Abstract
Serotonin (5-HT) regulates key processes in both vertebrates and invertebrates. Previously, four 5-HT receptors that contributed to the 5-HT modulation of egg laying were identified in Caenorhabditis elegans. Therefore, to assess potential receptor interactions, we generated animals containing combinations of null alleles for each receptor, especially animals expressing only individual 5-HT receptors. 5-HT-stimulated egg laying and egg retention correlated well with different combinations of predicted excitatory and inhibitory serotonergic inputs. For example, 5-HT did not stimulate egg laying in ser-1, ser-7, or ser-7 ser-1 null animals, and ser-7 ser-1 animals retained more eggs than wild-type animals. In contrast, 5-HT-stimulated egg laying in ser-4;mod-1 animals was greater than in wild-type animals, and ser-4;mod-1 animals retained fewer eggs than wild-type animals. Surprisingly, ser-4;mod-1;ser-7 ser-1 animals retained the same number of eggs as wild-type animals and exhibited significant 5-HT-stimulated egg laying that was dependent on a previously uncharacterized receptor, SER-5. 5-HT-stimulated egg laying was absent in ser-5;ser-4;mod-1;ser-7 ser-1 animals, and these animals retained more eggs than either wild-type or ser-4;mod-1;ser-7 ser-1 animals. The 5-HT sensitivity of egg laying could be restored by ser-5 muscle expression. Together, these results highlight the dual excitatory/inhibitory serotonergic inputs that combine to modulate egg laying.
IN mammals, serotonin (5-HT) regulates diverse central and peripheral responses, including feeding, mood, perception, and aggression, and a variety of drugs appear to modulate serotonergic signaling in the treatment of schizophrenia, depression, and migraine. Clearly, changes in serotonergic signaling can have profound effects on behavior. Mammalian 5-HT receptors are divided into seven subfamilies (5-HT1-7) and 14 subtypes on the basis of amino acid sequence, pharmacology, and coupling specificity (Hoyer et al. 1994; Bockaert et al. 2006). However, although all of the mammalian 5-HT receptors appear to have been identified, much still remains to be learned about how they are integrated in complex multi-level signaling pathways.
5-HT also is an important neuromodulator in invertebrates. For example, in the nematode model Caenorhabditis elegans, 5-HT is one of the “food is at hand” signals for starved animals. 5-HT levels are elevated on food (bacteria), resulting in the inhibition of locomotion and the stimulation of pharyngeal pumping and egg laying, as well as the modulation of more complex behaviors, including the enhanced slowing response, forward/reverse movement, chemosensation, and aversive olfactory learning (Horvitz et al. 1982; Sawin et al. 2000; Sze et al. 2000; Chao et al. 2004; Zhang et al. 2005). Until recently, our understanding of serotonergic signaling in C. elegans was gleaned largely from the study of tph-1 null animals that lack tryptophan hydroxylase, essential for 5-HT synthesis, or from the incubation of animals in high levels of 5-HT, on the assumption that these concentrations were necessary to overcome the relative impermeability of the nematode cuticle (Sze et al. 2000). Previously, four 5-HT receptors were characterized in C. elegans: three G-protein-coupled receptors, SER-1, SER-4, and SER-7, which appear to couple to Gαq, Gαo, and Gαs, respectively, and a novel 5-HT-gated Cl− channel, MOD-1 (Olde and McCombie 1997; Hamdan et al. 1999; Ranganathan et al. 2000; Hobson et al. 2003). More recently, serotonergic signaling has been examined in animals containing null mutations in the genes encoding these 5-HT receptors. For example, both SER-1 and SER-7 are required for 5-HT-stimulated egg laying, SER-7 for 5-HT-stimulated pharyngeal pumping, SER-1 for male tail curling, and MOD-1 for the 5-HT inhibition of locomotion and modulation of aversive olfactory learning (Carnell et al. 2005; Dempsey et al. 2005; Hobson et al. 2006; Ranganathan et al. 2000; Zhang et al. 2005, respectively). More importantly, 5-HT can have both excitatory and inhibitory inputs into the same process. For example, both ser-1 and ser-7 appear to be essential for 5-HT-stimulated egg laying, but in either ser-1 or ser-7 null backgrounds, 5-HT actually inhibits egg laying through a pathway involving mod-1 and ser-4 (Carnell et al. 2005; Hobson et al. 2006).
Therefore, to further define potential interactions among these 5-HT receptors, we prepared animals containing combinations of null alleles for these four 5-HT receptors and examined 5-HT-dependent egg laying, a simple behavior with complex regulation by 5-HT. Most importantly, we generated animals that expressed only individual 5-HT receptors from their native promoters, minimizing the need for subtype-specific ligands to examine receptor function. Interestingly, ser-4;mod-1;ser-7 ser-1 quadruple null animals (Q4ser-5) were viable and still exhibited significant 5-HT-dependent egg laying, suggesting that additional 5-HT receptor(s) remained to be identified. Indeed, using a combination of bioinformatics, RNA interference (RNAi), and null mutants, we tentatively identified a fifth amine receptor, SER-5 (F16D3.7), involved in the serotonergic modulation of egg laying. 5-HT-stimulated egg laying was completely abolished in ser-5;ser-4;mod-1;ser-7 ser-1 quintuple null animals (Q5) and could be rescued to the levels observed in ser-4;mod-1;ser-7 ser-1 animals by ser-5 expression in muscle. Interestingly, ser-4;mod-1 and ser-7 ser-1 null animals exhibited more obvious egg-laying defects than wild-type or ser-4;mod-1;ser-7 ser-1 animals, emphasizing the delicate balance of serotonergic signaling. These observations confirm that the serotonergic modulation of egg laying involves both excitatory and inhibitory inputs mediated by at least five different receptors, three of which, SER-1, SER-7, and SER-5, appear to function in muscle. Together, these studies highlight the utility of the C. elegans model system for studying the interactions of multiple receptors in aminergic modulation of both simple and complex behaviors.
MATERIALS AND METHODS
Culture, maintenance of strains, and reagents:
C. elegans animals were cultured by standard methods at 20° (Brenner 1974). All experiments used well-fed adults grown on standard nematode growth medium (NGM) seeded with the Escherichia coli strain OP50 (Brenner 1974). The N2 Bristol strain was used as wild type in all assays. The ser-7(tm1325), ser-7(tm1728), ser-5(tm2654), and ser-5(tm2647) strains were obtained from the National Bioresources Project (Tokyo Women's Medical University, Tokyo); ser-7(tm1325) ser-1(ok345) (DA2109) double mutants were a kind gift from Leon Avery (University of Texas Southwestern Medical Center, Dallas); and all other strains were obtained from the Caenorhabditis Genetics Center (Oklahoma Medical Research Foundation). All chemicals were purchased from Sigma-Aldrich (St. Louis). 5-HT creatinine sulfate was used for all assays requiring serotonin (Sigma-Aldrich).
Creation of strains containing null mutations in multiple genes encoding 5-HT receptors:
Putative null alleles are available for genes encoding each of the four currently identified C. elegans 5-HT receptors: ser-4(ok512)III, mod-1(ok103)V, ser-7(tm1325)X, and ser-1(ok345)X. All mutant animals were backcrossed with the N2 Bristol strain at least five times before use in assays or crosses. Using standard genetic techniques, mod-1(ok103) animals were crossed with ser-4(ok512) animals to create ser-4(ok512);mod-1(ok103) (OT179) double mutants. ser-4;mod-1 (OT179) double mutants were then crossed into ser-7(tm1325) ser-1(ok345) (DA2109) animals to create ser-4;mod-1;ser-7 ser-1 (OT182) quadruple mutants. To create the ser-5;ser-4;mod-1;ser-7 ser-1 quintuple mutant (Q5), ser-5(tm2654)I was outcrossed (five times) and then crossed into ser-7(tm1325) ser-1(ok345) (DA2109) animals or ser-4;mod-1 animals to create ser-5;ser-7 ser-1 (RWK1) or ser-5;ser-4;mod-1 (RWK2) triple mutants, respectively. ser-5;ser-7 ser-1 animals were then crossed into ser-4;mod-1;ser-7 ser-1 animals to create ser-5;ser-4;mod-1;ser-7 ser-1 (RWK3) quintuple mutants. ser-5;ser-4;mod-1;ser-7 ser-1 (RWK3) mutants were crossed with either ser-5;ser-7 ser-1 (RWK1) or ser-5;ser-4;mod-1 (RWK2) triple mutants to create ser-5;ser-4;mod-1;ser-7 (RWK14) and ser-5;ser-4;mod-1;ser-1 (RWK15) or ser-5;mod-1;ser-7 ser-1 (RWK16) and ser-5;ser-4;ser-7 ser-1 (RWK17) quadruple mutants, respectively. All mutant animals were screened by PCR with primers flanking the deletions to confirm the crosses. Animals containing mutant alleles have correspondingly smaller PCR products relative to wild type. To confirm that the mutant animals were homozygous for the deletion, a second PCR reaction was conducted using the forward primer flanking the deletion and a reverse primer internal for the deletion. Homozygous mutants produced no PCR product in this reaction.
Rescue contructs and strains:
All rescue constructs were created by overlap fusion PCR (Hobert 2002). For overlap PCR, constructs were pooled from multiple reactions and were co-injected with myo-3p∷gfp, unc-119p∷gfp or rol-6 and carrier DNA (to 100 ng) into gonads of wild-type and null mutant animals by standard techniques (Mello and Fire 1995). Multiple lines for each construct examined. The RWK4 ser-5;ser-4;mod-1;ser-7 ser-1 fvEx1[myo-3p∷ser-5] expresses a ser-5 transgene in body-wall and vulval muscle of ser-5;ser-4;mod-1;ser-7 ser-1 null animals (Okkema et al. 1993). The myo-3p∷ser-5∷gfp transgene includes the myo-3 promoter, the ser-5 cDNA, and the unc-54 3′-UTR and was injected into the gonads of ser-5;ser-4;mod-1;ser-7 ser-1 animals at 10 ng/μl along with the co-injection marker unc-119∷gfp. FY750 ser-5(tm2654)grEx162[ser-5p∷ser-5] expresses a full-length ser-5 transgene in ser-5 null animals, including the ser-5 3′-UTR. The ser-5p∷ser-5 transgene was created by PCR fusion, including a 5-kb ser-5 promoter using SER-5F (CCCCCGAAGGTAATGGAAA) and SER-5R (TGGGCAGTTATTTTTGGGATTC) to produce an 8.6-kb product.
Localization of SER-5 expression:
A ser-5 full-length translational fusion was created by overlap fusion PCR (Hobert 2002). To create the full-length ser-5∷gfp transgene with sequence coding for GFP inserted into the predicted C-terminal tail 10 aa from the stop codon (exon 7), three different PCR fragments were fused by two rounds of PCR. Primers were designed to amplify the 5′-end of the gene ∼5 kb upstream of the ATG (SER-5 A1 F1: CTTACCTGTAGAATGGCACAAACCC), including the full-length gene and portion of the exon 7 (SER-5 B1 R: caactccagtgaaaagttcttctcctttactcatAGTAATGAGCCCAACAACTTTGGTTC; sequence representing the gfp overlap is in lowercase). A second set of primers was designed to amplify a short gfp overlap with the ser-5 3′-end of the gene, including the last 10 aa and 3′-UTR ∼1 kb downstream of the stop codon (SER-5 C1 F: ttggaattcgctagccggccataccagCCTGATCATAAGTTGCAGACAGTAGC and SER-5 D1 R: CTTTATGAACCTATGTTGTGTTGTGAC). A full-length amplified gfp fragment was then fused in frame with the ser-5 3′ product. Using nested primers, this resulting product was combined and amplified with the ser-5 5′ fragment to generate a full-length gene product and its endogenous 3′-UTR with GFP inserted in the C terminus of the receptor. PCR products were pooled from at least three separate PCR reactions (5 ng total) and were co-injected either alone or with rol-6 plasmid (pRF4) and carrier DNA into gonads of ser-5 (tm2564) null mutant animals to create the RWK7 ser-5(tm2654) fvEx2 [ser-5p∷ser-5∷gfp] transgenic line. At least five transformed lines were analyzed.
DiD staining of amphidial sensory neurons:
Uptake of dye in living animals was assayed as described (Herman and Hedgecock 1990). Briefly, stock solution (1 mm) of 1,1′-dioctadecyl-3,3,3′,3′-tetrametylindodicarbocyanine (DiD) (Invitrogen/Molecular Probes, Eugene, OR) was diluted 1:200 in M9 buffer. Using a 96-well microtiter plate, animals were immersed in wells containing 100 μl of diluted DiD and allowed to incubate for 1 hr at room temperature. Animals were then transferred to a fresh NGM plate seeded with OP50 and allowed to crawl on the bacterial lawn for 1–2 hr to destain. Animals were then put on agarose pads with 20 mm sodium azide and visualized by confocal microscopy using the appropriate filters.
Behavioral assays:
Egg laying was assayed at 20°–22°, as described by Hobson et al. (2006) unless stated otherwise. Young adult animals were synchronized by picking fourth-stage larvae 24 hr before assay. For assays in the absence of bacteria, animals were transferred to fresh bacteria-free NGM and allowed to move away from any transferred bacteria prior to replating and assay. 5-HT-stimulated egg laying was measured by placing animals on NGM plates with or without 5-HT (26 mm) in the presence or absence of bacteria. To assess egg retention, individual animals were dissolved in 1% hypochlorite, and released eggs were counted. To stage freshly laid eggs, animals were plated on NGM seeded with bacteria and left for 45 min, and the stage of eggs was determined as described by Koelle and Horvitz (1996). Locomotion was assayed as described by Sawin et al. (2000). Briefly, synchronized young adult animals were transferred to bacteria-free NGM to prevent bacterial carryover and then to NGM plates containing no drug or 15 mm 5-HT, and body bends/20 sec were counted after 20 min.
Identification of the ser-5 cDNA:
The full-length ser-5 cDNA sequence was generated by 5′ and 3′ RACE using gene-specific primers and a C. elegans cDNA pool prepared using a Marathon cDNA amplification kit (BD Biosciences Clontech, Palo Alto, CA). Total RNA was isolated from mixed-staged N2 worms with Trizol (Invitrogen, Carlsbad, CA), and poly(A)+ RNA was prepared with the PolyATract mRNA isolation system (Promega, Madison, WI). The cDNAs were synthesized from poly(A)+ RNA and ligated with adapter primer 1 (AP1) and adapter primer 2 (AP2). Multiple fragments were subcloned into PCR 2.1 (Invitrogen) and sequenced (Plant and Microbe Genomics Facility, Columbus, OH). The full-length ser-5 cDNA was ∼1.4 kb and differed significantly from that described in the Wormbase database. For expression, ser-5 was amplified using two gene-specific primers—ser-5 F (5′-ATGTTTCAAGTCTCCGATGGAGATGGC) and ser-5 R (5′-CGAAGCCACTGTCTGCAACTGGAA)—and subcloned into pDisplay (Invitrogen).
Sequence alignments and production of phylogenetic tree:
To remove hypervariable regions, each vertebrate and invertebrate 5-HT receptor sequence was truncated at the N terminus [3 residues before transmembrane I (TMI)], the third intracellular loop (8 residues after TMV and 6 residues before TMVI), and the C terminus (12 residues after TMVII). The initial alignment was performed with the modified sequences using the MegAlign program (DNAStar, Madison, WI) with ClustalW parameters set to optimize protein sequence alignments (multiple alignment parameters: gap opening penalty of 15 and gap extension penalty of 0.3; pairwise alignment parameters: gap opening penalty of 35 and gap extension penalty of 0.75). The resulting alignment was then fine-tuned manually. All alignments are available upon request. DNAStar also performed the neighbor-joining analysis, followed by bootstrapping (1000 replicates, random seed) to produce the tree reported here. The C. elegans GAR-1 sequence was utilized as an outgroup. Accession numbers for all sequences used are listed in the legend to Figure 3.
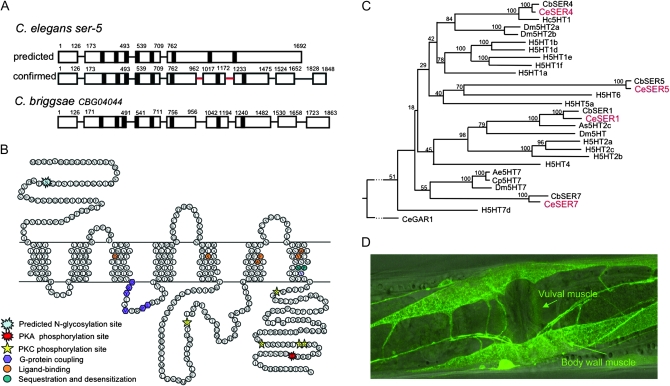
Figure 3.—
Genomic structure, predicted amino acid sequence, phylogenetic relationships, and muscle expression of ser-5. (A) New ser-5 genomic structure predicted from RACE. ser-5 contains eight exons and seven introns and exhibits an intron/exon pattern similar to that predicted for its C. briggsae homolog, CBG04044. Intron positions conserved in the human 5-HT6 receptor are indicated by a red line. (B) Predicted amino acid sequence and membrane-spanning model of SER-5. Amino acids potentially involved in ligand binding, G-protein coupling, sequestration, and desensitization are illustrated (Moro et al. 1993; Barak et al. 1994; Roth et al. 1997). Potential PKC, PKA, and palmitolylation sites were identified using Scanprosite. (C) Unrooted phylogenetic tree of previously characterized vertebrate and invertebrate 5-HT receptors. Sequences were modified to remove hypervariable regions by deletion of the N termini, 5 amino acids before the first predicted transmembrane domains; the third intracellular loops, 5 amino acids after and before predicted transmembrane domains 5 and 6, respectively; and the C termini, 10 amino acids following predicted transmembrane domain 7. Annotated sequences were initially aligned using MegAlign in DNAStar with Clustal W using default parameters and fine-tuned by hand. Bootstrapping was undertaken in DNAStar (1000 replicates with random seed). Homo sapiens (H): H5HT-1a (CAA40962), H5HT1b (P28222), H5HT1d (P28221), H5HT1e (CAA77558), H5HT1f (AAA36605), H5HT2a (CAA40963), H5HT2b (CAA54513), H5HT2c (AAF35842), H5HT4 (Q13639), H5HT5a (CAA57168), H5HT6 (AAA92622), Hs5HT7 (CAH69965). C. elegans (Ce) in red: CeSER1 (NP_001024728), CeSER4 (NP_497452), CeSER7 (NP_741730), and CeSER5 (our unpublished results). C. briggsae (Cb): CbSER-1 (CAE69959), CbSER4 (CAE69091), CbSER7 (CAE58847), and CbSER5 (CAE60436). Aedes egyptii (Ae): Ae5HT7 (AAG49292). Drosophila melanogaster (Dm): Dm5HT7 (NP_524599), Dm5HT2a (NP_725849), Dm5HT2b (CAA77571), and Dm5HT (NP_730859). Haemonchus contortus (Hc): Hc5HT1e (AAO45883). Ascaris suum (As): As5HT-2c (AAC78396). (D) Expression of a full-length ser-5p∷ser-5∷gfp translational fusion in muscle. Wild-type animals expressing a full-length ser-5 transgene with sequence coding for GFP inserted into the predicted SER-5 C terminus were examined for GFP fluorescence, as described in materials and methods.
RESULTS
The 5-HT stimulation of egg laying is mediated by multiple 5-HT receptors and involves both excitatory and inhibitory inputs:
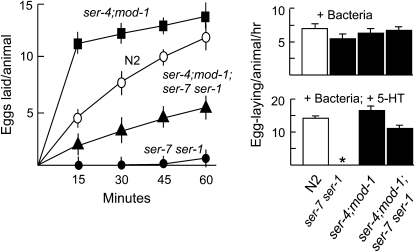
Each of the four previously identified C. elegans 5-HT receptors plays a role in modulating 5-HT-stimulated egg laying (SER-1, SER-4, SER-7, and MOD-1). For example, SER-1 and SER-7 are expressed in vulval muscle and stimulate egg laying; i.e., 5-HT does not stimulate egg laying in animals with null mutations in either ser-1 or ser-7 (Carnell et al. 2005; Dempsey et al. 2005; Hobson et al. 2006). In contrast, 5-HT inhibits egg laying on bacteria in ser-1 or ser-7 null backgrounds through a pathway that appears to involve both mod-1 and ser-4, as null mutations in either (or both; see Figure 1) of these genes abolishes the 5-HT inhibition of egg laying on bacteria observed in ser-1 or ser-7 null backgrounds (Carnell et al. 2005; Hobson et al. 2006). Therefore, to examine the potential interactions of these 5-HT receptors in the modulation of egg laying, we generated mutant animals containing combinations of null alleles for the genes encoding each of these receptors, including a quadruple null mutant (ser-4;mod-1;ser-7 ser-1), lacking all of the previously identified 5-HT receptors. The ser-4;mod-1;ser-7 ser-1 animals were viable, although they failed to respond to 5-HT in most locomotory, feeding, and behavioral assays (data not shown). All ser-4, mod-1 or ser-4;mod-1 null animals laid eggs more rapidly on 5-HT than wild-type animals, and this increased 5-HT responsiveness was more much apparent earlier in the egg-laying assays (Figure 1; data not shown; Komuniecki et al. 2004). For example, after 15 min on 5-HT, ser-4;mod-1 and wild-type animals had laid 12.3 ± 3.2 and 4.8 ± 1.1 eggs, respectively, while after 60 min they had laid 13.6 ± 2.6 and 11.7 ± 1.8 eggs, respectively (Figure 1). Together, these results support earlier observations that SER-4 and MOD-1 provide inhibitory input into the egg-laying process (Komuniecki et al. 2004; Carnell et al. 2005; Dempsey et al. 2005). Surprisingly, although 5-HT did not stimulate egg laying in ser-1, ser-7 or ser-7 ser-1 animals, 5-HT still significantly stimulated egg laying in ser-4;mod-1;ser-7 ser-1 animals (Figure 1 and Figure 2; Hobson et al. 2006). This result strongly suggests that the inability of the ser-1, ser-7 and ser-7 ser-1 animals to respond to 5-HT is due not only to the potentially decreased responsiveness of the vulval muscle, but also to the serotonergic inhibition of egg laying mediated by ser-4 and/or by mod-1. In addition, the demonstration of significant 5-HT-stimulated egg laying in the ser-4;mod-1;ser-7 ser-1 null animals also suggests that, in the absence of inhibitory serotonergic input through SER-4 and/or MOD-1, 5-HT can stimulate egg laying through additional, as yet unidentified, 5-HT receptor(s).
Figure 1.—
Effects of 5-HT on egg laying in animals expressing combinations of 5-HT receptors. Egg laying was assayed on NGM agar plates in the presence or absence of 5-HT or bacteria (E. coli OP50), as described in materials and methods. At least three trials with 15 animals/trial were performed for each strain. Data presented as mean ±SE and analyzed by two-tailed Student's t-test. *P < 0.001, significantly different from wild-type animals examined under identical conditions. ○, N2; ▪, ser-4;mod-1; •, ser-7 ser-1; and ▴, ser-4;mod-1;ser-7 ser-1.
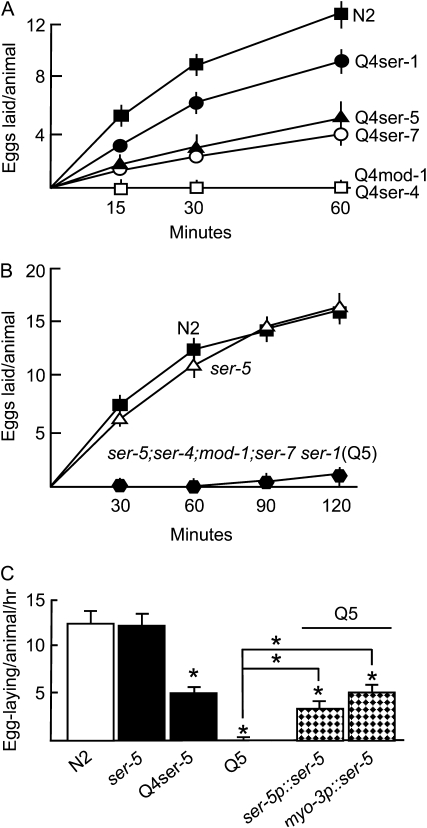
Figure 2.—
The muscle expression of SER-5 is essential for 5-HT-stimulated egg laying in ser-4;mod-1;ser-7 ser-1 null animals. Egg laying was assayed on NGM agar plates in the presence of 5-HT as described in materials and methods (A–C). At least three trials with 15 animals/trial were performed for each strain. Data presented as mean ±SE and analyzed by two-tailed Student's t-test. *P < 0.001, significantly different from wild-type animals examined under identical conditions. ▪, N2; ▵, ser-5(tm2654); •, Q4ser-1, ser-5;ser-4;mod-1;ser-7; ▴, Q4ser-5, ser-4;mod-1;ser-7 ser-1; ○, Q4ser-7, ser-5;ser-4;mod-1;ser-1; □, Q4mod-1, ser-5;ser-4;ser-7 ser-1; □, Q4ser-4, ser-5;mod-1;ser-7 ser-1;  , Q5, ser-5; ser-4;mod-1;ser-7 ser-1. Hatchmarked bars in C: Q5 animals rescued with the transgenes indicated.
, Q5, ser-5; ser-4;mod-1;ser-7 ser-1. Hatchmarked bars in C: Q5 animals rescued with the transgenes indicated.
Identification of an additional G-protein-coupled receptor, SER-5, involved in the serotonergic stimulation of egg laying in ser-4;mod-1;ser-7 ser-1 animals:
To identify additional amine receptor(s) responsible for the 5-HT-stimulated egg laying observed in ser-4;mod-1;ser-7 ser-1 animals, wild-type, RNAi-sensitive rrf-3(pk1269), and ser-4;mod-1;ser-7 ser-1 animals were screened using RNAi for predicted, previously uncharacterized, C. elegans biogenic amine receptors that might be involved in 5-HT-stimulated egg laying (F14D12.6, Y54G2A.35, F16D3.7, and TYRA-3). Using this approach, we identified a putative 5-HT receptor, F16D3.7 (SER-5), which was potentially involved in 5-HT-stimulated egg laying in ser-4;mod-1;ser-7 ser-1 animals (data not shown; Carre-Pierrat et al. 2006). To confirm a role of SER-5 in the serotonergic modulation of egg laying, 5-HT-stimulated egg laying was examined in ser-5(tm2654) animals (Figure 2). The ser-5(tm2654) animals contain a 348-bp deletion in exon 4, resulting in deletion of a portion of TMV, the third intracellular loop, and half of TMVI. Therefore, ser-5(tm2654) is a predicted null allele, as TMs V and VI are required for ligand binding (Strader et al. 1989; Choudhary et al. 1993; Almaula et al. 1996; Roth et al. 1997) and for G-protein coupling (Moro et al. 1993). Under standard conditions, ser-5 null animals exhibited wild-type responses to 5-HT in egg-laying assays (Figure 2). However, since the 5-HT stimulation of egg laying in ser-4;mod-1;ser-7 ser-1 animals was significantly less than that observed in wild-type animals, we reasoned that any potential role of SER-5 in wild-type animals might be obscured by the presence of more robust serotonergic inputs from the other 5-HT receptors. Therefore, 5-HT-stimulated egg laying was also examined in quadruple null animals (Q4) expressing only individual receptors and in ser-5;ser-4;mod-1;ser-7 ser-1 quintuple null animals (Q5) lacking all five receptors (Figure 2). Quadruple null animals expressing only ser-1 (Q4ser-1), ser-7 (Q4ser-7), or ser-5 (Q4ser-5) alone all exhibited significant, but varying, degrees of 5-HT-stimulated egg laying (with ser-1 > ser-5 = ser-7), suggesting that serotonergic signaling through each of these receptors individually was capable of stimulating egg laying (Figure 2). In contrast, as predicted, quadruple null animals expressing only ser-4 (Q4ser-4), mod-1 (Q4mod-1), or Q5 quintuple null animals failed to lay eggs on 5-HT, supporting our hypothesis that ser-5 was involved in the 5-HT-dependent egg laying observed above in the ser-4;mod-1;ser-7 ser-1 null animals. In addition, these data suggest that all of the major 5-HT receptors involved in the serotonergic modulation of egg laying have been identified (Figure 2).
No ser-5 ESTs are present in the database. Therefore, we attempted to confirm the predicted SER-5 amino acid sequence by 5′ and 3′ RACE using multiple cDNA pools from mixed-stage animals. Surprisingly, the ser-5 cDNA sequence and predicted intron/exon splicing pattern of the ser-5 gene differed significantly from that predicted in Wormbase (Figure 3). On the basis of our RACE data, the “new” ser-5 genomic sequence contains eight exons and seven introns and closely resembles that of its putative Caenorhabditis briggsae ortholog (CBG04044, accession no. CAE60436). The predicted SER-5 amino acid sequence contains seven putative transmembrane regions that include conserved amino acids involved in biogenic amine binding (Figure 3B; Roth et al. 1997). In addition, SER-5 contains the highly conserved DRY motif in the second intracellular loop, potential protein kinase A (PKA) and protein kinase C (PKC) phosphorylation sites, and the NPXXY motif within TM7 that has been implicated in receptor desensitization and internalization (Figure 3B; Moro et al. 1993; Barak et al. 1994).
On the basis of an analysis of all previously characterized 5-HT receptor sequences, the predicted SER-5 amino acid sequence is most identical to those of mammalian 5-HT6 receptors (Figure 3, A–C). For example, SER-5 was 35% identical to the human 5-HT6 receptor within the seven predicted transmembrane domains (Figure 3B). In addition, both SER-5 and 5-HT6 receptors have short third intracellular loops (67 and 60 amino acids, respectively) with BBXXB296 (where B is a basic amino acid) motifs at the C termini of the loop that in other G-protein-coupled receptors (GPCRs) can confer constitutive activity and a conserved PLRYK169 motif in the second intracellular loop that is not found in any other 5-HT receptor subtypes (Kohen et al. 2001; Purohit et al. 2003; Mitchell and Neumaier 2005). Although the phylogenetic tree was generated using truncated amino acid sequences with the more variable N and C termini and third intracellular loops deleted, similar results were obtained using the full-length receptors (data not shown). Interestingly, all of the 5-HT receptors clustered according to their documented G-protein coupling, regardless of phylogenetic origin, validating the utility of the tree and supporting the identification of SER-5 as a Gαs-coupled 5-HT receptor (Figure 3C). Finally, the genes encoding the mammalian 5-HT6 receptors contain two introns within the sequence encoding the putative third intracellular and third extracellular loops. The positions of both introns are conserved in ser-5 (Figure 3A).
The muscle expression of SER-5 rescues 5-HT-stimulated egg laying in ser-5;ser-4;mod-1;ser-7 ser-1 null animals:
Carre-Pierrat et al. (2006) reported that SER-5 was expressed in both neurons and body-wall/vulval muscle on the basis of fluorescence from a transcriptional GFP reporter that included ∼3.5 kb upstream of the putative start codon. We have confirmed these results using a full-length ser-5 transgene that has sequence coding for GFP inserted into the predicted SER-5 C terminus. In addition to extensive neuronal fluorescence, we observed robust fluorescence in body-wall muscle that extended into the muscle arms and weak and variable fluorescence in vulval muscle (Figure 3D). Given these observations, we attempted to rescue 5-HT-stimulated egg laying in Q5 null animals by expressing SER-5 directly in muscle using the myo-3 promoter (Figure 2). Indeed, the muscle expression of SER-5 in the Q5 animals restored 5-HT-stimulated egg laying to levels observed in Q4ser-5 animals (Figure 2). Together, these results suggest that the muscle expression of SER-5 is sufficient for the serotonergic modulation of egg laying observed above in Q4ser-5 animals.
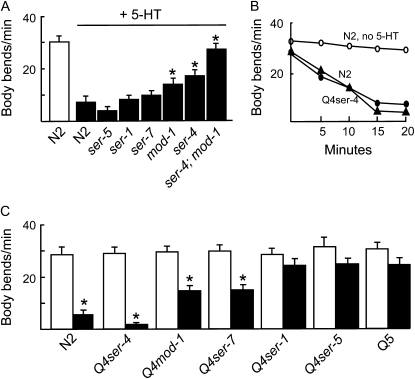
Since SER-5 is expressed in both body-wall and vulval muscle, we also examined whether SER-5 was involved in the serotonergic modulation of locomotion, especially since, in addition to modulating the rate of egg laying, 5-HT also appears to modulate the pattern of egg-laying events, coordinating locomotion with egg laying (Horvitz et al. 1982; Waggoner et al. 1998). The effects of 5-HT on locomotion were measured in animals with null mutations in individual 5-HT receptors and in quadruple null animals expressing only individual 5-HT receptors, as described above for egg laying (Figure 4). SER-5 did not appear to play a major role in the modulation of locomotory rate. For example, 5-HT slowed ser-5 null animals to the same extent as wild-type animals. In addition, Q4ser-5 animals that express only SER-5 and Q5 animals that lack all five 5-HT receptors were equally resistant to the 5-HT-dependent inhibition of locomotion. In contrast, both ser-4 and mod-1 appeared to be involved in the 5-HT-dependent inhibition of locomotion; i.e., 5-HT did not inhibit locomotion in ser-4;mod-1 double mutants and inhibited Q4ser-4 animals that express only SER-4 as rapidly as wild-type animals (Figure 4). These results suggest that, in contrast to SER-4 or MOD-1, SER-5 does not contribute directly to the modulation of locomotory rate.
Figure 4.—
Effects of 5-HT on locomotion. Animals were plated on NGM and body bends/minute were counted in the presence (solid) or absence (open) of 5-HT, as described in materials and methods. Locomotion was assayed after 20 min on 15 mm 5-HT (A–C). Data are presented as mean ±SE. Each group included at least 15 animals and each experiment was performed in triplicate. An asterisk indicates results significantly different from N2 in the presence of 5-HT (P < 0.001). Q4ser-5, ser-4;mod-1;ser-7 ser-1 null animals; Q4ser-4, ser-5;mod-1;ser-7 ser-1 null animals; Q4mod-1, ser-5;ser-4;ser-7 ser-1 null animals; Q4ser-1, ser-5;ser-4;mod-1;ser-7 null animals; Q4ser-7, ser-5;ser-4;mod-1;ser-1 null animals; Q5, ser-5;ser-4;mod-1;ser-7 ser-1 null animals.
Effects of serotonergic signaling on egg retention:
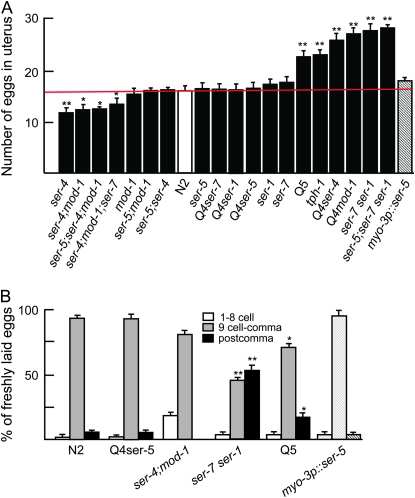
The role of dual stimulatory/inhibitory serotonergic signaling in the regulation of egg laying is supported by data on egg retention (Figure 5A). For example, most animals lacking both SER-1 and SER-7 (ser-7 ser-1, ser-5;ser7 ser-1, Q4ser-4, Q4mod-1, and Q5 mutants) were egg laying defective (Egl-d) and retained significantly more eggs than wild-type animals, suggesting that, in the absence of both ser-1 and ser-7, inhibitory serotonergic input predominated (Figure 5A). Similarly, animals lacking both SER-1 and SER-7 and either or both SER-4 or MOD-1 also retained significantly more eggs than tph-1 null animals that lack significant amounts of 5-HT on the basis of HPLC and immunostaining, supporting an inhibitory role for SER-4 and MOD-1 in the egg-laying circuit. In contrast, animals lacking SER-4, but retaining two of the three stimulatory 5-HT receptors (ser-4, ser-4;mod-1, ser-5;ser-4;mod-1, or ser-4;mod-1;ser-7) were egg laying constitutive and retained significantly fewer eggs than wild-type animals, further supporting an inhibitory role for SER-4 in the egg-laying circuit (Figure 5A). Interestingly, Q4 animals expressing only ser-1, ser-5, or ser-7 were not egg laying and retained the same number of eggs as wild-type animals, highlighting the delicate balance of serotonergic input that regulates egg laying (Figure 5A). As predicted on the basis of this observation, the egg-retention phenotype in the Q5 animals could be rescued by the muscle expression of either ser-5 or ser-7 (Figure 5A; data not shown). Finally, both Q5 animals that lack all known 5-HT receptors and tph-1 null animals that lack most, if not all, serotonergic signaling were Egl-d and retained about the same number of eggs, supporting the suggestion that all of the major 5-HT receptors involved in the egg-laying circuit had been identified (Zhang et al. 2005).
Figure 5.—
Egg retention and stages of freshly laid eggs in animals expressing combinations of 5-HT receptors. (A) Animals were picked as fourth-stage larvae and grown for 24 hr, and the number of eggs in individual uteri were counted, as described in materials and methods. (B) Staged adults were plated on NGM seeded with bacteria and left for 45 min, and the stage of eggs was determined. Data are presented as mean ±SE (n = 75) and analyzed by two-tailed Student's t-test. *P < 0.005 and **P < 0.001: significantly different from wild-type animals examined under identical conditions. Q4ser-1, ser-5;ser-4;mod-1;ser-7; Q4ser-4, ser-5;mod-1;ser-7 ser-1; Q4ser-5, ser-4;mod-1;ser-7 ser-1, Q4ser-7, ser-5;ser-4;mod-1;ser-1; Q4mod-1, ser-5;ser-4;ser-7 ser-1; Q5, ser-5; ser-4;mod-1;ser-7 ser-1. Hatchmarked bars: Q5 animals rescued with the transgenes indicated.
As predicted from the egg retention data, wild-type and Q4ser-5 animals laid most of their eggs at the comma stage (Figure 5B). In contrast, ser-4;mod-1 animals laid a significantly higher proportion of early embryos (one- to eight-cell embryos) and ser-7 ser-1 and Q5 animals laid a significantly higher proportion of late-stage embryos (post comma, Figure 5B). These differences in egg laying were not the result of defects in fecundity, as all of the animals used in this study produced similar numbers of eggs (data not shown).
Taken together, these observations suggest that serotonergic input into the regulation of egg laying is complex, that both excitatory and inhibitory serotonergic inputs are involved, and that additional redundant regulatory pathways are probably operative.
DISCUSSION
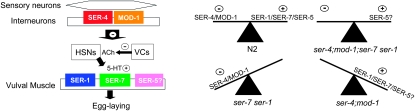
Serotonin modulates most key processes in C. elegans and this study highlights previous observations on the complexity of serotonergic signaling in the regulation of egg laying. To summarize, (1) at least five different amine receptors are involved in the 5-HT stimulation of egg laying, (2) serotonergic input can be both excitatory and inhibitory, (3) serotonergic signaling appears to overlap with other modulatory pathways, and (4) 5-HT appears to function at multiple levels within the egg-laying circuit and may modulate both the release of neurotransmitters/neuromodulators and the activity state of vulval muscle. For example, SER-1, SER-7, and the putative 5-HT receptor, SER-5, are all expressed in vulval muscle and the muscle expression of each rescues 5-HT-insensitive egg-laying phenotypes; SER-1 and SER-7 in ser-1 and ser-7 null animals, respectively, and SER-5 in Q5 null animals (Figure 6; Carnell et al. 2005; Hobson et al. 2006; Xiao et al. 2006). In contrast, the expression of both SER-4 and MOD-1 appears to be exclusively neuronal and neither 5-HT receptor appears to be expressed in vulval muscle or the HSN/VC neurons that control egg laying, suggesting that SER-4 and MOD-1 act upstream to inhibit the egg-laying neurons directly and/or to regulate the release of additional modulators (Mendel et al. 1995; Segalat et al. 1995; Lackner et al. 1999; Bany et al. 2003; Moresco and Koelle 2004; Carnell et al. 2005; Hobson et al. 2006). Fluorescence from a ser-4∷gfp transgene has been observed primarily in the RIB and RIS head interneurons and the PVT and either DVA or DVC tail interneurons (as well as a few other potentially faintly fluorescing neurons in the nerve ring (Tsalik et al. 2003). Similarly, a mod-1∷gfp transgene appears to be expressed in the AIA, AIB, AIY, RID, and probably AIZ interneurons, as well as additional interneurons in the head, ventral cord, and tail (Ranganathan et al. 2000; Wenick and Hobert 2004; Zhang et al. 2005). However, the definitive site(s) of SER-4 and MOD-1 action and their roles in the modulation of egg laying remain to be determined. In addition, it also will be important to separate the effects of 5-HT directly on the egg-laying machinery and secondarily on locomotion (Horvitz et al. 1982; Waggoner et al. 1998). For example, these 5-HT receptors modulate many aspects of locomotion, including rate and reversal frequency, and previous studies have demonstrated a relationship between egg laying and locomotion; i.e., eggs are laid in clusters and the velocity of forward movement increases significantly immediately (30 sec) prior to an egg-laying event (Hardaker et al. 2001). This increase in velocity requires the AVF interneurons and 5-HT, as it is absent in tph-1 null animals that lack 5-HT. Clearly, additional studies correlating the serotonergic modulation of egg laying and locomotion are warranted.
Figure 6.—
Dual excitatory and inhibitory inputs modulate 5-HT-stimulated egg laying through at least five different receptors. Left: Putative localization of 5-HT receptors in the egg-laying circuit. None of the 5-HT receptors appear to be expressed directly in the egg-laying neurons (HSNs/VCs). Instead, SER-4 and MOD-1 appear to be expressed upstream in interneurons in the nerve ring, SER-1 and SER-7 function directly in vulval muscle, and SER-5 is expressed in both body-wall and vulval muscle, although its definitive site of action remains to be determined. Right: Both excitatory and inhibitory serotonergic inputs modulate egg laying. The serotonergic regulation of egg laying involves a delicate balance of excitatory and inhibitory inputs. In ser-4;mod-1;ser-7 ser-1 animals, egg laying on bacteria appears to be wild type, most probably due to redundant regulatory pathways. In contrast, in the absence of either inhibitory or excitatory serotonergic input, the balance is tipped and ser-4;mod-1 or ser-7 ser-1 double mutants exhibit more obvious egg-laying phenotypes than either wild-type animals or mutant animals lacking most serotonergic signaling.
Dual excitatory and inhibitory serotonergic modulation of egg laying:
This study confirms that dual excitatory and inhibitory serotonergic inputs combine to modulate egg laying in C. elegans, as suggested by other workers, and demonstrates that the lack of 5-HT-stimulated egg laying in ser-1 and ser-7 null animals results in large part from unchecked inhibitory serotonergic inputs mediated by SER-4 and MOD-1 (Carnell et al. 2005; Dempsey et al. 2005; Hobson et al. 2006). In other organisms, interactions between 5-HT receptors in single cells or tissues have been described, but few studies have defined a circuit with both inhibitory and stimulatory serotonergic inputs into a single pathway. Recently, multiple serotonergic inputs regulating bladder contraction have been suggested on the basis of pharmacological analyses. For example, acetylcholine release from isolated detrusor muscle strips appears to be stimulated by Gαs-coupled 5-HT4 and 5-HT7 receptors and inhibited by Gαi/o-coupled 5-HT1 receptors (D'Agostino et al. 2006). Similarly, low concentrations of 5-HT (1 nm) inhibit and higher concentrations stimulate heart rate in Drosophila larvae, suggesting differential inputs from 5-HT receptors with potentially different affinities for 5-HT (Dasari and Cooper 2006). Although a number of additional studies in invertebrates have used mammalian 5-HT subtype-specific agonists and antagonists to predict the involvement of multiple 5-HT receptors in the regulation of various behaviors, these observations should be interpreted with caution. Ligands specific for vertebrate 5-HT receptor subtypes can bind with dramatically different affinities and specificities to their invertebrate counterparts. For example, α-methyl-5-HT, a potent agonist of mammalian 5-HT2 receptors is also a potent agonist of SER-7, while ketanserin, a 5-HT2 antagonist, binds with very low affinity to invertebrate 5-HT2-like receptors (Hamdan et al. 1999; R. J. Hobson and R. Komuniecki, unpublished observations). Similarly, 5-CT, a potent agonist of mammalian 5-HT1 and 5-HT7, does not activate SER-7 (Hobson et al. 2003). The absence of selective ligands for invertebrate 5-HT receptors highlights the utility for genetic model systems, such as C. elegans, in the study of multi-level, multi-receptor, serotonergic regulation in invertebrates, since individual 5-HT receptors can be readily expressed in individual cells in a multitude of different mutant backgrounds. Most importantly, animals expressing only individual 5-HT receptors from their native promoters can be generated. For example, the quadruple null animals used in the present study presumably express only single 5-HT receptors and therefore the effects of low levels of exogenous 5-HT on these animals will be mediated exclusively by these individual receptors, minimizing the need for receptor-specific ligands.
The identification of the individual excitatory/inhibitory 5-HT receptors involved in the modulation of egg laying are in agreement with the complex roles proposed for individual G-proteins in the regulation of egg laying (Bany et al. 2003; Bastiani et al. 2003; Shyn et al. 2003; Dempsey et al. 2005). For example, the direct modulation of vulval muscle calcium dynamics by 5-HT is dependent on EGL-30, supporting a direct role for SER-1/EGL-30 in vulval muscle (Brundage et al. 1996; Bany et al. 2003; Bastiani et al. 2003; Shyn et al. 2003; Carnell et al. 2005; Hobson et al. 2006). GOA-1 appears to play multiple roles in the modulation of egg laying. In vulval muscle, GOA-1 is not involved directly in responses to 5-HT, but instead antagonizes the effects of 5-HT-stimulated calcium transients, potentially through a number of uncharacterized Gαo-coupled receptors (Bastiani et al. 2003; Shyn et al. 2003). In the HSNs, GAR-2, a Gαo-coupled muscarinic receptor and GOA-1 appear to mediate the effects of acetylcholine released by the VCs and to inhibit the release of 5-HT and perhaps other neurotransmitters from the HSNs (Bany et al. 2003). Finally, GOA-1 is also essential for the 5-HT-dependent silencing of the HSNs, presumably through the Gαo-coupled SER-4 (Bany et al. 2003; Shyn et al. 2003). However, it is unclear if SER-4 and GOA-1 act directly in the HSNs or in neurons innervating the HSNs, especially since SER-4 expression appears to be limited to a small number of interneurons, and is not observed in the HSNs. In fact, it is not clear if the serotonergic silencing of the HSNs is direct or involves the release of other unidentified neuromodulators.
Role of SER-5 in egg laying:
SER-5 appears to be responsible for the 5-HT-stimulated egg laying observed in ser-4;mod-1;ser-7 ser-1 null animals, suggesting that SER-5 may be a 5-HT receptor. As noted above, SER-5 is most identical to mammalian 5-HT6 Gαs-coupled receptors. However, since the 5-HT specificity of SER-5 has yet to be demonstrated directly after heterologous expression, it is possible that an additional, as yet unidentified, 5-HT receptor is involved in the release of another neuromodulator that then activates SER-5 in muscle. We consider this to be a remote possibility, given (1) the close alignment of the new SER-5 predicted amino acid sequence with other 5-HT receptors; (2) the utility of the tree in predicting the G-protein coupling of all previously characterized 5-HT receptors; and (3) the identification of a second 5-HT-stimulated behavior that is also dependent on SER-5, i.e., 5-HT-dependent increases in aversive responses to dilute octanol (Figures 2 and 3; G. Harris and R. Komuniecki, unpublished results). Unfortunately, attempts to characterize the pharmacology and coupling of SER-5 directly from heterologous expression in a number of mammalian cell lines have been unsuccessful. On the basis of the immunolocalization of the FLAG-tagged receptor, SER-5 appears to be expressed at low levels and retained in the endoplasmic reticulum (R. Komuniecki, unpublished results). The heterologous expression of SER-5 may problematic; for example, many mammalian olfactory GPCRs are resistant to heterologous expression and appear to require additional ancillary proteins for proper membrane localization (Brady and Limbird 2002; Tan et al. 2004; Luttrell 2006). Interestingly, Carre-Pierrat et al. (2006) reported that 5-HT did not stimulate cAMP levels after the heterologous expression of SER-5 in COS-7 cells. However, it is unclear which SER-5 sequence was examined in these studies, given our RACE results described above, and whether the receptor was actually surface expressed.
Role of other regulatory pathways in the modulation of egg laying:
Both Q4ser-5 and Q5 animals were viable and superficially wild type with respect to locomotion and feeding, although exogenous 5-HT did not inhibit locomotion. In addition, both mutant animals appeared to acutely upregulate egg laying when starved animals are placed on bacteria in a manner similar to that observed in wild-type animals (R. Komuniecki, unpublished results). Indeed, Q4ser-5 animals retained the same number of eggs at the same developmental stage as wild-type animals, suggesting that in the absence of most serotonergic signaling, egg laying on bacteria was wild type and that overlapping signaling pathways, mediated by other modulators, also regulate egg laying in wild-type animals. Indeed, animals with mutations in peptidergic signaling are also egg laying defective. For example, egl-21 null animals that lack an enzyme essential for processing FMRFamide peptides also exhibit a severe egg-laying-defective phenotype, and in many systems it has been suggested that peptidergic signaling may be a prerequisite for effective serotonergic modulation (Waggoner et al. 2000; Jacob and Kaplan 2003; Schafer 2006). This observation highlights the potentially important role of other ligands in the modulation of egg laying.
To summarize, it is clear from recently published work and from the analyses outlined above that at least five receptors, operating at multiple levels within the egg-laying circuit, relay nutrition status and other sensory information to fine-tune egg laying through a delicate balance of both excitatory and inhibitory serotonergic inputs. Such complexity probably evolved to ensure that egg laying is rapidly and specifically initiated only under the appropriate environmental conditions.
Acknowledgments
We thank Bruce Bamber and Robert Steven for critical discussion of the manuscript, S. Mitani at the National Bioresources Project, and the Caenorhabditis elegans Genetics Center, which is supported by the National Institutes of Health (NIH) National Center for Research Resources, for strains. This work was supported by NIH grant AI-145147 awarded to R.W.K. and funds from the Joan L. and Julius H. Jacobson Biomedical Professorship.
References
- Almaula, N., B. J. Ebersole, D. Zhang, H. Weinstein and S. Sealfon, 1996. Mapping the binding site pocket of the 5-hydroxytryptamine2A receptor. J. Biol. Chem. 271 14672–14675. [DOI] [PubMed] [Google Scholar]
- Bany, I. A., M. Q. Dong and M. R. Koelle, 2003. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J. Neurosci. 23(22): 8060–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak, L. S., M. Tiberi, N. J. Freedman, M. Kwatra, R. J. Lefkowitz et al., 1994. A highly conserved tyrosine residue in G-protein coupled receptors is required for agonist-mediated β2-adrenergic receptor sequestration. J. Biol. Chem. 269 2790–2796. [PubMed] [Google Scholar]
- Bastiani, C. A., S. Gharib, M. I. Simon and P. W. Sternberg, 2003. Caenorhabditis elegans Gαq regulates egg-laying behavior via a PLCβ-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics 165 1805–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert, J., S. Claeysen, C. Becamel, A. Dumis and P. Marin, 2006. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 326 553–572. [DOI] [PubMed] [Google Scholar]
- Brady, A. E., and L. E. Limbird, 2002. G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell Signal. 14(4): 297–309. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage, L., L. Avery, A. Katz, U. Kim, J. E. Mendel et al., 1996. Mutations in C. elegans Gαq gene disrupt movement, egg-laying and viability. Neuron 16 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnell, L., J. Illi, S. W. Hong and S. L. McIntire, 2005. The G-protein coupled receptor SER-1 regulates egg-laying and male mating behaviors in Caenorhabditis elegans. J. Neurosci. 25 10671–10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre-Pierrat, M., D. Baillie, R. Johnsen, R. Hyde, A. Hart et al., 2006. Characterization of the Caenorhabditis elegans G-protein coupled serotonin receptors. Invert. Neurosci. 6 189–205. [DOI] [PubMed] [Google Scholar]
- Chao, D., H. Komatsu, H. S. Fukuto, H. M. Dionne and A. C. Hart, 2004. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc. Natl. Acad. Sci. USA 101 15512–15517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary, M. S., S. Craig and B. L. Roth, 1993. A single point mutation of a conserved phenylalanine abolishes 4[125I] iodo-(2,5 dimethoxy) phenylidopropylamine and [3H]-mesulergine but not [3H]-ketanserin binding to 5-HT2 receptors. Mol. Pharmacol. 43 755–761. [PubMed] [Google Scholar]
- D'Agostino, G., A. M. Condino, P. Gallinari, G. P. Franceschetti and M. Tonini, 2006. Characterization of prejunctional serotonin receptors modulating [3H]acetylcholine release in the human detrusor. J. Pharmacol. Exp. Ther. 316 129–135. [DOI] [PubMed] [Google Scholar]
- Dasari, S., and R. L. Cooper, 2006. Direct influence of serotonin on the larval heart of Drosophila melanogaster. J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol. 176 349–357. [DOI] [PubMed] [Google Scholar]
- Dempsey, C. M., S. M. Mackenzie, A. Gargus, G. Blanco and J. Y. Sze, 2005. 5HT, fluoxetine, imipramine and dopamine target distinct 5HT receptor signaling to modulate Caenorhabditis elegans egg-laying behavior. Genetics 169 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan, F. F., M. D. Ungrin, M. Abramovitz and P. Ribeiro, 1999. Characterization of a novel serotonin receptor from Caenorhabditis elegans: cloning and expression of two spliceB variants. J. Neurochem. 72 1372–1383. [DOI] [PubMed] [Google Scholar]
- Hardaker, L. A., E. Singer, R. Kerr, G. Zhou and W. R. Schafer, 2001. Serotonin modulates locomotory behavior and coordinates egg-laying and movement in Caenorhabditis elegans. J. Neurobiol. 49(4): 303–313. [DOI] [PubMed] [Google Scholar]
- Herman, R. K., and E. M. Hedgecock, 1990. Limitationof the size of the vulval primordium of Caenorhabditis elegans by lin-15 expression in surrounding hypodermis. Nature 348(6297): 169–171. [DOI] [PubMed] [Google Scholar]
- Hobert, O., 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32(4): 728–730. [DOI] [PubMed] [Google Scholar]
- Hobson, R. J., J. Geng, A. D. Gray and R. W. Komuniecki, 2003. SER-7B, a constitutively active Galphas coupled 5-HT7-like receptor expressed in the Caenorhabditis elegans M4 pharyngeal motorneuron. J. Neurochem. 87 22–29. [DOI] [PubMed] [Google Scholar]
- Hobson, R. J., V. M. Hapiak, H. Xiao, K. L. Buehrer, P. R. Komuniecki et al., 2006. SER-7, a Caenorhabditis elegans 5-HT7-like receptor, is essential for the 5-HT stimulation of pharyngeal pumping and egg laying. Genetics 172 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz, H. R., M. Chalfie, C. Trent, J. E. Sulston and P. D. Evans, 1982. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216 1012–1014. [DOI] [PubMed] [Google Scholar]
- Hoyer, D., D. E. Clarke, J. R. Fozard, P. R. Hartig, G. R. Martin et al., 1994. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol. Rev. 46 157–203. [PubMed] [Google Scholar]
- Jacob, F., and J. M. Kaplan, 2003. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J. Neurosci. 23 2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle, M. R., and H. R. Horvitz, 1996. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell 84 115–125. [DOI] [PubMed] [Google Scholar]
- Kohen, R., L. A. Fashingbauer, D. E. A. Heidmann, C. R. Guthrie and M. W. Hamblin, 2001. Cloning of the mouse 5-HT6 serotonin receptor and mutagenesis studies of the third cytoplasmic loop. Mol. Brain Res. 90 110–117. [DOI] [PubMed] [Google Scholar]
- Komuniecki, R. W., R. J. Hobson, E. B. Rex, V. M. Hapiak and P. R. Komuniecki, 2004. Biogenic amine receptors in parasitic nematodes: What can be learned from Caenorhabditis elegans? Mol. Biochem. Parasitol. 137(1): 1–11. [DOI] [PubMed] [Google Scholar]
- Lackner, M. R., S. J. Nurrish and J. M. Kaplan, 1999. Facilitation of synaptic transmission by EGL-30 Gqα and EGL-8 PLCβ: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24 335–346. [DOI] [PubMed] [Google Scholar]
- Luttrell, L. M., 2006. Transmembrane signaling by G protein-coupled receptors. Methods Mol. Biol. 332 3–49. [DOI] [PubMed] [Google Scholar]
- Mello, C., and A. Fire, 1995. DNA transformation. Methods Cell Biol. 48 451–482. [PubMed] [Google Scholar]
- Mendel, J. E., H. C. Korswagen, K. S. Liu, Y. M. Hajdu-Cronin, M. I. Simon et al., 1995. Participation of the protein Go in multiple aspects of behavior in C. elegans. Science 267 1652–1655. [DOI] [PubMed] [Google Scholar]
- Mitchell, E. S., and J. F. Neumaier, 2005. 5-HT6 receptors: a novel target for cognitive enhancement. Pharmocol. Ther. 108 320–333. [DOI] [PubMed] [Google Scholar]
- Moresco, J. J., and M. R. Koelle, 2004. Activation of EGL-47, a Galpha(o)-coupled receptor, inhibits function of hermaphrodite-specific motor neurons to regulate Caenorhabditis elegans egg-laying behavior. J. Neurosci. 24 8522–8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro, O., J. Lameh, P. Hogger and W. Sadee, 1993. Hydrophobic amino acids in the i2 loop plays a key role in receptor-G-protein coupling. J. Biol. Chem. 268 22273–22276. [PubMed] [Google Scholar]
- Okkema, P.G., S. W. Harrison, V. Plunger, A. Aryana and A. Fire, 1993. Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135 385–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde, B., and W. R. McCombie, 1997. Molecular cloning and functional expression of a serotonin receptor from Caenorhabditis elegans. J. Mol. Neurosci. 8 53–62. [DOI] [PubMed] [Google Scholar]
- Purohit, A., K. Herrick-Davis and M. Teitler, 2003. Creation, expression, and characterization of a constitutively active mutant of the human serotonin 5HT-6 receptor. Synapse 47 218–224. [DOI] [PubMed] [Google Scholar]
- Ranganathan, R., S. C. Cannon and H. R. Horvitz, 2000. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 408 470–475. [DOI] [PubMed] [Google Scholar]
- Roth, B. L., M. S. Shoham, M. S. Choudhary and N. Khan, 1997. Identification of conserved aromatic residues essential for agonist binding and second messenger production at 5-hydroxytryptamine2A receptors. Mol. Pharmacol. 52 259–266. [DOI] [PubMed] [Google Scholar]
- Sawin, E. R., R. Ranganathan and H. R. Horvitz, 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26 619–631. [DOI] [PubMed] [Google Scholar]
- Schafer, W. F., 2006. Genetics of egg-laying in worms. Annu. Rev. Genet. 40 487–509. [DOI] [PubMed] [Google Scholar]
- Segalat, L., D. A. Elkes and J. M. Kaplan, 1995. Modulation of serotonin-controlled behaviors in C. elegans. Science 267 1652–1655. [DOI] [PubMed] [Google Scholar]
- Shyn, S. I., R. Kerr and W. R. Schafer, 2003. Serotonin and Go modulate functional states of neurons and muscles controlling C. elegans egg-laying behavior. Curr. Biol. 13 1910–1915. [DOI] [PubMed] [Google Scholar]
- Strader, C. D., M. R. Candelore, W. S. Hill, I. S. Sigal and A. F. Dixon, 1989. Identification of two serine residues involved in agonist activation of the β-adrenergic receptor. J. Biol. Chem. 264 572–578. [PubMed] [Google Scholar]
- Sze, J. Y., M. Victor, C. Loer, Y. Shi and G. Ruvkun, 2000. Food and metabolic signaling defects in a Caenorhabditis elegans serotonin synthesis mutant. Nature 403 560–564. [DOI] [PubMed] [Google Scholar]
- Tan, C. M., A. E. Brady, H. H. Nickols, Q. Wang and L. E. Limbird, 2004. Membrane trafficking of G-protein-coupled receptors. Annu. Rev. Pharmocol. Toxicol. 44 559–609. [DOI] [PubMed] [Google Scholar]
- Tsalik, E. L., T. Niacaris, A. S. Wenick, K. Pau, L. Avery et al., 2003. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev. Biol. 263 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner, L. E., G. T. Zhou, R. W. Schafer and W. R. Schafer, 1998. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron 21 203–214. [DOI] [PubMed] [Google Scholar]
- Waggoner, L. E., L. A. Hardaker, S. Golik and W. R. Schafer, 2000. Effect of a neuropeptide gene on behavioral states in Caenorhabditis elegans. Genetics 154 1181–1192. [DOI] [PMC free article] [PubMed]
- Wenick, A. S., and O. Hobert, 2004. Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev. Cell 6 757–770. [DOI] [PubMed] [Google Scholar]
- Xiao, H., V. M. Hapiak, K. A. Smith, L. Lin, R. J. Hobson et al., 2006. SER-1, a Caenorhabditis elegans 5-HT(2)-like receptor, and a multi-PDZ domain containing protein (MPZ-1) interact in vulval muscle to facilitate serotonin-stimulated egg-laying. Dev. Biol. 298 379–391. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., H. Lu and C. I. Bargmann, 2005. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438 179–184. [DOI] [PubMed] [Google Scholar]