Abstract
The self-transmissible plasmid pUO1 from Delftia acidovorans strain B carries two haloacetate-catabolic transposons, TnHad1 and TnHad2, and the mer genes for resistance to mercury. The complete 67,066-bp sequence of pUO1 revealed that the mer genes were also carried by two Tn402/Tn5053-like transposons, Tn4671 and Tn4672, and that the pUO1 backbone regions shared 99% identity to those of the archetype IncP-1β plasmid R751. Comparison of pUO1 with three other IncP-1β plasmids illustrated the importance of transposon insertion in the diversity and evolution of this group of plasmids. Mutational analysis of the four outermost residues in the inverted repeats (IRs) of TnHad2, a Tn21-related transposon, revealed a crucial role of the second residue of its IRs in transposition.
It has been demonstrated that bacterial genes for the degradation of various xenobiotic compounds are often loaded on transposable elements that are mainly classified into two major groups: class I and class II transposons (14). The class I transposon is a mobile element in which a short DNA segment is flanked by two copies of an insertion sequence (IS). The class II transposon usually carries transposase (TnpA), resolvase (TnpR), and a resolution (res) site between short terminal inverted repeats (IRs) and transposes by a two-step process—cointegration and resolution (3, 9). Broad-host-range and self-transmissible plasmids are well known to contribute to the wide dissemination of these catabolic transposons (13). The successful conjugal transfer of these plasmids facilitates the spread of the catabolic transposons and at times promotes the evolution of novel catabolic pathways by genetic rearrangements (15). A 67-kb self-transmissible and broad-host-range but not-well characterized plasmid, pUO1, from Delftia acidovorans strain B carries, in addition to the mer genes for resistance to mercury, two haloacetate dehalogenase genes (dehH1 and dehH2) on the two transposons, TnHad1 and TnHad2 (Fig. 1A) (10). TnHad1, with a size of 8.9 kb, is a class I transposon that carries dehH2 between two directly repeated copies of IS1071 (8), and dehH1 and TnHad1 are loaded on a 15.6-kb class II transposon, TnHad2, that lacks the tnpA and tnpR genes (Fig. 1A) (10). TnHad2 has the 38-bp terminal IRs, IR1 and IR3, and the res site (Fig. 1A and 2A), each of which shares extensive homology to the corresponding sequence of Tn21, and the supply in trans of the Tn21 transposition genes enables the complete transposition of TnHad2 (10). The sequence of IR2, which is highly homologous to IR1, is also located very close to the res site, although the 5′ end of IR2 on pUO1 is occupied by the C residue (Fig. 1A and 2A). In our previous study (10), we replaced this C residue with the T residue, and the mini-TnHad2 derivative flanked by this IR2 derivative (5′-TGGG end) and IR1 (5′-GGGG end) (Fig. 2B) was found to transpose at a high frequency in the presence of the Tn21 tnpA gene. This finding was unexpected, since (i) the outermost four G residues of the IRs are conserved in the naturally occurring and experimentally mobile class II transposons, and (ii) these residues in Tn3 and its related transposon, γδ (= Tn1000), have been reported to be very crucial in the cointegration step (1, 7).
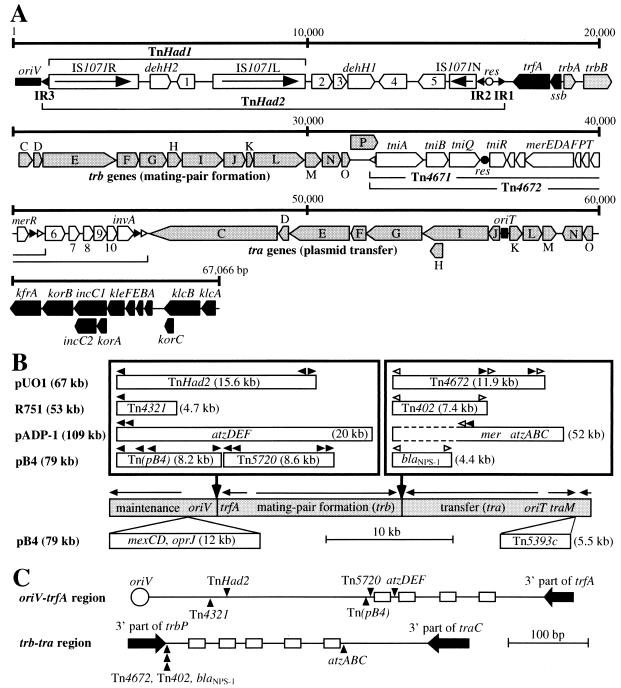
FIG. 1.
Structure of pUO1 and comparison with other IncP-1β plasmids. The black arrowhead represents the 38-bp IR highly homologous to that of Tn21, and the white arrowhead indicates the 25-bp IR of the Tn402/Tn5053-related transposons. (A) Structure of pUO1. The white and black circles show the res sites in TnHad2 and Tn4671, respectively. The arrow indicates the transcriptional direction of the tnpA gene in IS1071. The oriV (origin of vegetative replication) and oriT (origin of plasmid transfer) sequences are shown by black boxes. The pentagon indicates the size and orientation of the gene. Black pentagons are the genes for the maintenance (replication, regulation, partition, stable inheritance) of plasmid, and shaded pentagons are those for the conjugal transfer. See references 6 and 12 for the detailed functions of the proteins encoded in these backbone regions. mer, genes for resistance to mercury; tni, genes for transposition of Tn4671. Abbreviations 1 to 10 represent the ORFs orf1 to orf10, whose functions are unclear. (B) Comparison of the four IncP-1β plasmids. The shaded bar indicates the backbone of the IncP-1β plasmids. The horizontal arrow shows the transcriptional direction of coding regions (A). The DNA inserts situated at the similar positions are boxed. Additional DNA insertions upstream of oriV and downstream of traM of pB4 are depicted below the backbone. Tn5720 covers Tn5719, and Tn(pB4) is a putative class II transposon that was not described in the original report (11). The 52-kb fragment on pADP-1 is flanked by two truncated versions of IS1071 (see text for details). trfA, gene for plasmid replication and regulation; atzABCDEF, genes for atrazine degradation; blaNPS-1, β-lactamase NPS-1 gene; and mexCD and oprJ, genes for multidrug efflux system. Sequence data for R751, pADP-1, and pB4 are compiled from DDBJ/EMBL/GenBank databases: R751, U67194; pADP-1, U66917; and pB4, AJ431260. (C) Comparison of the insertion sites of transposons and other phenotypic genes. The figures represent the oriV-trfA and trb-tra regions of pUO1, and the insertion sites of transposon orvarious phenotypic genes are drawn so as to locate on the pUO1 backbone. Vertical arrowhead indicates the insertion site of the foreign DNA, and the box represents the conserved 20-bp IR sequence (see text for details) on pUO1. This 20-bp sequence has been reported to be present in the corresponding regions on the three other plasmids (the oriV-trfA region: five copies on R751 and four copies on pADP-1 and pB4; and the trb-tra region: four and five copies on R751 and pB4, respectively) (6, 11, 12).
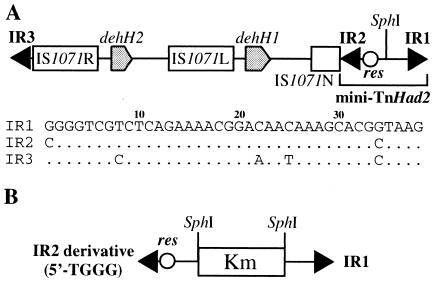
FIG. 2.
Schematic structures of TnHad2 and a mini-TnHad2 derivative. (A) Structure of TnHad2. The sequences of the three IRs are shown below the figure, and the dot indicates the nucleotide identical to that of IR1. The 38-bp IR2 sequence redefined in this study is depicted in the figure, and the IR2 sequence in our previous report (10) had been defined to the 37-bp sequence that corresponds to base positions from 2 to 38 in this figure. This revision is based on our observation in this study that the first C residue of IR2 was incorporated into the fragment that transposed onto a target replicon. In this study, the rightmost 818-bp fragment of TnHad2 containing IR1, IR2, and the res site is designated mini-TnHad2. (B) Structure of the mini-TnHad2 derivative on pMS015. The details to construct this plasmid have been described previously (10). Note that the IR2 derivative on this plasmid has the T, but not C, residue at the first position.
In this study, we determined the complete nucleotide sequence of pUO1 to know its detailed structure. We also investigated the roles of the four outermost residues of the mini-TnHad2 IRs in transposition, because such analyses had been limited to only Tn3 and γδ, whose transposases are not able to catalyze the transposition of the Tn21-related transposons (3, 9).
The Escherichia coli strains DH5α and HB101 (2) used in this study were cultivated at 37°C in Luria broth (LB) and LB agar. The following agents were added to the media: chloramphenicol, 50 μg/ml; kanamycin, 50 μg/ml; streptomycin, 50 μg/ml; and sulfathiazole, 350 μg/ml. Established protocols were employed for the preparation of plasmid DNA, DNA digestion with restriction endonucleases, ligation, gel electrophoresis, transformation of E. coli cells, DNA sequencing, and PCR (2).
Genetic organization of pUO1.
To understand the basic properties of pUO1, its complete sequence with a size of 67,066 bp (accession no. AB063332 in DDBJ/EMBL/GenBank databases) was determined by standard methods (2). Computer analysis with the software programs GENETYX 10 (SDC, Inc., Tokyo, Japan) and BLAST 2 (National Institute of Genetics, Mishima, Japan) led to identification of 70 putative open reading frames (ORFs) on pUO1 (Fig. 1A). Homology searches clarified that pUO1 was a member of the IncP-1β plasmids, since the backbone regions of pUO1 encoding its replication, maintenance, and transfer functions and their regulation shared more than 99% nucleotide identity with the corresponding segments of two well-characterized broad-host-range IncP-1β plasmids, a trimethoprim resistance plasmid, R751 (12), and an atrazine-catabolic plasmid, pADP-1 (6). The mer genes on pUO1 were located within two Tn402/Tn5053-related transposons designated Tn4671 (8,448 bp) and Tn4672 (11,914 bp), and both elements shared one end (Fig. 1A). The Tn4671 sequence was quite similar (92%) to that of an 8,447-bp mercury resistance transposon, Tn5053, on the chromosome of Xanthomonas sp. strain W17 (4). We were able to detect the intramolecular transposition of Tn4671 in pUO1 (data not shown). The Tn4672 region outside of Tn4671 carried six putative ORFs (orf6 to orf10 and invA) (Fig. 1A). While the predicted invA product with a size of 185 amino acid residues showed >91% identity to a hypothetical DNA invertase from Rhodospirillum rubrum S-1 (accession no. D17434) and two putative invertases from a drug resistance IncP-1β plasmid, pB4 (accession no. AJ431260) (11), no functions of the orf6 to orf10 products were defined by the database searches. The Tn402/Tn5053-related transposons have been reported to generate a 5-bp duplication of the target sequence upon transposition (4). Neither Tn4671 nor Tn4672 had such duplications at their external junctions, indicating that the putative duplication might have been lost by unknown DNA rearrangements.
Comparison of pUO1 with three other completely sequenced IncP-1β plasmids, R751 (12), pADP-1 (6), and pB4 (11), revealed that all four of the plasmids had “foreign” DNA fragments (i.e., transposons and various phenotypic genes) at very close positions between oriV and trfA and between the trb and tra clusters on the IncP-1β backbone (Fig. 1B and C). The DNA inserts in the oriV-trfA regions on these plasmids had the Tn21-like 38-bp IR sequences at one or both ends (Fig. 1B). It is therefore most likely that the insertion of the Tn21-related transposons or their ancestors into the oriV-trfA region of a common plasmid must have led to physical separation of the IncP-1β backbone. The DNA inserts in the trb-tra region on the four plasmids had the Tn402/Tn5053-related transposons or their remnants (Fig. 1B). It is noteworthy that these inserts on pUO1 and pB4 were situated at an identical position on the backbone and that this position was only 3 bp apart from the insertion site of Tn402 on R751 (data not shown) (Fig. 1C). We noticed that the right and left ends of the 52-kb insert on pADP-1 carried the terminal 68- and 476-bp sequences, respectively, of IS1071, indicating that the 52-kb insert might have been present as a huge IS1071-composite transposon on the ancestor of this plasmid. Thorsted et al. (12) and two other groups (6, 11) have proposed that several copies of a conserved 20-bp inverted repeat sequence (consensus = CATCGCCANNTCYGRCGATG) residing in the oriV-trfA and trb-tra regions on R751, pB4, and pADP-1 might have been associated with the insertion of the foreign DNA fragments. pUO1 also had four and five copies of this conserved sequence in the former and latter regions, respectively (Fig. 1C), supporting the proposal by Thorsted et al. and others and providing a better understanding for the evolution and establishment of various IncP-1β plasmids.
Transposition of the mini-TnHad2 derivatives.
As described above, the four outermost G residues in the IRs are conserved in the class II transposons (3). However, the first 5′ position of IR2 of mini-TnHad2 was occupied by the C residue, generating the 5′-CGGG end (Fig. 2A) (10). We have previously replaced this C residue with the T residue, and this mutant version of mini-TnHad2 with an additional insert of a kanamycin resistance (Kmr) gene was cloned into a p15A-based Cmr vector, pSTV29 (TAKARA BIO), to obtain pMS015 (Fig. 2B) (10). The mini-TnHad2 derivative on pMS015 has been found to still transpose efficiently in the presence of the Tn21 transposase, generating the transposed fragment having IR1 (5′-GGGG end) and the mutant IR2 (5′-TGGG end) at the extremities (10). This observation has provided us a possibility that the Tn21 transposase might be able to catalyze the transposition of the mini-TnHad2 derivatives that have various mutations in the four outermost positions of the IRs. To investigate this possibility in this study, additional mini-TnHad2 derivatives with various mutations in the four outermost residues of IR1 and IR2 were constructed by PCR using pMS015 as the template and various IR1- and IR2-based sequences as the primers. The PCR products were cloned in pSTV29 or its related vector, pSTV28, to obtain the plasmids listed in Table 1; the details to construct these plasmids will be provided upon request. Each of these plasmids was introduced into a DH5α derivative harboring R388 (16), a conjugal and Sur plasmid free of transposons, and pMT1252 (10), a pBR322 derivative carrying the transposition genes of Tn21. The DH5α derivative carrying the three plasmids was used as a donor to mate with an Smr strain, HB101, on a membrane filter, and the transposition of the mini-TnHad2 derivatives onto R388 was, as described previously (10), examined by selecting and analyzing the Kmr Smr transconjugants.
TABLE 1.
Transposition of the mini-TnHad2 derivatives with the mutant end(s)a
| Expt | Donor of Tn | Terminal residues (IR1 end +IR2 end)b | Relative transposition frequencyc |
|---|---|---|---|
| 1 | pMS015G | 5′-GGGG + 5′-GGGG | 1.0 |
| 2 | pMS015A | 5′-GGGG + 5′-AGGG | 1.6 × 10−1 |
| 3 | pMS015C | 5′-GGGG + 5′-CGGG | 1.6 × 10−1 |
| 4 | pMS015d | 5′-GGGG + 5′-TGGG | 4.6 × 10−1 |
| 5 | pMS0191 | 5′-CGGG + 5′-CGGG | 1.3 × 10−1 |
| 6 | pMS0196 | 5′-GAGG + 5′-GAGG | 1.2 × 10−3 |
| 7 | pMS0197 | 5′-GTGG + 5′-GTGG | <7.6 × 10−7 |
| 8 | pMS0192 | 5′-GCGG + 5′-GCGG | <5.3 × 10−7 |
| 9 | pMS0195 | 5′-GGGG + 5′-GCGG | 1.8 × 10−2 |
| 10 | pMS0193 | 5′-GGCG + 5′-GGCG | 2.0 × 10−3 |
| 11 | pMS0194 | 5′-GGGC + 5′-GGGC | 6.5 × 10−3 |
| 12 | pMS0198 | 5′-CGCC + 5′-CGCC | 3.6 × 10−4 |
Transposition of the mini-TnHad2 derivatives from the pMS plasmid to R388 was investigated in the presence of pMT1252, a pBR322-based plasmid carrying the transposition genes of Tn21. The average transposition frequency at least from three independent experiments was expressed as the number of the Kmr Smr transconjugants per the Sur Smr (R388) transconjugants. Sequence analysis demonstrated that all of the transposed regions inserted at various sites on R388 (i) had the mutant ends that were located on the donor plasmids and (ii) generated a 5-bp duplication of the target sequences (data not shown). The control experiments with no supply of the Tn21 transposition genes were performed using pBR322 instead of pMT1252, and no transposition of the mini-TnHad2 derivatives was detected at all (at the transposition frequency of <10−8).
For simplicity, only the four outermost residues of the IRs are indicated, and the complete sequences of the wild-type IR1 and IR2 are shown in Fig. 2A.
The transposition frequency of the mini-TnHad2 derivative flanked by the two 5′-GGGG ends (on pMS015G) was 1.3 × 10−1, and this value was taken as 1.0 to calculate the relative transposition frequency.
Construction of pMS015 has been described previously (10).
The four plasmids pMS015G, pMS015A, pMS015C, and pMS015 were used to investigate the effect of the mutations at the first residue of IR2. All of the mini-TnHad2 derivatives on the four plasmids were transposable in the presence of the Tn21 transposase, and the transposition frequencies of the mini-TnHad2 derivatives from the latter three plasmids were two- to sixfold lower than that from pMS015G (Table 1, experiments 1 to 4). These results clearly indicated that the presence of either one of the four possible nucleotides at the first position of IR2 permitted the efficient transposition. Efficient transposition was still observed in the pMS0191-loaded mini-TnHad2 derivative that was flanked by the two 5′-CGGG ends (Table 1, experiment 5), confirming that the transposition of mini-TnHad2 did not require the presence of the G residue at the first position in both IRs. This contrasted with the report that a G-to-T mutation at either one of the three outermost G residues in both IRs in γδ led to the complete abolishment of transposition (7). To know whether the critical roles of the second and third G residues of the γδ IRs in transposition were the cases with the IRs of mini-TnHad2, we first introduced various mutations into the second position of both IRs of mini-TnHad2 to obtain the three derivative transposons that carried the two 5′-GAGG, 5′-GTGG, and 5′-GCGG ends on pMS0196, pMS0197, and pMS0192, respectively (Table 1, experiments 6 to 8). The mini-TnHad2 derivative on pMS0196 (5′-GAGG) transposed at the frequency 800-fold lower than that on pMS015G, and the two transposons on pMS0197 (5′-GTGG) and pMS0192 (5′-GCGG) did not transpose at all. Replacement of the second C residue of the pMS0192-specified IR1 mutant with the G residue gave rise to pMS0195 (Table 1, experiment 9), and this mini-TnHad2 derivative with the 5-GGGG and 5′-GCGG ends exhibited the transposability at the frequency only 50-fold lower than that on pMS015G. These results indicated that (i) the presence of the A, but not T or C, residue at the second position in both IRs still permitted the transposition albeit at the much lower frequency, and (ii) the presence of the G residue at the second position at least in one IR allowed the efficient transposition. To examine the role of the third G residue in the IR, we next constructed pMS0193 in which both IRs of mini-TnHad2 were changed to the 5′-GGCG ends (Table 1, experiment 10). The resulting mini-TnHad2 derivative transposed at the frequency 500-fold lower than that on pMS015G. This was a unique property associated with mini-TnHad2, because a mutant of γδ having the two 5′-GGTG ends has been reported not to be transposable at all (7). Taking into consideration that the fourth G residue is conserved in the IRs of the class II transposons (3, 9), we also investigated the importance of the fourth G residue in the mini-TnHad2 IRs. The mini-TnHad2 derivative having the two 5′-GGGC ends on pMS0194 transposed at the frequency 150-fold lower than that on pMS015G (Table 1, experiment 11), demonstrating that the transposition of mini-TnHad2 did not absolutely require the presence of the G residue at the fourth position in both IRs.
On the basis of (i) the efficient transposition of the mini-TnHad2 derivative with the two 5′-CGGG ends, (ii) the inefficient but clearly detectable transposition of the derivative with the two 5′-GGCG or 5′-GGGC ends, and (iii) no transposition of the derivative with the two 5′-GCGG ends, we constructed pMS0198 in which the mini-TnHad2 derivative was flanked by the two 5′-CGCC ends (Table 1, experiment 12). The resulting transposon having these ends still moved at the frequency 2,800-fold lower than that on pMS015G, and this result reinforced the unique characteristics of mini-TnHad2 in that the presence of the G residue at the second positions was critical for transposition. It has been reported that the Tn21 transposase was able to catalyze the cointegration reaction even when its inner 20-bp sequence of the IR was replaced by that of Tn3 (5). This nonstrict property of the Tn21 transposase might also be associated with its recognition of the four outermost residues of mini-TnHad2, hence leading to our successful detection of the transposition of the mini-TnHad2 derivative having the 5′-CGCC end in both IRs. The drastic decrease in the transposition frequencies of several mini-TnHad2 mutants might be ascribed to either or both (i) inefficient binding of the Tn21 transposase to the mutant IRs and (ii) inefficient but still successful single-stranded break of the 3′ ends of the mini-TnHad2 mutants by the Tn21 transposase in the initial step of the cointegration reaction (3, 9). Biochemical purification of the Tn21 transposase and subsequent in vitro assay of its binding to various mutant IRs of mini-TnHad2 will elucidate more detailed interaction of the transposase with the IR.
Acknowledgments
This work was supported by Grants-in-Aid from Ministry of Education, Culture, Sports, Science and Technology and The Ministry of Agriculture, Forestry, and Fisheries (HC-03-2323-1), Japan.
REFERENCES
- 1.Amemura-Maekawa, J., and E. Ohtsubo. 1991. Functional analysis of the two domains in the terminal inverted repeat sequence required for transposition of Tn3. Gene 103:11-16. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1988. Current protocols in molecular biology. Wiley, New York, N.Y.
- 3.Grindley, N. D. F. 2002. The movement of Tn3-like elements: transposition and cointegrate resolution, p. 272-302. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 4.Kholodii, G., S. Z. Mindlin, I. A. Bass, O. V. Yurieva, S. V. Minakhina, and V. G. Nikiforov. 1995. Four genes, two ends, and a res region are involved in transposition of Tn5053: a paradigm for a novel family of transposons carrying either a mer operon or an integron. Mol. Microbiol. 17:1189-1200. [DOI] [PubMed] [Google Scholar]
- 5.Martin, C., J. Grinsted, and F. de la Cruz. 1989. Effects of variation of inverted-repeat sequences on reactions mediated by the transposase of Tn21. J. Bacteriol. 171:3996-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez, B., J. Tomkins, L. P. Wackett, R. Wing, and M. J. Sadowsky. 2001. Complete nucleotide sequence and organization of the atrazine catabolic plasmid pADP-1 from Pseudomonas sp. strain ADP. J. Bacteriol. 183:5684-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May, E. W., and N. D. Grindley. 1995. A functional analysis of the inverted repeat of the γδ transposable element. J. Mol. Biol. 247:578-587. [DOI] [PubMed] [Google Scholar]
- 8.Nakatsu, C., J. Ng, R. Singh, N. Straus, and C. Wyndham. 1991. Chlorobenzoate catabolic transposon Tn5271 is a composite class I element with flanking class II insertion sequences. Proc. Natl. Acad. Sci. USA 88:8312-8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherratt, D. 1989. Tn3 and related transposable elements: site-specific recombination and transposition, p. 163-184. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
- 10.Sota, M., M. Endo, K. Nitta, H. Kawasaki, and M. Tsuda. 2002. Characterization of a class II defective transposon carrying two haloacetate dehalogenase genes from Delftia acidovorans plasmid pUO1. Appl. Environ. Microbiol. 68:2307-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tauch, A., A. Schluter, N. Bischoff, A. Goesmann, F. Meyer, and A. Pühler. 2003. The 79,370-bp conjugative plasmid pB4 consists of an IncP-1β backbone loaded with a chromate resistance transposon, the strA-strB streptomycin resistance gene pair, the oxacillinase gene blaNPS-1, and a tripartite antibiotic efflux system of the resistance-nodulation-division family. Mol. Gen. Genomics 268:570-584. [DOI] [PubMed] [Google Scholar]
- 12.Thorsted, P. B., D. P. Macartney, P. Akhtar, A. S. Haines, N. Ali, P. Davidson, T. Stafford, M. J. Pocklington, W. Pansegrau, B. M. Wilkins, E. Lanka, and C. M. Thomas. 1998. Complete sequence of the IncPβ plasmid R751: implications for evolution and organisation of the IncP backbone. J. Mol. Biol. 282:969-990. [DOI] [PubMed] [Google Scholar]
- 13.Top, E. M., Y. Moenne-Loccoz, T. Pembroke, and C. M. Thomas. 2000. Phenotypic traits conferred by plasmids, p. 249-286. In C. M. Thomas (ed.), The horizontal gene pool. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 14.Tsuda, M., H. M. Tan, A. Nishi, and K. Furukawa. 1999. Mobile catabolic genes in bacteria. J. Biosci. Bioeng. 87:401-410. [DOI] [PubMed] [Google Scholar]
- 15.van der Meer, J. R., W. M. de Vos, S. Harayama, and A. J. B. Zehnder. 1992. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol. Rev. 56:677-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward, J. M., and J. Grinsted. 1982. Physical and genetic analysis of the Inc-W group plasmids R388, Sa, and R7K. Plasmid 7:239-250. [DOI] [PubMed] [Google Scholar]