Abstract
A genomic collection of haploid Saccharomyces cerevisiae deletion strains provides a unique resource for systematic analysis of gene interactions. Double-mutant haploid strains can be constructed by the synthetic genetic array (SGA) method, wherein a query mutation is introduced by mating to mutant arrays, selection of diploid double mutants, induction of meiosis, and selection of recombinant haploid double-mutant progeny. The mechanism of haploid selection is mating-type-regulated auxotrophy (MRA), by which prototrophy is restricted to a particular haploid genotype generated only as a result of meiosis. MRA escape leads to false-negative genetic interaction results because postmeiotic haploids that are supposed to be under negative selection instead proliferate and mate, forming diploids that are heterozygous at interacting loci, masking phenotypes that would be observed in a pure haploid double-mutant culture. This work identified factors that reduce MRA escape, including insertion of terminator and repressor sequences upstream of the MRA cassette, deletion of silent mating-type loci, and utilization of α-type instead of a-type MRA. Modifications engineered to reduce haploid MRA escape reduced false negative results in SGA-type analysis, resulting in >95% sensitivity for detecting gene–gene interactions.
GENE–gene interaction means a phenotype attributable to variation in one gene depends upon the allele status of a second gene (Barton and Keightley 2002). Relatively little is known about the overall contribution of gene interactions to natural genotype–phenotype variation. Systematic analysis of genetic interactions in experimental organisms could help in this regard to infer how naturally occurring genetic variation is expressed phenotypically (Hartman et al. 2001). In Saccharomyces cerevisiae, deletion of all nonessential genes on a single haploid genetic background enables genomewide assessment of gene interactions by parallel analysis of all mutants under various genetic or environmental conditions (Winzeler et al. 1999; Giaever et al. 2002). If performed comprehensively, genetic interaction networks can provide a more global perspective of cellular processes than is possible with studies that focus on only a few or a biased set of genes (Tong et al. 2004). The Boone laboratory developed the synthetic genetic array (SGA) methodology to introduce second mutations into the yeast gene deletion strain array, thereby enabling systematic analysis of gene–gene interactions on a genomewide scale (Tong et al. 2001; Tong and Boone 2006). To construct double mutants by SGA, a “query” strain, harboring a second mutation, is mated to the entire array of yeast gene deletion strains, thus creating a new array of heterozygous diploid double mutants. Sporulation and meiotic recombination produce haploid double mutants, which are recovered by media selection. Haploid selection is based on mating-type-regulated auxotrophy (MRA), which results from placing an auxotrophy-complementing gene under control of a mating-type-regulated promoter (referred to here as the “MRA cassette”). The MRA cassette is activated for prototrophy in the opposite mating type of the query strain; thus only after meiosis are haploids created that obtain the MRA cassette from the query strain and the permissive MAT allele from the deletion array.
The Burke laboratory reported low sensitivity of SGA for detecting known synthetic lethal interactions and described gene conversion between the mating-type-regulated HIS3 auxotrophy and the his3Δ1 allele as a responsible mechanism (Daniel et al. 2006). Replacement of HIS3 with Schizosaccharomyces pombe his5+ in the MRA cassette eliminated gene conversion and improved sensitivity of SGA analysis.
Similarly, the goal of our study was to improve the purity of haploid double-mutant strains and thus the sensitivity of SGA-type analysis, by reducing formation of heterozygous diploids. Our strategy focused on eliminating haploid MRA escape. We reasoned that MRA escape would allow haploids under negative selection to proliferate and form heterozygous “masking diploids” [having interacting mutations suppressed by complementing wild-type (WT) alleles]. Importantly, we discovered that diploid cells have a very high MRA escape frequency. This led to the idea that reducing haploid MRA escape, by minimizing mating between postmeiotic progeny cells, would improve SGA. Compared to the strains we began the study with, increased MRA stringency led to an 80–95% reduction in masking of genetic interactions. Thus, MRA escape is an indicator of the likely efficiency of a query strain for SGA-type construction of haploid double mutants, because MRA stringency reduces formation of masking diploids.
The majority of global gene interaction studies have been qualitative. However, quantitative analysis of interactions provides additional benefits for identifying gene modules (e.g., pathways or protein complexes) based on a shared pattern and strength of interactions among constituent genes (Hartman and Tippery 2004; Hartman 2007; St Onge et al. 2007; Roguev et al. 2008). Thus, in addition to qualitative studies, increased purity of SGA-derived haploid double mutants will further enable quantitative precision and accuracy for genomewide genetic interaction studies (Shah et al. 2007; Mani et al. 2008).
MATERIALS AND METHODS
Strains:
S. cerevisiae strains used in this study are listed in Table 1. These were derived from a strain generously provided by the Boone laboratory (CBY5565b). The steps involved in strain development are illustrated in supplemental Figure S1 and detailed in accompanying supplemental Table S1.
TABLE 1.
List of yeast strains used in this study
| Strain name | Relevant genotype | Acquired from |
|---|---|---|
| i. | ||
| CBY5565b | MATα his3Δ1 leu2Δ0 ura3Δ0 met17Δ0 lyp1Δ0 mfα1Δ∷PMFα1-LEU2 can1Δ0∷PMFA1-HIS3 | Boone laboratory |
| CBY7092b | MATα his3Δ1 leu2Δ0 ura3Δ0 met17Δ0 lyp1Δ0 can1Δ0∷PSTE2-his5+ | Burke laboratory |
| BY4741a | MATahis3Δ1 leu2Δ0 ura3Δ0 met17Δ0 | Research Genetics |
| BY4742b | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Research Genetics |
| Y15110-3-4.1b | MATα his3Δ1 leu2Δ0 ura3Δ0 met17Δ0 can1Δ0∷PMFA1-HIS3 | This study |
| Y15111b | MATα his3Δ1 leu2Δ0 ura3Δ0 met17Δ0 can1Δ0∷KanMX-TTEF1-PMFA1-HIS3 | This study |
| Y15114b | MATα his3Δ1 leu2Δ0 ura3Δ0 met17Δ0 can1Δ0∷KanMX-PGAL1-TADH1-PMFA1-HIS3 | This study |
| Y15116a | MATahis3Δ1 leu2Δ0 ura3Δ0 met17Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-HIS3 | This study |
| Y15117b | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-HIS3 | This study |
| ii. | ||
| Y15118a | MATahis3Δ1 leu2Δ0 ura3Δ0 met17Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-LEU | This study |
| Y15125-1.3b | MATα his3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-LEU2 | This study |
| Y15121a | MATahis3Δ1 leu2Δ0 ura3Δ0 met17Δ0 lys2Δ0∷PGAL1-TADH1-PMFA1-HIS3 | This study |
| Y15122a | MATahis3Δ1 leu2Δ0 ura3Δ0 met17Δ0 lys2Δ0∷PGAL1-TADH1-PMFA1-LEU2 | This study |
| Y15126-1.4a | MATahis3Δ1 leu2Δ0 ura3Δ0 met17Δ0 lys2Δ0∷PGAL1-TADH1-PMFA1-HIS3 | This study |
| Y15124-2.4b | MATα his3Δ1 leu2Δ0 ura3Δ0 met17Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-HIS3 | This study |
| Y15132-2-2.1b | MATα his3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-LEU2 | This study |
| Y15132-2-2.4b | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0∷PGAL1-TADH1-PMFA1-HIS3 | This study |
| Y15132-2-2.3a | MATahis3Δ1 leu2Δ0 ura3Δ0 met17Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-LEU2 | This study |
| Y15127-2.2a | MATahis3Δ1 leu2Δ0 ura3Δ0 lys2Δ0∷PGAL1-TADH1-PMFA1-LEU2 | This study |
| Y15131-1-5.1b | MATα his3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-HIS3 | This study |
| Y15131-1-6.3b | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0∷PGAL1-TADH1-PMFA1-LEU2 | This study |
| iii. | ||
| Y15571a | MATahis3Δ1 leu2Δ0 ura3Δ0 met17Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-his5+ | This study |
| Y15147b | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 hmrΔ0∷URA3ca | This study |
| Y15572-2-10.3b | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-his5+ | This study |
| Y15572-3-30.3b | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-his5+ hmrΔ0∷URA3ca | This study |
| iv. | ||
| Y15573b | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 can1Δ0∷PGAL1-TADH1-PMFα1-his5+ | This study |
| Y15574-3.4a | MATahis3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 can1Δ0∷PGAL1-TADH1-PMFα1-his5+ | This study |
| v. | ||
| CBY5257b | MATα his3Δ1 leu2Δ0 ura3Δ0 met17Δ0 lyp1Δ0 can1Δ0∷PMFA1-HIS3-PMFα1-LEU2 | Boone laboratory |
| Y15575-6.2b | MATα his3Δ1 leu2Δ0 ura3Δ0 met17Δ0 lyp1Δ0 | This study |
| Y15575-2.1a | MATahis3Δ1 leu2Δ0 ura3Δ0 met17Δ0 lyp1Δ0 | This study |
| Y15576-15.3a | MATahis3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-his5+ lyp1Δ0 | This study |
| Y15576-12.4b | MATα his3Δ1 leu2Δ0 ura3Δ0 met17Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-his5+ lyp1Δ0 hmrΔ0∷URA3ca | This study |
| Y15578-1.2b | MATα his3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-his5+ lyp1Δ0 hmrΔ0∷URA3ca | This study |
| Y15577-2.1a | MATahis3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PMFα1-his5+ lyp1Δ0 | This study |
| vi. | ||
| Y15584-4a | MATahis3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PSTE2-his5+ lyp1Δ0 | This study |
| Y15591-5.2b | MATα his3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PSTE2-his5+ lyp1Δ0 hmrΔ0∷URA3ca | This study |
| Y15591-15.3b | MATα his3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PSTE2-his5+ lyp1Δ0 hmrΔ0∷URA3ca | This study |
| Y15591-21.4b | MATα his3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PSTE2-his5+ lyp1Δ0 hmrΔ0∷URA3ca | This study |
| Y15583-13.2b | MATα his3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-his5+ lyp1Δ0 | This study |
| Y15586b | MATα his3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PSTE3-his5+ lyp1Δ0 | This study |
| vii. | ||
| Y15580a | MATahis3Δ1 leu2Δ0 ura3Δ0 met17Δ0 hmlΔ0∷URA3ca | This study |
| Y15588-10.2a | MATahis3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PSTE3-his5+ lyp1Δ0 hmlΔ0∷URA3ca | This study |
| Y15579-4a | MATahis3Δ1 leu2Δ0 ura3Δ0 can1Δ0∷PGAL1-TADH1-PMFα1-his5+lyp1Δ0 hmlΔ0∷URA3ca | This study |
| Diploids | ||
| Y15124a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 LYS2/lys2Δ0 met17Δ0/MET17can1Δ0∷PGAL1-TADH1-PMFA1-HIS3/CAN1 | This study |
| Y15125a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 LYS2/lys2Δ0 met17Δ0/MET17can1Δ0∷PGAL1-TADH1-PMFA1-LEU2/CAN1 | This study |
| Y15126a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 lys2Δ0∷PGAL1-TADH1-PMFA1-HIS3/lys2Δ0 met17Δ0/MET17 | This study |
| Y15127a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met17Δ0/MET17 lys2Δ0∷PGAL1-TADH1-PMFA1-LEU2/lys2Δ0 | This study |
| Y15131a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 MET17/met17Δ0 CAN1/can1Δ0∷PGAL1-ADH11term-PMFA1-HIS3 lys2Δ0∷PGAL1-TADH1-PMFA1-LEU2/LYS2 | This study |
| Y15132a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met17Δ0/MET17 CAN1/can1Δ0∷PGAL1-TADH1-PMFA1-LEU2 lys2Δ0∷PGAL1-TADH1-PMFA1-HIS3/LYS2 | This study |
| Y15572a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 LYS2/lys2Δ0 met17Δ0/MET17can1Δ0∷PGAL1-TADH1-PMFA1-his5+/CAN1 HMR/hmrΔ0∷URA3ca | This study |
| Y15574a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 LYS2/lys2Δ0 can1Δ0∷PGAL1-TADH1-PMFα1-his5+ | This study |
| Y15575a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met17Δ0/met17Δ0 LYP1/lyp1Δ0 CAN1/can1Δ0∷PMFA1-HIS3-PMFα1-LEU2 | This study |
| Y15576a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met17Δ0/MET17 lyp1Δ0/LYP1LYS2/lys2Δ0 CAN1/can1Δ0∷PGAL1-TADH1-PMFA1-his5+ HMR/hmrΔ0∷URA3ca | This study |
| Y15577a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 lys2Δ0/LYS2 MET17/met17Δ0 can1Δ0∷PGAL1-TADH1-PMFα1-his5+/CAN1 lyp1Δ0/LYP1 | This study |
| Y15578a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 MET17/met17Δ0 can1Δ0∷PGAL1-TADH1-PMFA1-his5+/can1Δ0∷PGAL1TADH1-PMFA1-his5+lyp1Δ0/lyp1Δ0 HMR/hmrΔ0∷URA3ca | This study |
| Y15583a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 CAN1/can1Δ0∷PGAL1-TADH1-PMFA1-his5+HMR/hmrΔ0∷URA3ca LYP1/lyp1Δ0 | This study |
| Y15588a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met17Δ0/MET17 CAN1/can1Δ0∷PGAL1-TADH1-PSTE3-his5+hmlΔ0∷URA3ca/HML LYP1/lyp1Δ0 | This study |
| Y15591a/b | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 LYS2/lys2Δ0 can1Δ0∷PGAL1-TADH1-PSTE2-his5+/CAN1 HMR/hmrΔ0∷URA3ca lyp1Δ0/LYP1 | This study |
Roman numerals are referenced to supplemental Figure S1, supplemental Table S1, and materials and methods.
(i) Integration of transcriptional terminator/repressor sequences upstream of the MRA cassette:
Strains CBY5565b and BY4741a were mated (step a), sporulated, and dissected (step b) to obtain Y15110-3-4.1b. The TEF terminator sequence was amplified from Longtine plasmid 1 (Longtine et al. 1998) using primer pair P1/P2 and was chromosomally integrated between the 3′ end of the CAN1 promoter and the can1Δ0∷PMFA1-HIS3 MRA cassette by transformation of Y15110-3-4.1b (step c), yielding strain Y15111b (supplemental Table S1). Similarly, the PGAL1-TADH1 sequence was amplified from Longtine plasmid 19 using primer pair P3/P4 and integrated at the same location, creating strain Y15114b (step d). To remove KanMX linkage and G418 resistance, the PGAL1-TADH1-PMFA1-HIS3 cassette was amplified from Y15114b using primer pair P5/P6 and chromosomally integrated into BY4741a by knocking out CAN1, creating a can1Δ0∷PGAL1-TADH1-PMFA1-HIS3 cassette in strain Y15116a (step e). BY4742b was similarly transformed to create Y15117b. All subsequent cassettes (see supplemental Figure S1 and supplemental Table S1) incorporated the upstream PGAL1-TADH1 sequence.
(ii) Construction of MRA cassettes with different auxotrophies and integration at different loci:
LEU2 was PCR amplified with primer pair P7/P8 and transformed into Y15116a, creating a can1Δ0∷PMFA1-LEU2 cassette in strain Y15118a (step f). The PMFA1-HIS3 and PMFA1-LEU2 cassettes were amplified with primer pair P9/P10 and used to knock out LYS2 (steps g and h) in BY4741a, yielding strains Y15121a (lys2Δ0∷PMFA1-HIS3) and Y15122a (lys2Δ0∷PMFA1-LEU2). Y15118a (can1Δ0∷PMFA1-LEU2) was backcrossed with BY4742b (step i) to switch the mating type, yielding Y15125-1.3b. Y15121a (lys2Δ0∷PMFA1-HIS3) was backcrossed with BY4742b to obtain Y15126-1.4a. The strains Y15125-1.3b (can1Δ0∷PMFA1-LEU2) and Y15126-1.4a (lys2Δ0∷PMFA1-HIS3) were mated to obtain the diploid Y15132a/b, which was sporulated and dissected to obtain the strains Y15132-2-2.1b (can1Δ0∷PMFA1-LEU2), Y15132-2-2.4b (lys2Δ0∷PMFA1-HIS3), and Y15132-2-2.3a (can1Δ0∷PMFA1-LEU2). Y15122a was backcrossed with BY4742 to obtain Y15127-2.2a. Y15116a was backcrossed with BY4742b to obtain Y15124-2.4b. Y15127-2.2a was crossed with Y15124-2.4b to obtain Y15131-1-5.1b (can1Δ0∷PMFA1-HIS3) and Y15131-1-6.3b (lys2Δ0∷PMFA1-LEU2).
(iii) Substitution of S. pombe his5+ for HIS3 and deletion of silent MATa locus:
S. pombe his5+ was PCR amplified from the plasmid p3xHA-his5+ (made by Sean Munro; acquired via the Bedwell laboratory) using primer pair P11/P12 with flanking homology to the MFA1 promoter and 3′-UTR of CAN1 and transformed into Y15132-2-2.3a (PMFA1-LEU2) to create a PMFA1-his5+ cassette in Y15571a (step j). An hmraΔ0∷URA3ca allele was made by PCR amplification of Candida albicans URA3 (URA3ca) from pAU112 (made by Sandy Johnson; acquired via the Gottschling laboratory) with primers P13/P14 and integration into 4742b, yielding Y15147b (step k). Y15571a was crossed with Y15147b (hmraΔ0∷URA3ca) to obtain Y15572-2-10.3b (PMFA1-his5+/HMRa) and Y15572-3-30.3b (PMFA1-his5+/HMRa).
(iv) Creation of a PMFα1-his5+ cassette:
The MFα1 promoter was PCR amplified using primer pair P15/P16 and integrated into strain Y15572-2-10.3b (PMFA1-HIS3) to create a PMFα1-his5+ cassette Y15573b (step l). Y15573b was backcrossed with BY4741a to obtain Y15574-3.4a.
(v) Introduction of lyp1Δ0 (recessive thialysine resistance):
The Boone laboratory strain CBY5257b containing a deletion of LYP1, the lysine permease (lyp1Δ0), was backcrossed with BY4741a to obtain Y15575-6.2b and Y15575-2.1a, each carrying lyp1Δ0 conferring recessive resistance to thialysine. Strain Y15572-3-30.3b (PMFA1-his5+/HMRa) was crossed to Y15575-2.1a (lyp1Δ0) to obtain Y15576-15.3a (PMFA1-his5+/lyp1Δ0/MET17/HMRa) and Y15576-12.4b (PMFA1-his5+/lyp1Δ0/met17Δ0/HMRa), which were crossed with one another to obtain Y15578-1.2b (PMFA1-his5+/lyp1Δ0/MET17/HMRa). Similarly, Y15574-3.4a (PMFα1-his5+) was crossed with Y15575-6.2b (lyp1Δ0) resulting in strain Y15577-2.1a (PMFα1-his5+/lyp1Δ0).
(vi) Creation of MRA cassettes with STE2 and STE3 promoters:
The STE2 promoter sequence was PCR amplified with primer pair P17/P18 and transformed into Y15577-2.1a (PMFα1-his5+), creating a PSTE2-his5+ cassette in Y15584-4a (step m). Y15584-4a was crossed with Y15147b (hmraΔ0∷URA3ca) to obtain Y15591-5.2b (PSTE2-his5+/HMRa). Conversely, Y15578-1.2b (PMFA1-his5+/HMRa) was backcrossed to BY4741a to obtain Y15583-13.2b (PMFA1-his5+/HMRa). The STE3 promoter sequence was PCR amplified with primer pair P19/P20 and transformed into Y15583-13.2b, creating a PSTE3-his5+ cassette in Y15586b (step n).
(vii) Incorporation of hmlαΔ0∷URA3ca allele:
URA3ca gene was PCR amplified from pAU112 using primer pair P21/P22 and transformed into BY4741a, creating an hmlαΔ0∷URA3ca allele in Y15580a (step o). Y15586b (PSTE3-his5+/HMLα) was crossed with Y15580a to obtain Y15588-10.2a (PSTE3-his5+/hmlαΔ0∷URA3ca). Step o was repeated for Y15577-2.1a (PMFα1-his5+/HMLα) to obtain Y15579-4a (PMFα1-his5+/hmlαΔ0∷URA3ca).
Yeast methods and molecular biology:
Standard methods were used for growing, transforming, mating, and sporulating/dissecting yeast strains (Burke et al. 2000; Gietz and Woods 2002). All DNA transformations were chromosomal integrations and correct targeting was confirmed by PCR. Cultures were grown at 30°. Pre-SPO5 (Codon et al. 1995) was used in addition to liquid sporulation media consisting of 1% potassium acetate, 0.01% uracil, 0.01% leucine, 0.01% histidine, 0.01% adenine, and 0.005% zinc chloride. Amino acids and filtered, sterilized solutions of l-canavanine (50 mg/liter), thialysine (S-aminoethyl-l-cysteine; SAEC) (50 mg/liter), 5-FOA (0.5–1 mg/liter), 3-aminotriazole (0.5–2 mm), or G418 (200 mg/liter) were added to cooled media (∼60°), after autoclaving. DNA isolation and PCR amplifications were performed according to standard protocols; primer sequences are given in supplemental Table S1. Mating-type tests were performed by coreplica-plating MRA escape colonies (after 3–4 days of growth on auxotrophic selection media) and a complementation tester strain (containing an ilv2 mutation from the Hartwell strain collection) to YPD for 1 day, followed by replica plating to B media, and imaged 1 day later. Tetrad dissection was performed after digestion of cell wall with lyticase (BioChemika), using a Carl Zeiss Axioskop 40 tetrad dissection microscope. SGA haploid selection was performed according to (Tong and Boone 2006).
RESULTS
Placement of transcriptional terminator and repressor sequences upstream of the MRA cassette reduces MRA escape:
We first assessed MRA escape of strain CBY5565b, using a colony papillation assay performed by plating 500 μl of an overnight YPD liquid culture onto appropriate dropout media, scoring colonies after 3–4 days. A mixed population of large and small colonies was observed, with a much higher frequency of smaller colonies (Figure 1A; results for each entire plate represented in Figure 1 are quantified in Table 2). CBY5565b was backcrossed with BY4741a (strain background for yeast gene deletion strain collection). Some variability in papillation between meiotic segregants was observed after replica-plating SC −HIS media (data not shown), so a segregant (Y15110-3-4.1b) without papillating colonies was carried forward in further studies (Figure 1B).
Figure 1.—
Effect of terminator and repressor sequences upstream of MRA cassette on frequency of small MRA escape colonies. Overnight grown cultures of (A) CBY5565b (no terminator), (B) Y15110-3-4.1b (no terminator), (C) Y15111b (TTEF1) and (D) Y15114b (PGAL1-TADH1) were normalized to same optical density, and 500 μl was plated on SC−His media. Plates were imaged after 3.5 days.
TABLE 2.
Effects of transcriptional terminator, repressor, auxotrophic gene, and chromosomal position of MRA cassette on MRA escape
| Colony size
|
|||||
|---|---|---|---|---|---|
| Figure | Strain | MRA factor tested | MRA escape total | Large | Small |
| 1A | CBY5565b | 1910 | 387 | 1523 | |
| 1B | Y15110-3-4.1b | Backcross of CBY5565b | 1562 | 338 | 1224 |
| 1C | Y15111b | TTEF1 | 1204 | 320 | 884 |
| 1D | Y15114b | PGAL1-TADH1 | 489 | 466 | 23 |
| S2A | Y15132-2-2.4b | lys2Δ0∷PMFA1-HIS3 | 203 | 155 | 48 |
| S2B | Y15121-1-5.1b | can1Δ0∷PMFA1-HIS3 | 330 | 283 | 47 |
| S2C | Y15131-1-6.3b | lys2Δ0∷PMFA1-LEU2 | 475 | 93 | 382 |
| S2D | Y15132-2-2.1b | can1Δ0∷PMFA1-LEU2 | 562 | 127 | 435 |
We reasoned that integration of a transcriptional terminator, between the CAN1 promoter sequence and the PMFA1-HIS3 cassette might reduce transcriptional read through and thereby reduce MRA escape. Terminators from the TEF1 and ADH1 genes were tested; the ADH1 terminator (TADH1) also had an upstream GAL1 promoter (PGAL1) sequence (Longtine et al. 1998). The TEF1 terminator sequence reduced formation of small MRA escape colonies by approximately half (Figure 1C, Table 2), whereas the PGAL1-TADH1 sequence led to ∼99% reduction of small colonies (Figure 1D, Table 2). Repression of low-level readthrough of HIS3 was previously inferred by 3-amino-triazole (3-AT) inhibition growth of small colonies (Daniel et al. 2006), which we confirmed (data not shown).
LEU2 exhibits higher MRA escape frequency than HIS3:
We next considered whether the particular auxotrophy employed in an MRA cassette might affect escape, on the idea that the absolute transcriptional requirement for prototrophy (given the same degree of leaky expression from a mating-type repressed promoter) could be different. To compare LEU2 and HIS3, the PGAL1-TADH1-PMFA1-HIS3 cassette was PCR amplified from Y15114b and transformed into BY4741a to create Y15116a (all subsequent cassettes incorporate the PGAL1-TADH1 sequence), and the LEU2 gene was introduced into Y15116a to create a PMFA1-LEU2 cassette (Y15118a). Y15131-1-5.1b (can1Δ0∷PMFA1-HIS3) and Y15132-2-2.1b 1b (can1Δ0∷PMFA1-LEU2) (derived from Y15116a and Y15118a, respectively) were compared with respect to frequency of MRA escape. Small colonies were reduced by approximately eightfold for strain Y15131-1-5.1b compared to Y15132-2-2 (Table 2 and supplemental Figure S2), suggesting a higher transcriptional requirement for HIS3 than LEU2. We also integrated MRA cassettes at the LYS2 locus in Y15131-6.3b (lys2Δ0∷PMFA1-LEU2) and in 15132-2-2.4b (lys2Δ0∷PMFA1-HIS3) to test for an effect of chromosomal position on MRA escape. The relative frequency of small colonies was similar for MRA cassettes integrated at either locus. Fewer large colonies were observed with integration at LYS2, but the effect was less than twofold (Table 2 and supplemental Figure S2).
Deletion of HMRa prevents MRA escape that occurs due to mating-type switching:
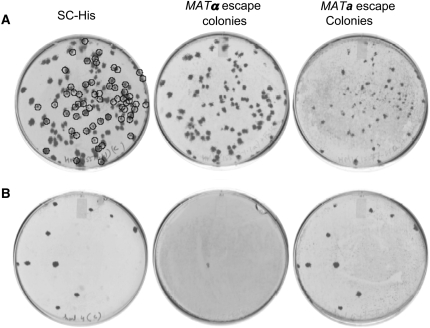
We tested mating types of MRA escape colonies to gain further insight. In general, small escape colonies exhibited the original mating type, while large escape colonies appeared either sterile or to have undergone mating-type switching (Figure 2A). The information for mating type switching is contained in silent loci on either end of chromosome III. HMLα contains transcription factors that regulate genes involved in expression of the MATα phenotype, whereas HMRa contains those required for expression of the MATa phenotype (Nasmyth 1982). Mating-type switching is deficient in the heterothallic laboratory strains in this study due to loss of function of the HO endonuclease. To prevent low-frequency HO-independent gene conversion involving the MAT and silent HMLα/HMRa, we knocked out the HMRa locus (containing the silenced MATA1 and MATA2 genes) in BY4742b, creating Y15147b. Y15147b (hmraΔ0∷URA3ca) was crossed with Y15571a to obtain Y15572-3-30.3b (PMFA1-his5+/hmraΔ0∷URA3ca). Deletion of HMRa eliminated mating-type switching and nearly all remaining MRA escape colonies were sterile (Figure 2B, Table 3).
Figure 2.—
Small a-regulated MRA escape colonies retain an α-mating type, while large MRA escape colonies are either sterile or a-mating type. Overnight grown cultures of strains (A) Y15110-3-4.1b (can1Δ0∷Pcan1-PMFA1-his3/HMRa) and (B) Y15572-3-30.3b (can1Δ0∷PGAL1-TADH1-PMFA1-his5+/hmraΔ0∷URA3ca) were first treated as in Figure 1. After 3.5 days, MRA escape colonies were replica plated to SC−HIS media, and coreplica plated with mating-type tester strains on YPD. After 1 day, strains tested for mating-type complementation were replica plated to media lacking all amino acids, and plates were imaged 1 day later.
TABLE 3.
Effects of MRA factors on MRA escape colony frequency and mating phenotypes
| MRA escape colony mating type
|
||||||
|---|---|---|---|---|---|---|
| Figure | Strain | MRA factors compared | Total | MATa | MATα | Sterile |
| 2A | 15110-3-4.1b | PMFA1-HIS3, HMR | 281 | 157 | 85 | 39 |
| 2B | Y15572-3-30.3b | PGAL1-TADH1-PMFA1-his5+, hmrΔ0∷URA3 | 103 | 0 | 7 | 96 |
| S3A | Y15578-1.2b | PGAL1-TADH1-PMFA1-his5+ | 94 | 3 | 4 | 87 |
| S3B | Y15591-5.2b | PGAL1-TADH1-PSTE2-his5+ | 80 | 2 | 16 | 62 |
| 3A | Y15577-2.1a | MATa, can1Δ0∷PMFa1-his5+/HMLα | 174 | 62 | 112 | 0 |
| 3B | Y15579-4a | MATa, can1Δ0∷PMFa1-his5+/hmlΔ0∷URA3 | 10 | 10 | 0 | 0 |
| 6A | Y15588-10.2a | MATα, can1Δ0∷PSTE3-his5+/hmlΔ0∷URA3 | 0 | |||
The STE2 and MFA1 promoters exhibit similar frequencies of MRA escape:
STE2 encodes the cell surface receptor for α-factor pheromone, and MFA1 encodes a-factor, both of which have expression restricted to MATa cells. We tested whether the STE2 or MFA1 promoter might exhibit lower MRA escape frequency. Y15591-5.2b (can1Δ0∷PSTE2-his5+/hmraΔ0∷URA3ca) was constructed and its MRA escape phenotype was compared to Y15578-1.2b (can1Δ0∷PMFA1-his5+/hmraΔ0∷URA3ca). MRA escape of the PSTE2-his5+ cassette was similar to PMFA1-his5+, although there was variability among different segregants isolated by tetrad dissection. Of potential importance was that Y15591 escape colonies were most frequently associated with a MATα MRA escape colony phenotype, rather than the sterile phenotype associated with 15578 (supplemental Figures S3 and S5 and Table 3).
α-Mating-type regulation, with deletion of HMLα, provides the most stringent MRA:
All SGA studies to our knowledge have used a MATα query strain and the MATa yeast gene deletion strains, selecting recombinant haploid double mutants based on MATa-regulated MRA. We speculated that the reverse strategy, mating a MATa query strain to the MATα yeast gene deletion strain array and relying on MATα-regulated prototrophy for haploid selection, might afford lower MRA escape. MATa-regulated genes are constitutively expressed in the absence of repression by MFα2, whereas MATα genes require induction by MFα1 (Nasmyth 1982). Since loss of gene function occurs more readily than gain of function (Olson 1999), MATα-repressed expression defaults to MATa, but MATa-regulated gene expression does not default to MATα. Thus, MATα-regulated auxotrophy might perform as well or better for SGA-type studies, while also providing a means of mutual validation of interactions.
To test this hypothesis, a MATa strain harboring a PMFα1-his5+ cassette (Y15577-2.1a) was constructed. Similar to the MATα query strain (Figure 2), we observed large MRA escape colonies due to mating-type switching of Y15577-2.1a. However, in contrast, there were no sterile escape colonies (Figure 3A). Analogous to the reduction of MRA escape by knocking out HMRa in a MATα strain harboring MATa-regulated prototrophy (Figure 2), deleting HMLα from Y15577-2.1 prevented MRA escape associated with mating-type switching (Figure 3B). As with PSTE2 and PMFA1 (supplemental Figure S3, Table 2), we tested STE3 promoter regulation in lieu of PMFα1 in the MRA cassette. The comparison revealed that a low rate of MRA escape in 15579-4a (PMFα1-his5+) (MRA escape = ∼1 × 10−7 cells) was reduced to no measurable escape (<1 × 10−8 cells) in 15588-10.2a (PSTE3-his5+) (Figures 3 and 6A, Table 3).
Figure 3.—
Escape from α-regulated MRA is reduced by deletion of HMLα and not associated with sterile MRA escape colonies. MRA escape and mating type of MRA escape colonies was measured for (A) Y15577-2.1a (can1Δ0∷PMFα1-his5+/HMLα) and (B) Y15579-4a (can1Δ0∷PMFα1-his5+/hmlΔ0∷URA3ca) as described in Figure 2. Note that MATa colonies (highlighted by circles) tend to be smaller, all colonies mate (i.e., no sterile colonies), and mating-type switching is eliminated by deletion of HMLα.
Figure 6.—
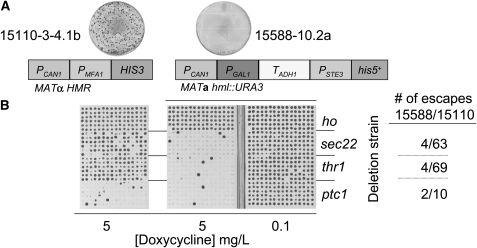
Reducing MRA escape increases sensitivity of gene–gene interaction analysis. (A) MRA escape is depicted for 15110-3-4.1b and 15588-10.2a by the petri dishes (MRA factors for each strain indicated below). (B) The frequency of false-negative results for known lethal interactions is reduced 80–95% in association with reduction of MRA escape. A chromosomal Tet-RNR1∷ClonNat “query” allele was introduced into 15110-3-4.1b and 15588-10.2a. Haploid double-mutant strains, involving hoΔ0∷KanMX (used as a “wild-type” negative control), sec22Δ0∷KanMX, thr1Δ0∷KanMX, or ptc1Δ0∷KanMX alleles were obtained in 96-replicate by the SGA method. Interactions were assessed by conditionally expressing RNR1 on C media + 0.1 μg/ml or 5 μg/ml doxycycline. False-negative interactions were reduced by 80% (ptc1) to 95% (thr1) for 15588-10.2a compared to 15110-3-4.1b.
Canavanine but not thialysine eradicates premeiotic heterozygous diploids:
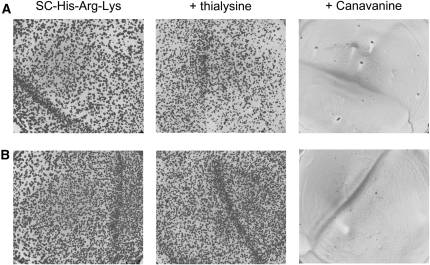
An important question, regarding how to minimize heterozygous diploid contamination in final haploid double-mutant cultures derived by the SGA method, is whether diploid escape events originate before or after sporulation. In theory, diploid cells should be histidine auxotrophs because mating-type-regulated genes are not expressed. However, we found that MRA escape was very high in diploids heterozygous for the MRA-cassette (Figure 4). MRA cassettes are integrated in the query strain by knocking out CAN1 (arginine permease), which introduces recessive canavanine resistance into premeiotic SGA heterozygous diploids. Canavanine (toxic arginine analog) is very efficient at eliminating unsporulated heterozygous diploid cells (CAN1/can1Δ0∷MRA-cassette) that are carried along after sporulation during haploid selection, which comes into play because sporulation efficiency is typically <10%. Thus canavanine eradicates premeiotic heterozygous diploids, preventing masking of genetic interactions observed for pure haploid double mutants. In contrast, deletion of LYP1 (lysine permease), which has been employed as a recessive drug resistance marker for negative selection against heterozygous diploids, using thialysine in combination with canavanine (Tong and Boone 2006), was not effective for selecting against lyp1Δ0/LYP1 diploids (Figure 4). To confirm this unexpected result, lyp1/LYP1 heterozygous, as well as the respective homozygous diploids and haploid strains were constructed independently and tested for thialysine sensitivity (supplemental Figure S4). The order of sensitivity to thialysine was LYP1 haploid > LYP1/LYP1 diploid ≫ lyp1/LYP1 diploid ≫ lyp1/lyp1 diploid = lyp1 haploid.
Figure 4.—
A high frequency of MRA escape observed in heterozygous diploids obtained by mating query and deletion array strains is eradicated by canavanine, but not thialysine. Diploid clones, heterozygous for lyp1Δ0/LYP1 and can1Δ0/CAN1 alleles were formed by mating hoΔ0∷KanMX strains (from Research Genetics, Huntsville, AL) with (A) Y15578-1.2b or (B) Y15588-10.2a. MRA escape was assayed on −HIS media, and −HIS media +thialysine or canavanine, as described in Figure 2.
Haploid cells under negative selection can form diploid MRA escape colonies:
Effective eradication of can1Δ0/CAN1 heterozygous diploids by canavanine indicated that diploid cells present in a final SGA haploid culture are probably homozygous can1Δ0/can1Δ0 (canavanine resistant) and thus originate from matings between postmeiotic haploids. To test the possibility of mating between haploid cells in the setting of negative selection by G418, ClonNat, and MRA, query and deletion strains were mixed and plated onto haploid selection media. Diploid cells formed readily when the query strain and deletion array strain were of opposite mating types (Figure 5A). Diploidy was evidenced by sporulation and absence of escape by either haploid if plated alone (not shown). Formation of diploid escape colonies could be prevented by treatment with 5-FOA when a query strain harboring URA3 was involved (Figure 5A). Diploids were also formed (though at much lower frequency) when query and deletion array strains of the same mating type were mixed, and these events were eliminated by deletion of the silent mating-type locus (Figure 5B).
Figure 5.—
Haploid cells under negative selection can mate to form heterozygous MRA escape colonies. The indicated SGA query strains harboring a Tet-RNR1∷ClonNat allele were mated with a ptc1Δ0∷KanMX deletion array strain of the (A) opposite or (B) same original mating type by cospotting overnight YPD cultures onto SGA haploid selective media lacking canavanine. A depicts 4-μl droplets of cultures that were cospotted. B (since these events were rare) depicts an area of a petri dish over which 100 μl of culture were coplated.
MRA escape is associated with masking of genetic interactions:
To test whether reduction in MRA escape translates to increased sensitivity to detect genetic interactions, we performed SGA with the strain we began the study with (15110-3.4.1b) and those we constructed with reduced MRA escape. We introduced a tetracycline-repressible allele of RNR1 into each query strain, as previously described (Hartman 2007). We next assembled a test array of 384 cultures, employing 96 cultures each for one negative control (hoΔ0∷KanMX serves as a wild-type control, since S288C is heterothallic), and three positive controls (genes known from unpublished data to be synthetic lethal with dial-down of RNR1). Double-mutant haploids were constructed by mating each respective query stain to the test array, followed by SGA selection (Tong and Boone 2006). Double mutants were selected in the absence of doxycycline (RNR1 expression “ON”). To test for genetic interactions, SGA-selected double-mutant haploids were transferred to media with doxycycline to reduce RNR1 expression (Figure 6). Strains with reduced MRA escape exhibited fewer instances of being able to grow in the presence of doxycycline, thus exhibiting greater sensitivity (reduced false positives) to detect true genetic interactions. The frequency of false positives for strains with reduced MRA escape varied between 2 and 4%, which was low compared to 10–70% for the original strain (exhibiting higher MRA escape) (Figure 6). Sensitivity was similar for the new MATa and MATα query strains (data not shown).
DISCUSSION
This study aimed to increase the haploid purity of double-mutant cultures obtained by the SGA method, and thus to improve precision and accuracy for measuring genetic interactions in high-throughput screens. We identified factors conferring escape from mating-type-regulated auxotrophy (MRA), the principle mechanism enabling media selection of haploids from a complex sporulation mixture. By focusing on MRA escape of the query strain (entailing a simple colony-forming assay) we could test MRA factors more quickly than by SGA, a more involved assay. As hoped, factors that reduced MRA escape also reduced formation of heterozygous diploid double mutants that mask gene interactions otherwise observed on a haploid background. In addition to increasing sensitivity in a qualitative sense, eliminating the masking effect of contaminating diploids will enhance the utility of quantitative phenotyping methods to precisely quantify interactions across the genome for SGA-derived double-mutant cell arrays (Shah et al. 2007). Diploid contamination undermines the distinction between strong (e.g., “synthetic lethal”), intermediate (e.g., “enhancing”), weak (e.g., “negligible”), and alleviating (e.g., “suppressor”) interactions, which quantitative phenotyping methods attempt to delineate (Hartman and Tippery 2004; Collins et al. 2006; Hartman 2007).
An unexpectedly high frequency of diploid escape revealed the importance of recessive drug resistance to canavanine for eliminating the presporulation population of heterozygous diploids (Figure 4). Moreover, to prevent postmeiotic haploid mating and formation of diploids with can1/can1 homozygous resistance to canavanine, MRA stringency is especially critical in that setting. Integration of the MRA cassette at the CAN1 locus (encoding the arginine permease) places it in the context of actively transcribed chromatin under conditions of MRA selection (media lacking arginine). Integration of a transcriptional terminator and GAL1 repressor between the CAN1 promoter and PMFA1-HIS3 cassette helped to silence readthrough transcription and thereby prevent small MRA escape colonies having the original query strain mating type. The effect of PGAL1 upstream of the MRA cassette is presumably due to binding of transcriptional repressor elements in the setting of glucose as the primary carbon source (Figure 1, Table 2). Consistent with this mechanism, strains harboring PGAL1 exhibited higher MRA escape when plated on galactose-containing media (data not shown). We next tested whether different auxotrophic genes, assuming the same degree of “leakiness” from a particular mating-type-regulated promoter, affect MRA stringency. In this regard, HIS3 exhibited fewer small MRA escape colonies than LEU2, indicating it has a higher transcriptional requirement for prototrophy (supplemental Figure S2, Table 2). LYS2 is an additional auxotrophy (not examined in this study) that could be investigated for future use with the MATα deletion strain collection.
Despite the 99% reduction in small MRA escape colonies observed after introducing PGAL1-TADH1 upstream of the MRA cassette, the frequency of large colonies was unchanged. Although less frequent, large MRA escape colonies accounted for a significant percentage of the total cellular mass of MRA escape (Figure 1). We found mating-type switching to be associated with large colony MRA escape in query strains of either mating type, and by knocking out the respective silent HM loci prevented mitotic gene conversion at the MAT locus, the presumed mechanism (Figures 2 and 3 and supplemental Figures S3 and S5). Numerous sterile MRA escape colonies remained after deletion of the HMRa locus from the MATα query strain, in contrast to the MATa query strain, which did not exhibit sterile colonies (Figures 2 and 3). A possible explanation for this difference is mutation of MFα2, which would derepress a-regulated genes in α-cells, but not affect α-regulated genes in a-cells (Nasmyth 1982). We do not have an explanation for the observation that the 15591 MRA cassette (PGAL1-TADH1-PSTE2-his5+) yielded α-mating escape colonies, while 15578 (PGAL1-TADH1-PMFA1-his5+) escape colonies were sterile. Nevertheless, PMFA1 may be favorable over PSTE2 for the MATα query strain, due to the sterile phenotype. On the other hand, PSTE3 may be preferable to PMFα1 for use in a MATa query strain, due to its lower MRA escape frequency.
Consistent with the idea that masking of genetic interactions results primarily from creation of diploid cells, sterile MRA escape observed for the 15578 (MATa PGAL1-TADH1-PMFA1-his5+) strain was not associated with an increase in diploid masking of interactions over the level observed for 15588 (MATα PGAL1-TADH1-PSTE3-his5+) (Figure 6 and data not shown). This can be explained, because only postmeiotic, nonsterile haploid cells can form diploid cells having homozygous canavanine resistance, dual G418/ClonNat resistance, masking wild-type allele(s), and a high frequency of MRA escape.
Taken together, our results suggest that masking of genetic interactions stems largely from unanticipated matings between postmeiotic haploid cells, which may be contributed in part by MRA escape. Haploid cells under negative selection during SGA are capable of mating, further supporting the notion that haploid MRA escape can contribute to masking of genetic interactions (Figure 5). Diploids that have acquired homozygous canavanine resistance and heterozygosity at interacting loci will have an even higher rate of escape (Figure 4).
In general, our study validates the premise that reducing MRA escape improves SGA efficiency (Figure 6). However, factors were only independently tested for their effects on MRA escape: they were not tested in incremental detail for effects on SGA because of the large number of factors, various ways of combining them, the relative difficulty and time demand for SGA analysis, and the strong rationale for MRA stringency correlating with SGA efficiency. As a result, some questions remain open regarding the degree to which MRA escape correlates with false-negative SGA results. For example, Daniel et al. (2006) published the benefit of the his5+ gene from S. pombe in SGA during the course of our studies, and additionally the STE2 promoter was adapted in lieu of the MFA1 promoter in the MRA cassette (Tong and Boone 2006). We tested a strain (CBY7092) containing a PSTE2-his5+ MRA cassette for MRA escape, and found it to also have reduced MRA escape, relative to 15110-3-4.1b. In theory, this reduction in MRA escape could be attributable to replacement of the MFA1 promoter with the STE2 promoter or replacement of the HIS3 gene with his5+ in the MRA cassette. Because we had already shown the benefit of the terminator (which this cassette did not employ), we did not test this strain directly by SGA. We note however that a query strain employing PGAL1-PADH1-PMFA1, exhibited less MRA escape than the one employing PGAL1-PADH1-PSTE2 (supplemental Figure S5). On this basis, we speculate that the reduction in MRA escape for CBY7092 is due to the his5+ gene product having reduced activity relative to the HIS3 gene product, and that (in the setting of PGAL1-PADH1) PMFA1 is a better choice than PSTE2. Because we introduced the ADH1 terminator and GAL1 repressor elements before testing the relative effects of PSTE2 and PMFA1 on MRA escape, the interpretation could be different if it were not used. We also note that the terminator/repressor element further reduced small MRA escape events when introduced to CBY7092 (supplemental Figure S5, supplemental Table S2). As another example, the effect of deleting the silent mating type was not tested independently by SGA. However, because the terminator/repressor element and silent mating-type locus impact different types of MRA escape, and by clearly different mechanisms, it is likely they contribute independently to the overall improvement observed when all are used together in SGA. Nevertheless, it is plausible that modifications that reduce MRA escape have more or less impact on SGA depending on the prior presence of other factors.
Another question raised stems from the observation of variability between the three different candidate genes in the number of false positives for Tet-RNR1 synthetic lethal pairs (Figure 6). This variability was observed for double mutants constructed by SGA with the original SGA query strain, but not the strains engineered for lower MRA escape (Figure 6). On the basis of only three examples, we cannot conclude how much variability might exist across the genome. Because we observed no such variability for the new strains, we have not pursued this further. A speculative explanation is that some genes impact mating-type regulation or histidine metabolism, such that query strains with a lower threshold for MRA escape are more variably affected by their deletion.
We observed that heterozygous diploid MRA escape cells were more sensitive to 3-AT than postmeiotic haploid cells having obtained the permissive mating type (data now shown). We also note that replacement of the silent mating-type locus with URA3 introduces 5-FOA sensitivity, which could be used to select against postmeiotic diploid cells (Figure 5B). Unfortunately, genetic linkage of the MAT locus to either silent mating-type locus is very weak (Klar et al. 1980). Thus, marking the silent mating-type loci in the query strain with URA3 does not insure selection against the query MAT locus, due to the high likelihood of meiotic crossover between the MAT and silent type loci on the homologous chromosome contributed by the deletion array strain. We did find that treatment of false-negative cultures with 5-FOA and 3-AT, such as those depicted in Figure 6, purified up to 80% of false-negatives cultures, such that they tested true in repeat analysis (data not shown). However, a tradeoff of using these agents is that 5-FOA and 3-AT are likely toxic to a variety of deletion strains (in our study the thr1 deletion strain was sensitive), systematically preventing analysis of such mutants in the presence of these additive (data not shown). Thus, the incremental benefit of additionally using 5-FOA and/or 3-AT in SGA haploid selection media would need to be evaluated for tradeoffs in toxicity to the overall collection of deletion mutants. Alternatively, assuming 3% false positives for the new strains, duplicates should reduce the false-positive rate to ∼0.1%. Moreover, duplicates can now be performed using MATa and MATα queries for cross-validation of hits, providing additional specificity and confidence in the validity of interactions.
A final consideration for optimizing the sensitivity of detecting genetic interactions by SGA-type construction of haploid double mutants is minimizing meiotic nondisjunction during sporulation, which leads to disomy (a homologous pair of chromosomes missegregating to a haploid cell) and masking of interactions. Following meiotic nondisjunction, a haploid spore can contain a synthetic lethal pair of genes (selected by ClonNat and G418 resistance), but in addition a disomic chromosome (carrying a WT copy of one of the pairs of genes), which would be maintained by selection for its suppression of the synthetic lethality. This is a problem of false negatives in the assay that seems to be largely dependent on the number of spores that are transferred to the haploid selection plate, and unrelated to MRA escape. Illustrating this point, SGA-type strategy has been employed recently to isolate disomic strains for nearly every chromosome, and the rate appeared to be ∼10−4 per meiosis (Zebrowski and Kaback 2008). The stability and viability of particular disomic chromosomes varies (Campbell et al. 1981), and thus could contribute a variable background rate of false-positive interactions. Ways to minimize disomy as a source of false-negative results include performing strain constructions in replicate and optimizing the number of tetrads transferred from sporulation media to haploid selection media. It is also plausible that genes affecting the fidelity of chromosome segregation might exhibit altered rates of disomic masking of genetic interactions and that rates will vary depending on the chromosomal locations of particular genes, due to differential stability of respective disomes.
The strategy for SGA that we present in Figure 6 yields double mutants with a deletion allele of one gene and a conditionally expressed allele of another. This approach offers some potential advantages. First, the double mutant should always be recovered, because conditional expression of the query gene suppresses synthetic lethality during haploid selection, distinguishing double mutants lost due to defects in mating or sporulation. Second, conditional expression should reduce masking disomes, which can be growth defective and thus outcompeted by nondisomic strains expressing the query gene during haploid selection. Once the query gene is dialed down to test for genetic interaction, disomic strains will have been diminished from the culture. Third, tetracycline-regulated conditional expression, employed in the context of genomewide SGA, can be utilized to quantify the relative strength of interaction exerted by different deletion genes assessing it at different levels of query gene expression (Hartman 2007). Incorporation of strategies that maximize the stringency of MRA, utilize conditional expression of query genes, and employ quantitative, high-throughput phenotyping (Shah et al. 2007), should provide more precise and accurate analysis of gene interaction networks.
Acknowledgments
The authors thank Lee Hartwell, David Bedwell, and referees for review of the manuscript and useful suggestions. We thank Charlie Boone and Dan Burke for providing strains and Jingyu Guo and Kim Gaston for technical assistance. Funding was provided by the National Institutes of Health (K08 CA90637, GM17709, and P30CA13148-35) and the Howard Hughes Medical Institute (Physician-Scientist Postdoctoral Fellowship and Early Career Awards to J.L.H.).
References
- Barton, N. H., and P. D. Keightley, 2002. Understanding quantitative genetic variation. Nat. Rev. Genet. 3 11–21. [DOI] [PubMed] [Google Scholar]
- Burke, D., D. Dawson and T. Stearns, 2000. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Campbell, D., J. S. Doctor, J. H. Feuersanger and M. M. Doolittle, 1981. Differential mitotic stability of yeast disomes derived from triploid meiosis. Genetics 98 239–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codon, A. C., J. M. Gasent-Ramirez and T. Benitez, 1995. Factors which affect the frequency of sporulation and tetrad formation in Saccharomyces cerevisiae baker's yeasts. Appl. Environ. Microbiol. 61 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, S. R., M. Schuldiner, N. J. Krogan and J. S. Weissman, 2006. A strategy for extracting and analyzing large-scale quantitative epistatic interaction data. Genome Biol. 7 R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel, J. A., J. Yoo, B. T. Bettinger, D. C. Amberg and D. J. Burke, 2006. Eliminating gene conversion improves high-throughput genetics in Saccharomyces cerevisiae. Genetics 172 709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles et al., 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418 387–391. [DOI] [PubMed] [Google Scholar]
- Gietz, R. D., and R. A. Woods, 2002. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350 87–96. [DOI] [PubMed] [Google Scholar]
- Hartman, J. L., IV, 2007. Buffering of deoxyribonucleotide pool homeostasis by threonine metabolism. Proc. Natl. Acad. Sci. USA 104 11700–11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman, J. L., IV, B. Garvik and L. Hartwell, 2001. Principles for the buffering of genetic variation. Science 291 1001–1004. [DOI] [PubMed] [Google Scholar]
- Hartman, J. L., IV, and N. P. Tippery, 2004. Systematic quantification of gene interactions by phenotypic array analysis. Genome Biol. 5 R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar, A. J., J. McIndoo, J. B. Hicks and J. N. Strathern, 1980. Precise mapping of the homothallism genes HML and HMR in Saccharomyces cerevisiae. Genetics 96 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M. S., A. McKenzie, III, D. J. Demarini, N. G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14 953–961. [DOI] [PubMed] [Google Scholar]
- Mani, R., R. P. St Onge, J. L. Hartman, IV, G. Giaever and F. P. Roth, 2008. Defining genetic interaction. Proc. Natl. Acad. Sci. USA 105 3461–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth, K. A., 1982. Molecular genetics of yeast mating type. Annu. Rev. Genet. 16 439–500. [DOI] [PubMed] [Google Scholar]
- Olson, M. V., 1999. When less is more: gene loss as an engine of evolutionary change. Am. J. Hum. Genet. 64 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roguev, A., S. Bandyopadhyay, M. Zofall, K. Zhang, T. Fischer et al., 2008. Conservation and rewiring of functional modules revealed by an epistasis map in fission yeast. Science 322 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, N. A., R. J. Laws, B. Wardman, L. P. Zhao and J. L. Hartman, IV, 2007. Accurate, precise modeling of cell proliferation kinetics from time-lapse imaging and automated image analysis of agar yeast culture arrays. BMC Syst. Biol. 1 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge, R. P., R. Mani, J. Oh, M. Proctor, E. Fung et al., 2007. Systematic pathway analysis using high-resolution fitness profiling of combinatorial gene deletions. Nat. Genet. 39 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, A. H., and C. Boone, 2006. Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol. Biol. 313 171–192. [DOI] [PubMed] [Google Scholar]
- Tong, A. H., M. Evangelista, A. B. Parsons, H. Xu, G. D. Bader et al., 2001. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294 2364–2368. [DOI] [PubMed] [Google Scholar]
- Tong, A. H., G. Lesage, G. D. Bader, H. Ding, H. Xu et al., 2004. Global mapping of the yeast genetic interaction network. Science 303 808–813. [DOI] [PubMed] [Google Scholar]
- Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson et al., 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285 901–906. [DOI] [PubMed] [Google Scholar]
- Zebrowski, D. C., and D. B. Kaback, 2008. A simple method for isolating disomic strains of Saccharomyces cerevisiae. Yeast 25 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]