (21, 37). Srs2 was also shown to disrupt Rad51 filaments formed on ssDNA (21, 37). Our results are consistent with the possibility that PcrA plays a similar role in the resolution of blocked recombination intermediates generated by the RecFOR proteins of the RecA pathway that may interfere with DNA replication. In addition, PcrA may also inhibit strand exchange catalyzed by the RecA protein or be required for the resolution of replication forks stalled at different regions of the chromosome. It is possible that the functions of PcrA in the above-described biochemical pathways and in UV repair and plasmid RC replication involve the recognition of structurally different substrates and specifically require either its 5′→3′ or its 3′→5′ helicase activity. Future studies should reveal the specific cellular pathways that are dependent upon the function of this essential helicase.
The various biochemical activities of PcrA may represent important new targets for the future development of drugs to combat B. anthracis and other gram-positive pathogens. The crystal structure of the B. stearothermophilus PcrA has been determined, and future studies on the structure-function relationship of the PcrA helicases could be important for rational drug design. Furthermore, several assays that may be amenable to high-throughput analysis could be utilized for PcrA, including ATPase and helicase assays with fluorescently labeled nucleotides.
Acknowledgments
We thank members of our laboratory for helpful discussions. We also thank Phil Hanna for providing the B. anthracis Sterne strain.
This work was supported in part by grants GM31685 and AI055929 from the National Institutes of Health. Sequencing of the B. anthracis genome at TIGR was accomplished with support from NIH, ONR, DOE, and DERA.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, and K. Struhl. 1988. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bird, L. E., S. Subramanya, and D. B. Wigley. 1998. Helicases: a unifying structural theme? Curr. Opin. Struct. Biol. 8:14-18. [DOI] [PubMed] [Google Scholar]
- 4.Bruand, C., and S. D. Ehrlich. 2000. UvrD-dependent replication of rolling-circle plasmids in Escherichia coli. Mol. Microbiol. 35:204-210. [DOI] [PubMed] [Google Scholar]
- 5.Bruck, I., and M. O'Donnell. 2000. The DNA replication machine of a gram-positive organism. J. Biol. Chem. 275:28971-28983. [DOI] [PubMed] [Google Scholar]
- 6.Carr, K. M., and J. M. Kaguni. 2001. Stoichiometry of DnaA and DnaB protein in initiation at the Escherichia coli chromosomal origin. J. Biol. Chem. 276:44919-44925. [DOI] [PubMed] [Google Scholar]
- 7.Chang, T.-L, A. Naqvi, S. P. Anand, M. G. Kramer, R. Munshi, and S. A. Khan. 2002. Biochemical characterization of the Staphylococcus aureus PcrA helicase and its role in plasmid rolling-circle replication. J. Biol. Chem. 277:45880-45886. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, W., J. Hsieh, K. M. Brendza, and T. M. Lohman. 2001. E. coli Rep oligomers are required to initiate DNA unwinding in vitro. J. Mol. Biol. 310:327-350. [DOI] [PubMed] [Google Scholar]
- 9.Delagoutte, E., and P. H. von Hippel. 2003. Helicase mechanisms and coupling of helicases within macromolecular machines. Part II: integration of helicases into cellular processes. Q. Rev. Biophys. 36:1-69. [DOI] [PubMed] [Google Scholar]
- 10.del Solar, G., R. Giraldo, M. J. Ruiz-Echevarria, M. Espinosa, and R. Diaz-Orejas. 1998. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 62:434-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillingham, M. S., D. B. Wigley, and M. R. Webb. 2000. Demonstration of single-stranded DNA translocation by PcrA helicase: measurement of step size and translocation speed. Biochemistry 39:205-212. [DOI] [PubMed] [Google Scholar]
- 12.Hall, M. C., and S. W. Matson. 1999. Helicase motifs: the engine that powers DNA unwinding. Mol. Microbiol. 34:867-877. [DOI] [PubMed] [Google Scholar]
- 13.Hanna, P. 1998. Anthrax pathogenesis and host response. Curr. Top. Microbiol. Immunol. 225:13-35. [DOI] [PubMed] [Google Scholar]
- 14.Iordanescu, S. 1993. Characterization of the Staphylococcus aureus chromosomal gene pcrA, identified by mutations affecting plasmid pT181 replication. Mol. Gen. Genet. 241:185-192. [DOI] [PubMed] [Google Scholar]
- 15.Iordanescu, S. 1993. Plasmid pT181-linked suppressors of the Staphylococcus aureus pcrA3 chromosomal mutation. J. Bacteriol. 175:3916-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin, R., M.-E. Fernandez-Beros, and R. P. Novick. 1997. Why is the initiation site of an AT-rich rolling circle plasmid at the tip of a GC-rich cruciform? EMBO J. 16:4456-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin, R., and R. P. Novick. 2001. Role of the double-strand origin cruciform in pT181 replication. Plasmid 46:95-105. [DOI] [PubMed] [Google Scholar]
- 18.Khan, S. A. 1997. Rolling-circle replication of bacterial plasmids. Microbiol. Mol. Biol. Rev. 61:442-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan, S. A. 2000. Plasmid rolling-circle replication: recent developments. Mol. Microbiol. 37:477-484. [DOI] [PubMed] [Google Scholar]
- 20.Koehler, T. M. 2002. Bacillus anthracis genetics and virulence gene regulation. Curr. Top. Microbiol. Immunol. 271:143-164. [DOI] [PubMed] [Google Scholar]
- 21.Krejci, L., S. V. Komen, Y. Li, J. Villemain, M. Sreedhar, H. Klein, T. Ellenberger, and P. Sung. 2003. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature 423:305-309. [DOI] [PubMed] [Google Scholar]
- 22.Lohman, T. M. 1993. Helicase-catalyzed DNA unwinding. J. Biol. Chem. 268:2269-2272. [PubMed] [Google Scholar]
- 23.Lohman, T. M., and K. P. Bjornson. 1996. Mechanisms of helicase-catalyzed DNA unwinding. Annu. Rev. Biochem. 65:169-214. [DOI] [PubMed] [Google Scholar]
- 24.Marians, K. J. 2000. Crawling and wiggling on DNA: structural insights to the mechanism of DNA unwinding by helicases. Structure 8:R227-R235. [DOI] [PubMed] [Google Scholar]
- 25.Mechanic, L. E., M. C. Hall, and S. W. Matson. 1999. Escherichia coli DNA helicase II is active as a monomer. J. Biol. Chem. 30:12488-12498. [DOI] [PubMed] [Google Scholar]
- 26.Messer, W., F. Blaesing, D. Jakimowicz, M. Krause, J. Majka, J. Nardmann, S. Schaper, H. Seitz, C. Speck, C. Weigel, G. Wegrzyn, M. Welzeck, and J. Zakrzewska-Czerwinska. 2001. Bacterial replication initiator DnaA. Rules for DnaA binding and roles of DnaA in origin unwinding and helicase loading. Biochimie 83:5-12. [DOI] [PubMed] [Google Scholar]
- 27.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 28.Moriya, S., Y. Imai, A. K. Hassan, and N. Ogasawara. 1999. Regulation of initiation of Bacillus subtilis chromosome replication. Plasmid 41:17-29. [DOI] [PubMed] [Google Scholar]
- 29.Petit, M. A., E. Dervyn, M. Rose, K. D. Entian, S. McGovern, S. D. Ehrlich, and C. Bruand. 1998. PcrA is an essential DNA helicase of Bacillus subtilis fulfilling functions both in repair and rolling-circle replication. Mol. Microbiol. 29:261-273. [DOI] [PubMed] [Google Scholar]
- 30.Petit, M. A., and S. D. Ehrlich. 2002. Essential bacterial helicases that counteract the toxicity of recombination proteins. EMBO J. 21:3137-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Read, T. D., S. L. Salzberg, M. Pop, M. Shumway, L. Umayam, L. Jiang, E. Holtzapple, J. D. Busch, K. L. Smith, J. M. Schupp, D. Solomon, P. Keim, and C. M. Fraser. 2002. Comparative genome sequencing for discovery of novel polymorphisms in Bacillus anthracis. Science 296:2028-2033. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Seitz, H., C. Weige, and W. Messer. 2000. The interaction domains of the DnaA and DnaB replication proteins of Escherichia coli. Mol. Microbiol. 37:1270-1279. [DOI] [PubMed] [Google Scholar]
- 34.Soultanas, P., M. S. Dillingham, F. Papadapoulos, S. E. Philips, C. D. Thomas, and D. B. Wigley. 1999. Plasmid replication initiator protein RepD increases the processivity of PcrA DNA helicase. Nucleic Acids Res. 27:1421-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soultanas, P., M. S. Dillingham, P. Wiley, M. R. Webb, and D. B. Wigley. 2000. Uncoupling DNA translocation and helicase activity in PcrA: direct evidence for an active mechanism. EMBO J. 19:3799-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soultanas, P., and D. B. Wigley. 2001. Unwinding the “Gordian knot” of helicase action. Trends Biochem. Sci. 26:47-54. [DOI] [PubMed] [Google Scholar]
- 37.Veaute, X., J. Jeusset, C. Soustelle, S. C. Kowalczykowski, E. L. Cam, and F. Fabre. 2003. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 423:309-312. [DOI] [PubMed] [Google Scholar]
- 38.Velankar, S. S., P. Soultanas, M. S. Dillingham, H. S. Subramanya, and D. B. Wigley. 1999. Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell 97:75-84. [DOI] [PubMed] [Google Scholar]