Abstract
Dicer1, an essential component of RNA interference and the microRNA pathway, has many important roles in the morphogenesis of developing tissues. Dicer1 null mice have been reported to die at E7.5; therefore it is impossible to study its function in adult tissues. We previously reported that Dicer1-hypomorphic mice, whose Dicer1 expression was reduced to 20% in all tissues, were unexpectedly viable. Here we analyzed these mice to ascertain whether the down-regulation of Dicer1 expression has any influence on adult tissues. Interestingly, all tissues of adult (8–10 week old) Dicer1-hypomorphic mice were histologically normal except for the pancreas, whose development was normal at the fetal and neonatal stages; however, morphologic abnormalities in Dicer1-hypomorphic mice were detected after 4 weeks of age. This suggested that Dicer1 is important for maintaining the adult pancreas.
Introduction
MicroRNA (miRNA) is small (∼22 nucleotides), non-coding RNA. Mature miRNA transcribed as long primary transcripts is processed to pre-miRNA in the nucleus by Drosha/DGC8 [1], and then processed in the cytoplasm by Dicer [2]. MiRNA is further incorporated into the RNA-inducing silencing complex (RISC), which includes Argonaute [3] to regulate gene expression via post-transcriptional repression. Over the past few years, more than 400 miRNAs have been identified, but their function is largely unknown. Several miRNAs exhibit tissue-specific or developmental stage-specific expression [4], [5], indicating that they have important roles in many biological processes.
Dicer1 encodes an RNaseIII endonuclease, a key enzyme that processes miRNA. It is broadly expressed in developing tissues, and several mutant alleles of Dicer1 have been generated in mice. Dicer1 seems to be critical in early development since loss of its function was lethal at embryonic day 7.5 [6]. Characterization of Dicer1 hypomorphic mice showed that the gene is required for embryonic angiogenesis [7]. Conditional inactivation of Dicer1 in the mouse limb bud mesenchyme [8], lung epithelium [9], epidermal hair follicle [10], and pancreas [11], T cell development and differentiation [12] led to the conclusion that Dicer1, which processes miRNA, is indispensable for the development and morphogenesis of these tissues.
We previously generated Dicer1-hypomorphic mice (homozygous Dicer1−/− mice) [13]. Complete loss of Dicer1 in mice results in early embryonic death [6]; however, our Dicer1-hypomorphic mice were viable [13]. To study the function of Dicer1 in the maintenance of homeostasis in adult tissues, we analyzed the adult tissues histologically and found abnormalities only in the pancreas. The phenotypes detected in the pancreas of Dicer1-hypomorphic mice might resemble the differentiation of endocrine precursor cells in adult pancreas.
The pancreas consists of three main tissue cell types: the endocrine cells (islet of Langerhans) which produce hormones such as insulin and glucagon; the exocrine acinar tissues which secrete digestive enzymes; and the branched duct. Numerous mechanisms that control the differentiation of endocrine and exocrine cells in the embryonic pancreas have been revealed [14], but how endocrine cells (especially insulin-producing β cells) are maintained in postnatal life has been controversial [15]. At E9.5, the endocrine cells of the pancreas arise from endocrine precursor cells, which express both glucagon and insulin and divide into distinct lineages such as glucagon or insulin-expressing cells. On the other hand, in the adult pancreas, it had been considered that there are no endocrine progenitor cells and that β cells are generated only by the replication of existing β cells, not from the differentiation of endocrine precursor cells (neogenesis) [16], [17]. However, several studies suggested that β cell differentiation from endocrine precursor cells can occur in adults in the regenerating pancreas after a partial pancreatectomy or duct ligation [18], [19], [20], [21]. In the regenerating pancreas, vigorous expansion of the β cell population was observed, and partial pancreatectomy and duct ligation has been a good model for regenerating endocrine cells. The phenotypes observed in Dicer1-hypomorphic mice suggested that Dicer1 regulates the endocrinal neogenesis in the adult pancreas. Previous study showed that Dicer1 is indispensable for normal development of the pancreas [11]; however, its function in the adult pancreas had not been elucidated. Here we report that Dicer1 also has important functions in the adult pancreas.
Results
Dicer1 expression was significantly reduced in all tissues of Dicer1-hypomorphic mice but histological abnormalities were only found in the pancreas
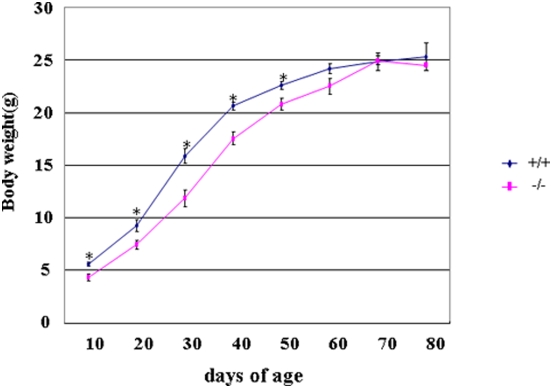
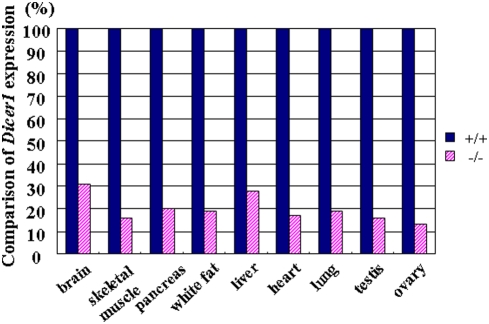
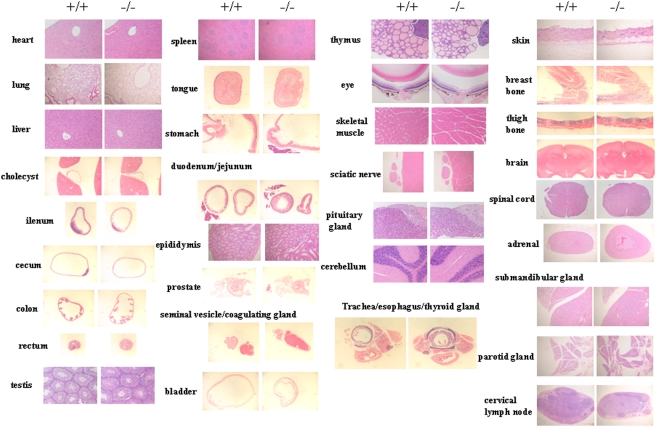
Dicer1-hypomophic mice (homozygous Dicer1−/− mice) showed a lower birth rate than expected by Mendelian rules [13]; however, they did not differ from their wild-type littermates in overall health. Although they showed slight growth retardation from 10 to 50 days of age, their body weight was similar to that of wild-type mice after 50 days of age (Fig. 1). A comparison of Dicer1 expression in nine tissues of adult mice revealed a 70–85% reduction in the hypomorphic mice (Fig. 2). Although we analyzed more than 40 tissues (Table 1), histological examination revealed no abnormalities in any tissues except the pancreas (Fig. 3); thus we focused on the pancreas of Dicer1-hypomorphic mice.
Figure 1. Body weight growth curves.
Male wild-type (+/+) and Dicer1-hypomorphic (−/−) mice were measured to determine the change in body weight from 10 to 80 days of age. *, P<0.05. n = 8–10 per group.
Figure 2. Comparison of Dicer1 expression in nine tissues between wild-type (+/+) and Dicer1-hypomorphic (−/−) mice.
The expression in the Dicer1-hypomorphic mice was normalized to that in the wild-type mice.
Table 1. The list of tissues with histological analysis (H&E assessment).
| lung | testis | eye boll | trachea |
| heart | epididymis | harderian gland | esophagus |
| kidney | prostate | skeletal muscle | thyroid gland |
| pancreas | seminal vesicle | sciatic nerve | liver |
| tongue | coagulating gland | skin | cholecyst |
| stomach | bladder | breast bone | spleen |
| duodenum | adrenal | femur | |
| jejunum | pituitary gland | cerebrum | |
| ileum | submandibular gland | hippocampus | |
| cecum | parotid gland | thalamus | |
| colon | thymus | cerebellum | |
| rectum | cervical lymph node | spinal cord |
Histological analysis of these tissues was performed in wild-type (+/+) (n = 2) and Dicer1-hypomorphic (−/−) mice (n = 4).
Figure 3. H&E-stained section of adult tissues of wild-type (+/+) (n = 2) and Dicer1-hypomorphic (−/−) mice (n = 4).
Dicer1 could be involved in differentiation of endocrine cells in adult pancreas
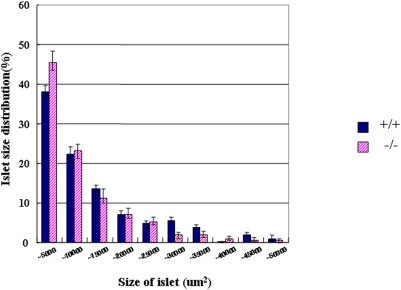
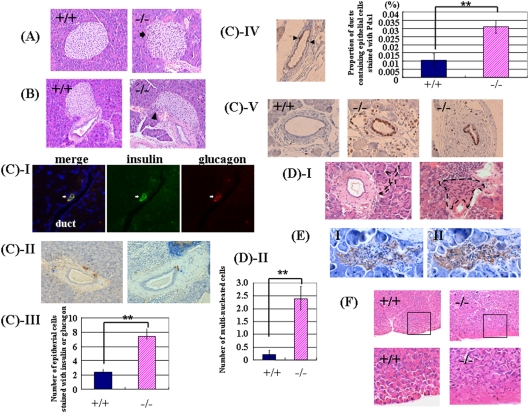
In Dicer1-hypomorphic mice, the size of the pancreas in adults (8–10 weeks of age) was nearly identical to that in the wild-type mice; however, there were more small islets (Fig. 4). In some of these islets, the distribution of islet cells and staining of nuclei were irregular (Fig. 5A). The boundary of islets and ducts was not clearly defined in the pancreas (Fig. 5B). Immunohistochemical analysis revealed mostly normal staining of insulin and glucagon at 8–10 weeks of age; however, the number of ductal epithelial cells stained with insulin or glucagon was significantly increased (Fig. 5C-III, P = 0.0051). In some models of pancreatic regeneration including partial pancreatectomy, insulin or glucagon-stained cells are present in the ductal epithelium, which had led to the idea that some endocrine cells differentiate in the ducts [18], [19], [20], [21]. Our observations in Dicer1-hypomorphic mice suggest that regeneration from the endocrine precursor cells took place in adulthood. Next we conducted a histological examination of the markers Pdx-1 and Ki67. The population of ducts containing Pdx-1-positive cells was significantly increased (Fig. 5C-IV, P = 0.009). Pdx1-positive cells in the ducts are possibly the adult progenitor cells [22], [23], and PDX-1 protein was detected in the pancreatic duct in adult rats after partial pancreatechtomy [21]. Surprisingly, abnormal staining of Ki67, which is a marker for proliferation of the cells, was detected in the pancreatic ducts in two of six Dicer1-hypomorphic mice (Fig. 5C-V). In some Ki-67-positive ducts, all the epithelial cells were stained. No such observations were found in wild-type mice.
Figure 4. Comparison of the size of islets in wild-type and Dicer1-hypomorphic mice.
The islets mass was measured in wild-type (blue bar) and Dicer1-hypomorphic mice (pink bar). The numbers of islets were examined in wild-type (n = 6) and Dicer1-hypomorphic (n = 6) mice, with six sections from each animal. The graph shows the percentage of islets in each size category.
Figure 5. Pancreas morphology in adult (8–10 weeks of age) Dicer1-hypomorphic mice.
A, B: Hematoxylin-eosin (H & E)-stained islets of the pancreas from an 8-week-old wild-type (+/+) mouse and Dicer1-hypomorphic (−/−) mice (*400). A: Arrows indicate an irregular distribution of islet cells. B: Arrowheads indicate that the boundary of islets and ducts was not clearly defined in the pancreas of Dicer1-hypomorphic mice. C: Immunohistochemistry of duct cells of Dicer1-hypomorphic mice. (I) Insulin (green) and glucagon (red) double-expressing cells were detected in the duct. (II) Insulin-positive cells (brown) and glucagon-positive cells (blue) were observed in the duct. (III) Comparison of the number of epithelial cells stained with both insulin and glucagon, only insulin, and only glucagon in wild-type and Dicer1-hypomorphic mice. These numbers were averaged from 6 animals, with six sections from each animal. **, P<0.01. (IV) Comparison of the proportion of ducts containing epithelial cells stained with Pdx1 in wild-type and Dicer1-hypomorphic mice. Arrowheads indicate the Pdx1-positive cells. *, P<0.01. (V) Abnormal staining of Ki67 was observed in the pancreas of Dicer1-hypomorphic mice. D: (I) H & E-stained multinuclear atypical cells in the pancreas of Dicer1-hypomorphic mice. The black dotted line indicates atypical multinuclear cells. (II) Comparison of the number of multi-nucleated cells in wild-type and Dicer1-hypomorphic mice. These numbers were averaged from 6 animals, with six sections from each animal. **, P<0.01. E: Immunohistochemistry of multinuclear atypical cells of adjacent sections of the pancreas of Dicer1-hypomorphic mice using anti-insulin (I) and anti-glucagon (II) antibodies. F: H & E-stained acinar cells. The rectangular areas outlined in the upper panels are magnified in the lower panels. An abnormal structure of exocrine cells was observed in the pancreas of Dicer1-hypomorphic mice.
Interestingly, cells morphologically different from either acinar or islet cells were observed in Dicer1-hypomorphic mice (Fig. 5D-I). Under a light microscope, some appeared to be syncytial multi-nucleated cells near the pancreatic duct and in acini. Numerous nuclei were distributed irregularly and were often clustered in the cells, which were all double-positive for insulin and glucagon (Fig. 5E). Cells double-positive for insulin and glucagon were significantly increased in Dicer1-hypomorphic mice compared to wild-type mice (Fig. 5D-II, P = 0.0019). In the exocrine portion of the pancreas of Dicer1-hypomorphic mice, most acini were morphologically normal, but some showed an irregular morphology (Fig. 5F). The shapes and position of the cells were irregular and the acinar structure was not organized. In normal acinar cells, zymogen granules are observed in the center of the acinus and the nucleus is located at its periphery.
We next investigated when the abnormal morphology appeared in the development of the pancreas in Dicer1-hypomorphic mice. For this purpose, a histological analysis was performed using E15.5 embryos, P1 mice, and 4-week-old mice. The pancreas of both wild-type and Dicer1-hypomorphic mice developed normally and endocrine and exocrine cells appeared morphologically normal at E15.5 and P1 (Fig. 6A, B); the same abnormalities observed in adult Dicer1-hypomorphic mice were detectable at 4 weeks of ages (Fig. 6C), although the number of abnormal cells was less than that found in adult Dicer1-hypomorphic mice. This suggested that the pancreas of Dicer1-hypomorphic mice developed normally after birth and abnormal cells appeared at around 4 weeks after birth, increasing with age.
Figure 6. Histological and immunohistochemical analysis of the pancreas at E15.5 (A) and P1 (B) of wild-type and Dicer1-hypomorphic mice.
Dicer1-hypomorphic mice show normal insulin (brown) and glucagon (blue) staining at E15.5 and P1. C: Histological abnormalities found in the pancreas of 4-week-old Dicer1-hypomorphic mice. (I) The endocrinal distribution was slightly irregular. The dotted line indicates the abnormal region of the islet. (II) Multi-nucleated cells were observed. The dotted line indicates multi-nucleated cells, which were also found in the pancreas of adult Dicer1-hypomorphic mice.
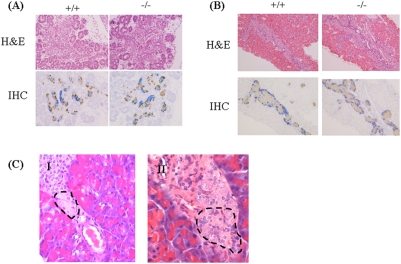
Surprisingly, the observations found in Dicer1-hypomorphic mice were quite similar to the histological findings in transgenic mice expressing a truncated type II activin receptor [24]. Therefore, we next investigated the expression of the activin type II receptor in the pancreas of Dicer1-hypomorphic mice. Two related receptors, ActRIIA and ActRIIB, were initially identified as type II receptors for activin [25], [26]. ActRIIA and ActRIIB have been reported to bind not only to activin [27], but also to other TGF-β family proteins, including BMP7 [28], GDF8 [29], Nodal [30], and GDF11 [31]. The precise role of the two activin receptors is still not clear. Real-time PCR analysis revealed that the expression of ActRIIA was slightly up-regulated in Dicer1-hypomorphic mice compared to wild-type mice, while the expression of ActRIIB did not differ (Fig. 7). Therefore, the abnormal morphology might be attributed to another signaling cascade.
Figure 7. Analysis of ActRIIA and ActRIIB expression.
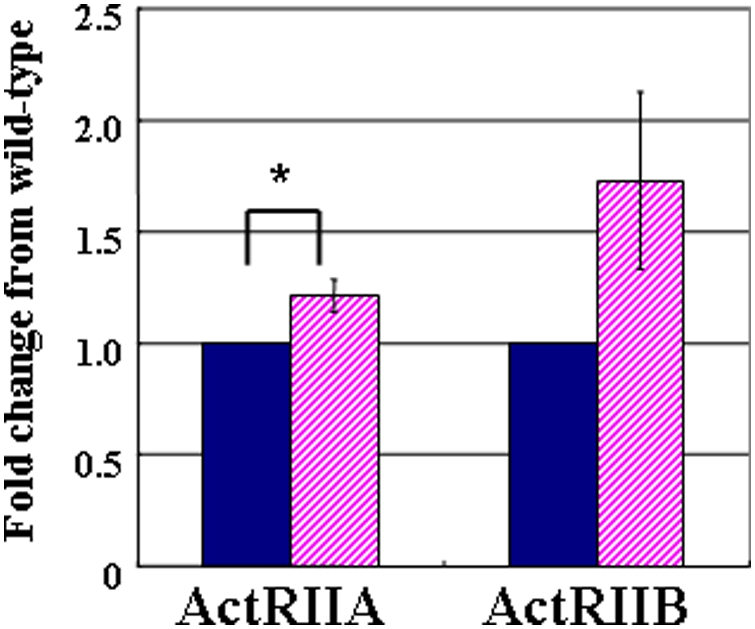
Data are expressed relative (n-fold) to the wild-type pancreas and correspond to the means and standard errors for three independent experiments performed in triplicate. *, P<0.05. wild-type n = 9, Dicer1-hypomorphic mice n = 9.
Detection of differential expressed miRNAs by microarray analysis
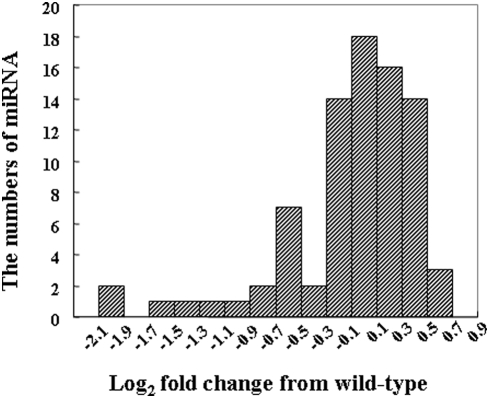
Because Dicer1 is required for the processing of miRNAs, the reduction of Dicer1 results in a decrease in miRNAs. To determine the differential expression of miRNAs in the pancreas of adult wild-type and Dicer1-hypomorphic mice, a miRNA microarray analysis was performed. The miRNAs of the pancreas of two wild-type and two Dicer1-hypomorphic mice were analyzed. Signals were very weak on hybridization with miRNA in the pancreas compared to other tissues; therefore a total of 83 miRNAs, which showed strong signals, were analyzed. Fig. 8 shows the change in the distribution of miRNA levels in Dicer1-hypomorphic mice compared to wild-type mice. Surprisingly, miRNA expression did not dramatically change in Dicer1-hypomorphic mice compared to the wild-type animals: however, 7% of miRNAs were down-regulated less than 0.5 fold. These miRNAs might function in maintaining the adult pancreas, but at present their relationship with the abnormal phenotype in Dicer1-hypomorphic mice is unclear. Why was only 7% of the miRNA expressed in pancreas attenuated? Dicer1 protein might catalyze processing of pre-miRNA differently dependent on the sequence when generating miRNA. The down-regulated miRNAs might be more difficult to process than the other miRNAs and thus significantly reduced Dicer1 expression might affect their generation.
Figure 8. The distribution of changes in miRNA levels in Dicer1-hypomorphic mice compared to wild-type mice.
Glucose metabolism was normal in Dicer1-hypomorphic mice
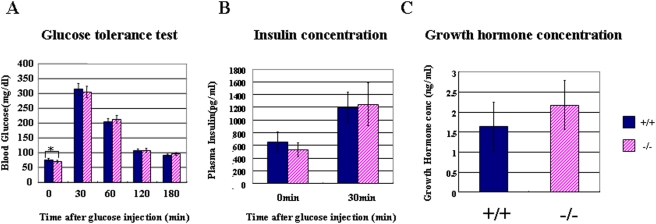
The hypo-expression of Dicer1 leads to abnormal endocrine cells, which might affect the function of the pancreas; therefore, we next investigated the metabolism of glucose in Dicer1-hypomorphic mice. Despite histological abnormalities in pancreatic islets, a glucose tolerance test showed that Dicer1-hypomorphic mice were able to clear glucose from the blood as efficiently as wild-type mice (Fig. 9A), and had insulin levels similar to wild-type mice (Fig. 9B). Despite no significant differences in the insulin content of serum after overnight fasting, Dicer1-hypomorphic mice showed a slightly reduced blood glucose level on fasting. Dicer1-hypomorphic mice were smaller than the wild-type mice before 50 days of age; therefore, we hypothesized that the growth hormone level affects fasting hypoglycemia. We checked the blood growth hormone level; however, we found no significant differences with wild-type mice (Fig. 9C). Thus, the growth hormone level is not responsible for fasting hypoglycemia in Dicer1-hypomorphic mice, probably due to an unknown mechanism involved in glucose metabolism in other tissues.
Figure 9. Glucose metabolism and growth hormone levels in wild-type and Dicer1-hypomorphic mice.
A: Glucose tolerance test. Fasted 8-week-old mice received an intraperitoneal injection of glucose (2 mg/g body weight). B: Insulin concentration. Plasma insulin was measured before and after the intraperitoneal glucose injection. C: Growth hormone concentration. Plasma growth hormone concentrations were measured in wild-type and Dicer1-hypomorphic mice. A, B: Values are expressed as the means±S.D. (n = 20 per group). C: Values are expressed as the means±S.D. (n = 10 per group).*, P<0.05.
Discussion
Dicer, the enzyme that generates miRNAs, has been reported to have quite important roles in a variety of developmental processes. In our Dicer1-hypomorphic mice, histological analysis (H&E assessment) showed that the abnormalities were found only in the pancreas and not in other tissues. However, we could not exclude the possibility that there are more minute structural abnormalities not detected with the H&E assessment, or functional abnormlities. In this study, we focused on the pancreas. The pancreatic-specific knockout of Dicer1 clarified that miRNA is required for the development of the pancreas in embryogenesis [11]. Our study suggested that Dicer1 also has important functions in maintaining the adult pancreas.
Histological abnormalities such as the irregular distribution of islet cells, and deviations from the typical structure of the acinus, were found in endocrine and exocrine cells in the adult pancreas of Dicer1-hypomorphic mice, although none of these abnormalities were detected before 4 weeks of age. Dicer1 is indispensable for normal pancreatic cell differentiation at embryogenesis [11]. The pancreas-specific Dicer1 knockout mice survived until birth but died before P3. These mice showed gross defects in all pancreatic lineages, and the formation of exocrine cells and duct cells, especially endocrine cells, was greatly impaired. Given that miRNA most probably plays essential roles in the morphogenesis of many tissues in a developing embryo [7], [8], [9], [10], [12], the gross defects in all pancreatic lineages observed upon removal of Dicer1 are not surprising. These mice died soon after birth; therefore, it is impossible to study the role Dicer may play in adult tissues.
The pancreas of our Dicer1-hypomorphic mice developed normally and the reduced expression of Dicer1 did not affect the development of the pancreas during embryogenesis or the neonatal stage. However, aberrant endocrine and exocrine cells could be detected after 4 weeks of age, and the number of abnormal regions seemed to increase with age. It is interesting that the developing pancreas during embryogenesis and the adult pancreas differ in sensitivity to the Dicer1 level. In other words, the reduction in Dicer1 only affects the maintaining of adult pancreas, not the normal development of the pancreas.
In addition, these observations, such as the increasing number of ductal epithelial cells stained positive for insulin, glucagon, and Pdx-1 in Dicer1-hypomorphic mice (Fig. 5D-III, IV), were also found in the regenerating pancreas [18], [19], [20], [21], suggesting that the differentiation of endocrine precursor cells (neogenesis) occurred in the adult pancreas. Moreover, a quite intriguing observation was the existence of unknown abnormal multi-nucleated cells in the pancreas that expressed both glucagon and insulin. In the developing pancreas, endocrine precursor cells first appeared at E9, and these cells expressed both glucagon and insulin [32], [33], then differentiated into insulin-producing cells or glucagon-producing cells. These cells in our adult Dicer1-hypomorphic mice resembled endocrine precursor cells in the fetal pancreas in terms of the expression of both glucagon and insulin. Therefore, Dicer1 might have roles in regulating endocrine precursor cells in the adult pancreas.
The proliferation of duct cells is increased in the regenerating pancreas compared to the normal adult pancreas [20]. However, in some Dicer1-hypomorphic mice, abnormal proliferation of duct epithelial cells was observed (Fig. 5D-V). These features were not detected in wild-type mice and could be caused by the reduction in Dicer1.
Surprisingly, these histological observations in Dicer1-hypomorphic mice were quite similar to the histological findings in transgenic mice expressing the truncated type II activin receptor [24]. However, as our Dicer1-hypomorphic mice showed only a 1.2-fold increase in ActRIIA in the pancreas, ActRIIA might not cause the abnormal phenotype.
Because Dicer1 has a key role in generating a large number of miRNAs, its removal results in a significant decrease in miRNAs. In the pancreas of our Dicer1-hypomorphic mice, the expression levels of miRNA changed slightly compared to wild-type level which is why the mice could survive. A complete loss of Dicer in mice results in early embryonic death. A large number of genes control the development or maintenance of the pancreas, and these genes might be a potential target of miRNAs. Even a slight change in miRNA expression might affect the gene expression, leading to the abnormal morphology in Dicer1-hypomorphic mice. Further exploration is necessary to investigate the relation between these genes and miRNA in the pancreas.
Our results suggest that Dicer1 functions in the adult pancreas and also raise the possibility that Dicer1 regulates the differentiation of endocrine precursor cells there. Further analysis is necessary to understand the mechanism behind the maintenance of each cell type in the adult pancreas, especially β cells.
Materials and Methods
Gene targeting and mice
We generated Dicer1-deficient mice from an ES cell (RFF266), which was obtained from Bay Genomics [34], carrying a gene trap insertion between exon 22 and exon 23, resulting in disruption of the second RNaseIII domain and loss of the double-stranded RNA-binding domain. A gene trap vector called pGT1Lxf, which has a splicing acceptor, was used to make this ES cell. Targeted clones were injected into blastocysts to generate chimeras. Five chimeras were generated and backcrossed with C57BL/6 mice. Dicer1 heterozygous mice were backcrossed with C57BL/6 mice for 12 generations. All animal experiments were approved by the Animal Research Ethics Board at the Gunma University.
Histochemistry and immunohistochemistry
Tissues and embryos were fixed overnight in formalin at 4°C and embedded in paraffin. Standard techniques were used for the embedding, sectioning and staining of tissues. Sections were cut at 5 µm. Immunohistochemistry was performed as follows: The slides were dewaxed and washed in PBS, and blocked in 1% BSA for 30 min. They were then incubated with primary antibodies overnight at 4°C in PBS containing 1% BSA, washed in PBS, and incubated with the appropriate secondary antibodies for 1 hour at room temperature. The slides were washed in PBS and mounted with Pristine Mount (Parma) with DAPI. The primary antibodies used were rabbit anti-glucagon (1∶200, DAKO) and guinea pig anti-insulin (1∶200, DAKO), rabbit Ki67 monoclonal antibody (Lab Vision), and Pdx1 (a gift from Christopher V. Wright (Vanderbilt University, Nashville)). The secondary antibodies were conjugated to rodamin (1∶200, Jackson) and Alexa 488 (1∶200, Molecular Probes). The slides were examined with a Nikon ECLIPSC TE300 and images were obtained with a LEICA DFC400 camera.
Measurement of islet area
ß-cell mass was measured using Image J software (NIH). Islet numbers and areas were averaged from 6 animals, with six sections from each animal, 250 µm apart.
RNA extraction and quantitative RT-PCR
Total RNA was prepared from isolated tissues using the RNeasy mini kit (Qiagen) with a modified protocol to purify total RNA containing miRNA from animal tissues. Differential gene expression was confirmed using the SYBR Premix Ex Taq (TAKARA) in accord with the manufacturer's instructions. The reaction was performed using the SYBER Green program on an ABI PRISM 7700 sequence detector system (Applied Biosystems). The expression of mRNA was normalized to that of GAPDH mRNA.
Primer sequences were as follows, ActRIIA: 5′-AGCGGAGCTGACAGTGATTT-3′, 5′-CATACACGCACAACACACCA-3′ ActRIIB: 5′-TGGACATCCATGAGGTGAGA-3′, 5′-CAGCAGCTGTAGTGGCTTCA-3′
miRNA microarray
Small RNAs were labeled with a miRNA labeling Reagent & Hybridization Kit (Agilent) based on the manufacturer's instructions. The Cy3-labeled RNA molecules were hybridized with a Mouse miRNA microarray (Agilent), consisting of control probes, mismatch probes, and 567 capture probes as registered and annotated in Sanger miRBase v10.1. A DNA MicroArray Scanner (Agilent) was used to scan images. The scanned images were analyzed with Agilent Feature Extractin Ver.9.5.3 (Agilent). Data were normalized globally per array. The net intensity values were normalized to per-chip median values.
Glucose tolerance test and insulin concentration
After overnight fasting, 2 mg/g (body weight) of glucose was administered intraperitoneally. Blood samples were drawn intraperitoneally from the tail at different times, and the blood glucose concentration was measured with an automatic blood glucose meter, Freestyle Freedom (NIPRO). Whole blood was collected and centrifuged, and the plasma was stored at −80°C. The insulin concentration was measured with an insulin measurement kit (Morinaga) in accordance with the manufacturer's instructions.
Growth hormone measurements
Growth hormone concentrations of wild-type and Dicer1-hypomorphic mice were measured with a rat/mouse growth hormone ELISA kit (LINCO Research).
Data analysis
Data were analyzed by the one-sample t-test and independent samples t-test. Data are the means±S.D.
Acknowledgments
We thank the Mutant Mouse Regional Resource Center (MMRRC) for providing RRF266 cells.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by the Japan Health Sciences Foundation (S.M. is a fellowship holder from the Japan Health Sciences Foundation), grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (I.H. and I.K.), the Ministry of Health, Japan Health Sciences Foundation, Labor and Welfare of Japan (T.O. and I.H.) and a grant from the Realization of Regenerative Medicine (I.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaij B, et al. The microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 3.Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 4.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of microRNA from mouse. Current Biology. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 5.Wienholds E, Kloosterman WP, Misaka E, Alvarez-Saavedra E, Berezikov E, et al. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 7.Yang WY, Yang DD, Songqing N, Sandusky GE, Zhang Q, et al. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 8.Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci USA. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essesntial for lung epithelium morphogenesis. Proc Natl Acad Sci USA. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, et al. The miRNA-processing enzyme Dicer is essential for morphogenesis and maintenance of hair follicles. Current Biology. 2006;16:1041–1046. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynn FC, Skewes-Cox BAP, Kosaka Y, McManus MT, Harfe BD, et al. MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes. 2007;56:2938–2945. doi: 10.2337/db07-0175. [DOI] [PubMed] [Google Scholar]
- 12.Cobb BS, Nesterova TB, Thompson E, Hertweck A, O'Connor E, et al. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med. 2005;20:1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukasawa M, Morita S, Kimura M, Horii T, Ochiya T, et al. Genomic imprinting in Dicer1-hypomorphic mice. Cytogenet Genome Res. 2006;113:138–143. doi: 10.1159/000090825. [DOI] [PubMed] [Google Scholar]
- 14.Habener JF, Kemp DM, Thomas MK. Minireview: Transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–1034. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- 15.Ackermann AM, Gannon M. Molecular regulation of pancreatic β-cell mass development, maintenance, and expansion. J Mol Endo. 2007;38:193–206. doi: 10.1677/JME-06-0053. [DOI] [PubMed] [Google Scholar]
- 16.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 17.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult β cells dose not involve specialized progenitors. Dev Cell. 2007;12:817–829. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regenerating of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42:1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 19.Bertelli E, Bendayan M. Intermediate endocrine-acinar pancreatic cells in duct ligation conditions. Am J Physiol Cell Physiol. 1997;273:C1641–C1649. doi: 10.1152/ajpcell.1997.273.5.C1641. [DOI] [PubMed] [Google Scholar]
- 20.Bonner-Weir S, Toschi E, Inada A, Reits P, Fonseca SY, et al. The pancreatic ductal epithelium serves as apotential pool of progenitor cells. Pediatric Diabetes. 2004;5:16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, et al. β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Kritzik MR, Jones E, Chen Z, Krakowski M, Krahl T, et al. Pdx-1 and Msx-2 expression in the regenerating and developing pancreas. J Endocrinol. 1999;163:523–530. doi: 10.1677/joe.0.1630523. [DOI] [PubMed] [Google Scholar]
- 23.Song SY, Gannon M, Wahington MK, Scoggins CR, Meszoely IM, et al. Pdx-1 expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1999;117:1416–1426. doi: 10.1016/s0016-5085(99)70292-1. [DOI] [PubMed] [Google Scholar]
- 24.Shiozaki S, Tajima T, Zhang YQ, Furukawa M, Nakazato Y, et al. Impaired differentiation of endocrine cells of pancreas in transgenic mouse expressing the truncated type II activin receptor. Biochimica et Biophysica Acta. 1999;1450:1–11. doi: 10.1016/s0167-4889(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 25.Attisano L, Wrana JL, Cheifetz S, Massague J. Novel activin receptors: dinsinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell. 1992;68:97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- 26.Mathews LS. Activin receptors and cellular signaling by the receptor serine kinase family. Endocr Rev. 1994;15:310–325. doi: 10.1210/edrv-15-3-310. [DOI] [PubMed] [Google Scholar]
- 27.Attisano L, Carcamo J, Ventura F, Weis FM, Massague J, et al. Identificatin of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993;75:671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita H, Ten Dijke P, Huylebroeck D, Sampath TK, Andries M, et al. Osteogenic protein-1 binds to activin type II receptors and induce certain activin-like effectors. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SJ, McPherron AC. Regulation of myostain activity and muscle growth. Proc Natl Acad Sci USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo C, Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell. 2001;7:949–957. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]
- 31.Oh SP, Yeo CY, Lee Y, Schrewe H, Whitman M, et al. Activin type IIA and type IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Gene Dev. 2002;16:2749–2754. doi: 10.1101/gad.1021802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gittes GK, Rutter WJ. Onset of cell-specific gene expression in the developing mouse pancreas. Proc Natl Acad Sci USA. 1992;89:1128–1132. doi: 10.1073/pnas.89.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118:1031–1039. doi: 10.1242/dev.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 34.Stryke D, Kawamoto M, Huang CC, Johns SJ, King LA. BayGenomics: a resorce of insertional mutations in mouse embryonic stem cells. Nucleic Acids Res. 2003;31:278–281. doi: 10.1093/nar/gkg064. [DOI] [PMC free article] [PubMed] [Google Scholar]