Abstract
We used P-element transposase-mediated “male recombination” between two P elements in trans to create genetic deletions that removed a number of loci, including the gene encoding the neuropeptide crustacean cardioactive peptide (CCAP). Two classes of recombinant chromosomes were produced. Approximately one-quarter were viable when homozygous or hemizygous, whereas the remaining lines caused homozygous and hemizygous lethality. Preliminary analyses using PCR and CCAP immunohistochemistry suggested that, whereas the DNA of the viable lines was largely intact, most lethal lines contained chromosomal deletions that were roughly bounded by the insertion sites of the two P elements used. Southern blot analyses of select lethal lines showed that the DNA flanking the deletion was indeed grossly intact whereas the intervening DNA could not be detected. Sequencing across the deletion in three of these lethal lines identified a single line bearing intact genomic DNA on either side of the deletion separated by 30 bp of P-element DNA. The method described here suggests a simple procedure for creating deletions with defined end points. Importantly, it can use preexisting P-element insertion strains and does not rely on the use of transposable elements that are engineered to cause specific DNA rearrangements.
UNDERSTANDING gene function is greatly aided by the availability of mutant alleles. In Drosophila a variety of methods presently exist that make use of engineered mobile elements to disrupt specific sequences (reviewed in Venken and Bellen 2005). They range from methods that allow for the targeted disruption of particular genes (e.g., Rong 2002; Gong and Golic 2003), to methods that permit the efficient identification of specific mutations from a large pool of mutagenized chromosomes (e.g., Ballinger and Benzer 1989; Kaiser and Goodwin 1990), to methods for producing tailor-made genetic deletions (e.g., Cooley et al. 1990; Huet et al. 2002; Parks et al. 2004; Ryder et al. 2004, 2007).
Drosophila P-mobile elements have been extensively used for insertional mutagenesis (e.g., Cooley et al. 1988; Rørth 1996; Deak et al. 1997; Spradling et al. 1999; Lukacsovich et al. 2001; Bellen et al. 2004) and many of the insertions have been molecularly mapped and are publicly available as part of the ongoing effort of the Berkeley Drosophila Genome Project Gene Disruption Project (Spradling et al. 1999; Bellen et al. 2004; cf. http://flypush.imgen.bcm.tmc.edu/pscreen/). In addition to their utility for insertional mutagenesis, these mapped P elements can be used to produce genetic deletions of surrounding loci. Many of these elements have been specifically constructed to make deletions with defined end points by including DNA recombinase recognition sites (e.g., Parks et al. 2004; Ryder et al. 2004, 2007). However, for most insertions, one must resort to using either imprecise excision (e.g., Tsubota and Schedl 1986; Salz et al. 1987) or P-induced “male recombination” (Preston et al. 1996). Yet, deletions produced using these methods are typically relatively small, or, at least, their size cannot be controlled. In contrast, the excision of two P elements in cis can result in the loss of the intervening DNA, thereby producing deletions with defined end points (Cooley et al. 1990). In their report, Cooley et al. (1990) did not recover the desired deletion when the two P elements were in trans. This result reduced the utility of the method for the isolation of small deletions since the parental P elements would first have to be placed in cis, a task that becomes increasingly difficult the closer the two P elements are to one another. More recent reports (Parks et al. 2004) have shown that deletions can be obtained from trans-heterozygous P elements. Although the end points of the deletions appeared to correspond to the positions of the P-element insertions, no analysis of the breakpoints was provided. Here, we report the first detailed characterization of the deletions produced by such a method. We show that the boundaries of these deletions are roughly defined by the two P elements and identify deletions that precisely remove only the intervening ∼40 kb of DNA. On the basis of these findings, we discuss various strategies that can be used to generate deletions with precise end points, using P elements in trans.
MATERIALS AND METHODS
Drosophila stocks:
All stocks were obtained from the Bloomington Drosophila Stock Center (http://flystocks.bio.indiana.edu/). They included the following: multiply marked chromosomes bearing the recessive eye color mutations claret1 (ca1) and/or scarlet1 (st1); Df(3R)23D1, ry506/TM3, Sb1 Ser1 (Granderath et al. 1999; breakpoints 94A3–4, 94D1–4; K. Cook, personal communication to FlyBase, http://flybase.bio.indiana.edu/), 2000; ∼800 kb based on information obtained from FlyBase 2000]; P-element stocks y1 w1118; P{lacW}l(3)L0580L0580/TM3, Ser1 (referred to here as “L”; insertion site at 94C1–2; origin, Berkeley Drosophila Genome Project) and w1118; P{GT1}CG17618BG02691 (referred to here as “B”; insertion site at 94C4; origin, Berkeley Drosophila Genome Project); and third chromosome “green” balancer w; Sb1/TM3, P{w+mC=ActGFP}JMR2, Ser1 (w; Sb/TM3, Ser, act-gfp; cf. Reichhart and Ferrandon 1998). Additional stocks not specifically listed were also obtained from this source. All cultures were raised on standard Drosophila cornmeal–agar medium and maintained at room temperature. Both L and B P-element stocks are viable when homozygous and are marked with the eye color gene mini-white.
Transposition of P elements in trans:
To cause P-induced male recombination between the L P element, P{lacW}l(3)L0580L0580, and the B P element, P{GT1}CG17618BG02691, the recessive eye color mutations st1 and ca1 were first recombined onto the L- and B-bearing chromosomes, respectively, using standard meiotic recombination.
P-induced male recombination between the resulting st1 L and B ca1-bearing chromosomes was then produced using the following scheme:
G0: Hobo(Δ2-3), CyO/+; B, ca1/Sb (males) × st1, L/TM3, Ser1 (virgin females)
G1: Hobo(Δ2-3), CyO/+; st1, L/B, ca1 (two males per vial) × st1, e1, ca1 (five virgin females per vial)
G2: st1, (L?), (B?), ca1/st1, e1, ca1 (single “st, ca” male from each vial) × TM3, Sb1, e/TM6B, Tb1, e (virgin females).
(?) indicates that the presence and condition of each P element was unknown.
Resulting st1, (L?), (B?), ca1/TM3, Sb1, e or st1, (L?), (B?), ca1/TM6B, Tb1, e males were then used to set up individual lines, w; st1, (L?), (B?), ca1/TM3, Ser, act-gfp.
G1 males have 1 copy of each P element in the presence of P transposase, supplied by Hobo(Δ2-3). G2 flies resulting from P-element transposase-induced male recombination were identified by virtue of being marked with both st and ca. A single such recombinant male from each vial was then used to establish independent lines, each balanced over TM3, Ser, act-gfp.
PCR analysis:
Ten st1, (L?), (B?) ca1/TM3, act-gfp, Ser flies and (10–20) st1, (L?), (B?), ca1/st1, (L?), (B?), ca1 homozygous third instar larvae (identified by their lack of GFP fluorescence) were collected from each line and frozen on dry ice.
DNA was extracted using the procedure of E. J. Rehm (BDGP protocol, cf. Methods under www.fruitfly.org) except that 10 flies or 10–20 third instar larvae were used, and the DNA was resuspended in a final volume of 50 μl.
One microliter of DNA was used for each 20-μl PCR reaction, which was run using the following conditions: 94° for 3 min; then 40 cycles of 94° for 45 sec, 58° for 1.5 min, and 72° for 1.5 min/kb of product; followed by 1 cycle at 72° for 1 min/kb of product. For most reactions, Taq polymerase was from Promega (Madison, WI). For reactions producing >3-kb products, Taq from Roche (Indianapolis) was used (Expand Long Template PCR system).
The primers used are listed in supplemental Table 1, and their position relative to the L and B P elements is illustrated in Figure 1. The size of the expected PCR product ranged from 670 to 980 bp (average 868 bp).
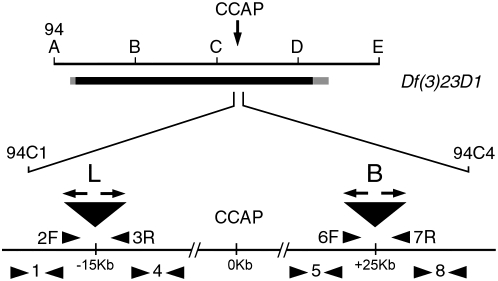
Figure 1.—
Genetic map of the CCAP gene region and location of the PCR primers used to characterize deletions produced by P-element-induced male recombination between the “L” and “B” P elements. The CCAP gene maps to 94C4 (3R, right arm of chromosome III). Large triangles represent the locations of the L and B P elements in the parental lines used for this study. Solid bar indicates the approximate genomic region deleted in Df(3R)23D1. Arrowheads indicate the relative location and direction of the PCR primers used (not to scale); “F” and “R” refer to forward and reverse primers, respectively. A single number indicates the number for that F + R primer pair; the arrows on the P elements represent the P-element-specific 31-bp terminal repeat.
Southern blot analysis:
Recombinant chromosomes, balanced over TM3, Ser, act-gfp, were crossed to Df(3R)23D1, ry506/TM3, Ser1, act-gfp. GFP-negative larvae were collected and stored at −80°. Parental lines were similarly crossed to Df(3R)23D1, ry506/TM3, Ser1, act-gfp and hemizygous adults were collected and stored at −80°. DNA was isolated from frozen tissue, using a glass homogenizer and the Genomic DNA Purification kit (Gentra Biosystems), following manufacturer's instructions.
Genomic DNA was EcoRI digested and 2–3 μg were loaded in each lane of a 0.7% agarose gel. Southern blotting was done onto positively charged nylon membranes using 20× SSC as described in Sambrook and Russell (2001). After blotting overnight, briefly rinsing in 6× SSC, and UV crosslinking, membranes were vigorously rinsed in distilled water, allowed to air dry, and stored at room temperature.
Probe templates were made using standard PCR protocols, using GoTaq polymerase (Promega) and BACR23F10 (obtained from the BACPAC Resource Center at Children's Hospital, Oakland Research Institute, Oakland, CA) as PCR template. The PCR primers used are listed in supplemental Table 2 and the positions of the resulting probes are indicated in Figure 3. Probe size ranged from 2.5 to 3.2 kb in length (average: 2.8 kb). PCR products were run on a low-melt agarose gel, and the bands of the correct size were excised and purified using the Wizard SV Gel and PCR Clean-Up system (Promega). Probes were made by labeling these DNA templates using the DIG DNA labeling kit (Roche Applied Science), following manufacturer's instructions.
Figure 3.—
Southern analyses of candidate deletions. (A) Schematic depicting the 94C1–4 region. Tick marks indicate EcoRI sites present in genomic DNA within the BAC clone BACR23F10 (GenBank accession no. AC009846); inverted triangles represent insertion of the “L” and “B” (parental) P elements, and “CCAP” indicates approximate location of the CCAP gene. Solid rectangles below the line mark the location of the hybridization probes used in Southern blotting. Results of Southern blots for the L and B parental lines (“Parental”) and for recombinant lines 12, 13, 36, 38, and 41 heterozygous with deletion of the region, Df(3)23D1 (see Figure 1) (“Recombinant”) using probes 1–6 are indicated immediately below the line; “+” indicates that a fragment(s) of the expected size(s) was obtained with a given probe, whereas “−” indicates that no hybridization was observed. (B) Representative Southern blots of EcoRI-digested DNA hybridized with probe 2 (left), probe 4 (middle), and probe 6 (right). DNA sources are shown at the top of each lane; approximate molecular weight is indicated to the left of each panel (in kilobases).
Prehybridizations and hybridizations were done for 2–4 hr and 14–18 hr, respectively, at 65° in 0.5 m sodium phosphate (pH 7.2), 1 mm EDTA, 7% SDS, and 1% BSA using a probe concentration of 25 μg/ml. After hybridizations, blots were rinsed at 65° in two changes of 2× SSC + 1% SDS, followed by two 30-min changes of 0.2× SSC + 0.1% SDS. All remaining steps were carried out at room temperature. Blots were rinsed briefly in MB buffer (0.1 m maleic acid, 0.15 m NaCl; pH 7.5) + 0.3% Tween-20 and then blocked in MB + 3% milk powder for 1 hr. After two brief rinses in MB + 0.3% Tween-20, blots were incubated for 30 min in alkaline phosphatase-conjugated anti-digoxygenin polyclonal serum (Roche Applied Science) diluted 1:20,000 in MB + 1% milk powder. The remaining rinses, incubation with CDP-Star reagent, and exposure to X-ray film were done as instructed by the manufacturer (Roche Applied Science).
Sequence analyses:
Primer pairs 2F and 7R (see Figure 1 and supplemental Table 1; respectively, 896 bp proximal to L and 673 bp distal to B) were used to amplify the intervening DNA in selected recombinants. The amplified product was purified using the QIAquick PCR purification kit (QIAGEN, Valencia, CA) and sequenced in the distal direction using primer AGT TTG TAG CCC TGC TGA CG (located 571 bp proximal to the L insertion site) and in the proximal direction using primer TTG TAA TAG TCC AGG GTT ACC G (located 261 bp distal to the B insertion site).
Immunohistochemistry:
Central nervous systems (CNSs) from homozygous st1, (L?), (B?), ca1/st1, (L?), (B?), ca1 or hemizygous st1, (L?), (B?), ca1/Df (3)23D1 third instar larvae (identified by their lack of GFP fluorescence from the TM3, Ser1, act-gfp balancer) were fixed >2hr at room temperature in buffered 4% paraformaldehyde or 1 hr at 4° in buffered 4% paraformaldehyde + 7% of a saturated aqueous picric acid solution. The tissues were then processed for crustacean cardioactive peptide (CCAP) immunohistochemistry as previously described (Park et al. 2003). Antibodies were rabbit anti-CCAP (used at 1:5000, a kind gift of Hans-Jürgen Agricola, University of Jena, Germany) and fluorescently labeled secondary antibodies obtained from Jackson ImmunoResearch and Molecular Probes (Eugene, OR). Preparations were dehydrated, mounted in DPX (Fluka, Buchs, Switzerland), and viewed under a conventional fluorescent microscope as well as under a confocal microscope (Leica DMR system). Four to five CNSs were fixed for each line, and, in every case, all CNSs within a group were either CCAP immunopositive or CCAP immunonegative (cf. Figure 2, B and C, respectively).
Figure 2.—
Characterization of three particular lethal P-induced male recombinant lines (nos. 12, 13, and 38). (A) PCR amplification using primers outside of the region between the “L” and “B” P elements (2F + P and P + 7R; see map below gel; “P” primer corresponds to the P-element-specific 31-bp terminal) yielded products of the expected lengths (∼900 and ∼700 bp, respectively) in lines 12 and 13, suggesting that these regions were intact in these two lines. Line 38 yielded the expected product from the 2F + P_31 reaction, while the P_31 + 7R reaction yielded no product, most likely due to lack of complete P_31 sequence remaining in this line (see Figure 4D). PCR amplification using the external primers 2F + 7R yielded no product from parental controls (not shown), due to the intervening 40 kb of DNA. However, the same reaction using DNA from lines 12, 13, and 38 yielded ∼7-, ∼4-, and ∼1.5-kb products, respectively, indicating that most of the DNA between the L and B P elements had been lost in these lines. Direct sequencing of these products supports this hypothesis (see Figure 4). (B) A lack of CCAP-IR in the CNS of a larva homozygous for the no. 12 recombinant chromosome confirms that the CCAP gene was deleted in this line. Homozygotes and hemizygotes from lines 13 and 38 were also immunonegative for CCAP (not shown). (C) CCAP-IR in the CNS of a no. 12/TM3 control larva, showing a normal pattern of immunoreactivity (cf. Park et al. 2003). Arrowheads point to prominently stained neurons in the brain (Br) and ventral nervous system (vns). Bar, 40 μm.
RESULTS
Chromosomes generated by P-element-induced male recombination of two P elements in trans:
The neuropeptide CCAP (at 94C4; Park et al. 2003; cf. Figure 1) plays an important role in the control of ecdysis, the behavior used by insects to shed their old cuticle at the end of the molt (reviewed in Ewer and Reynolds 2002). To more fully understand the role of this neuropeptide, we sought to isolate mutants that lacked a functional CCAP gene. As a first step, we were interested in isolating genetic deletions that included CCAP and were smaller than the Df(3R)23D1 deletion (breakpoints 94A3–4, 94D1–4), the smallest extant deletion for this region. We first screened for imprecise excisions of P-element strains P{lacW}l(3)L0580L0580 (L) and P{GT1}CG17618BG02691 (B), which carry insertions 15 kb proximal and 25 kb distal to CCAP, respectively, using standard procedures (Grigliatti 1998). Of ∼2000 excision lines obtained by mobilizing the L element (W. A. Hoose and J. Ewer, unpublished data) and ∼500 excision lines obtained using the B P-element insertion (J. Ewer, unpublished data), no line was isolated that carried a deletion >1.5 kb. For this reason we attempted to produce a deletion by inducing male recombination of these two P elements in trans (cf. Parks et al. 2004).
To do so the chromosome bearing the L insert was first marked with the proximal recessive eye color marker st1 and the chromosome bearing the B P element was marked with the distal recessive eye marker ca1, using standard meiotic recombination. These marked insertions were then placed in trans in the presence of a source of P transposase and the progeny screened for st1 ca1 male recombinants (see materials and methods). Forty-three independent “recombinant” chromosomes were isolated out of ∼20,000 flies screened (i.e., ∼0.2%) and were balanced as individual lines for further analyses.
The resulting chromosomes were then analyzed for viability when homozygous, as well as when heterozygous with Df(3R)23D1. In addition, the CNS of third instar larvae homozygous for each recombinant chromosome was processed for CCAP immunoreactivity (CCAP-IR) to determine if the DNA encoding this neuropeptide was still functional.
All 43 lines could readily be classified into two groups. Lines from the first group (12/43 = 28%) were viable as homozygotes and when heterozygous with Df(3)23D1. Homozygous larvae from all of these lines showed normal CCAP-IR (cf. Figure 2C; data summarized in Table 1; see Park et al. 2003). They are referred to as “homozygous viable lines,” below. On the other hand, recombinant lines from the second group (31/43 = 72%) were not viable when homozygous or heterozygous with Df(3)23D1. Homozygous recombinant animals from 28/31 of these lines died at the end the third larval instar with an elongated body and showed no CCAP-IR (Figure 2B; data summarized in Table 1), while the remaining 3/31 were embryonic lethal (these lines were not analyzed for CCAP-IR). These 31 lines are referred to as “homozygous lethal lines” below. Their characteristics, especially their lack of CCAP-IR as homozygotes, suggest that at least part of the DNA in the region bracketed by the L and B P elements had been deleted.
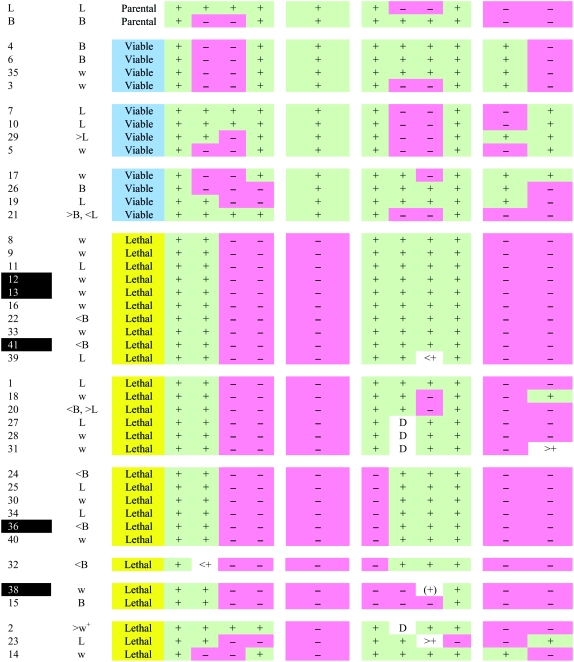
TABLE 1.
Summary of structure of P-induced male recombinants
Number of recombinant line. Lines were grouped into classes on the basis of their phenotypic characteristics. Black cells correspond to lines that were more fully analyzed (by Southern and sequence analyses).
Eye color of male progeny from a cross of recombinant to w flies. w, same as that of w− animals; L, B, similar to eye color of L or B control crosses, respectively; <B, lighter than that of B (lightest eye color); >L, darker than that of L (darkest eye color); w+, indistinguishable from that of w+ animals (≫L).
Viable (blue cells), lethal (yellow cells): lines in which animals homozygous or hemizygous for the recombinant chromosome lived until adulthood or died prior to adulthood, respectively.
Result of PCR amplification of product numbered in schematic, in homozygous or hemizygous adults (viable lines) or third instar larvae (lethal lines). Product of normal size, +, green cells; absence of product, −, rose-colored cells; <+, >+, product larger and smaller than expected, respectively; D, doublet; (+), faint band of correct size; ND, not done because these lines were embryonic lethal (see text for further details).
CCAP-IR of CNS of homozygous or hemizygous adults (viable lines) or third instar larvae (lethal lines). Normal CCAP-IR, +, green cells; no CCAP-IR, −, rose-colored cells; ND, not done because these lines were embryonic lethal (see text for further details).
The genomic region between the L and B P elements is predicted to encode 14 genes in addition to CCAP, 8 of which are of unknown function (cf. FlyBase). Thus, the simple grouping of recombinants into homozygous viable and homozygous lethal classes could hide a more complex picture. For this reason we sought to characterize further the nature of the lesions present in the recombinant lines. To do so we first used PCR to determine the gross integrity of the P-element ends and of the genomic DNA immediately flanking the insertion sites in each of the recombinant lines. For this we used DNA from heterozygous flies for testing for the presence of the inverted repeats on P-element ends and DNA from homozygous [or hemizygous using Df(3)23D1] larvae for testing the integrity of the DNA in the vicinity of the P-element insertion sites. This latter test was not done for the 3/43 lines that did not produce homozygous third instar larvae. The location of the primers used is indicated in Figure 1, and their sequences are listed in supplemental Table 1. In addition, flies bearing recombinant chromosomes were crossed to white (w) mutant flies to assess their eye color; this information was used to infer the integrity of the parental P elements (each carried the mini-white transgene and expressed a distinguishable eye color). Male progeny resulting from this cross were aged for 5 days, and their eye color was compared to that of the progeny obtained using the parental P-element strains. Eye color was classified as being indistinguishable from w (w), similar to that of L (L) or that of B (B), lighter than that of the lightest of these (B; <B), darker than that of L (>L), intermediate (>B <L), or indistinguishable from that of wild type (w+). The results of these analyses are summarized in Table 1.
Structure of homozygous viable P-element-induced male recombinant chromosomes:
Complementation tests using Df(3)23D1 and CCAP immunostaining suggested that the DNA in the region between the L and the B P-element insertion sites was mostly intact in the 12/43 homozygous viable lines. Our PCR results, summarized in Table 1 and discussed below, are consistent with this interpretation. In particular, all 12/12 recombinant chromosomes produced normal-sized products when testing for the integrity of the DNA immediately proximal to the L and the B insertion sites and immediately distal to the B insertion site (fragment 1, obtained with primer pair 1F + 1R; fragment 5, obtained with primer pair 5F + 5R; and fragment 8, obtained with primer pair 8F + 8R, respectively; see Figure 1 and Table 1 for a schematic showing the location of these fragments), and 10/12 lines amplified a normal fragment 4 (from DNA immediately distal to L, amplified using primer pair 4F + 4R; cf. Figure 1 and Table 1). These results together with the normal pattern of CCAP expression suggest that any genetic lesions present in these lines would likely be confined to P-element DNA and/or to DNA in the immediate vicinity of the P elements. Small deletions or duplications (cf. Preston et al. 1996) would, nevertheless, not have been detected by our tests.
Homozygous viable recombinant chromosomes could be further subdivided into three broad classes, on the basis of the integrity of the L and B P elements. A first class (4/12) contained recombinants where a precise or a near precise excision of the L P element had occurred. This diagnosis was based on the fact that DNA from these chromosomes failed to produce bands with primers 2F and P_31 (product 2, cf. Table 1) and P_31 and 3R (product 3, cf. Table 1) and that the 2F + 3R primer pair produced a band that was the same size as that obtained using DNA from wild-type flies. These chromosomes also produced normal-sized products from genomic DNA flanking the L P-element insertion (products 1 and 4, obtained using pairs 1F + 1R and 4F + 4R, respectively; cf. Figure 1 and Table 1), suggesting this DNA was intact. In two of these four recombinants the other (B) P element was likely intact, on the basis of the production of normal-sized products with primer pairs 6F + P_31 (product 6, cf. Table 1) and P_31 and 7R (product 7, cf. Table 1) and the expression of a B eye color. In the remaining two lines, at least part of the B P element had been lost, because products 6 and 7 were not obtained or the flies expressed a w eye color. Interestingly, the 6F + 7R primer pair failed to produce an amplification product in all four lines, indicating that either a significant fragment of the B P element was still present or DNA that included the 6F or 7R sequences had been deleted.
A second class of viable recombinants was essentially equivalent to the first, except that in this case it was the B P element that appeared to have been excised. They all failed to amplify products 6 and 7 (cf. Table 1), which depend on the presence of the P-element terminal inverted repeat, and amplified a wild-type-size product using primers 6F + 7R, indicating a precise (or near precise) excision of the B P element. Similarly to what was seen for the first class, the other (in this case L) P element appeared to be intact in some (two of four) of these recombinants, on the basis of the presence of the P-element ends (products 2 and 3; cf. Table 1) and the expression of L eye color.
The remaining 4/12 homozygous viable lines produced a more complicated constellation of PCR products. Three of these lines were similar to the first class, with most of the changes involving the L P-element insertion site, whereas in the fourth line most of the changes were detected around the B P-element insertion site.
Structure of homozygous lethal P-element-induced male recombinant chromosomes:
The structure of the viable recombinants suggests that the lesions present in these lines were likely confined to the DNA in the immediate vicinity of the L and B P elements. Their structure suggests that they arose through a simple male recombination event involving one of the two P elements and the corresponding DNA on the homolog (Preston and Engels 1996; Preston et al. 1996); further tests will be needed to confirm our preliminary assessment of the structure of these recombinants. As detailed below, the majority of the lethal recombinants were, by contrast, likely produced by male recombination events involving the region around the L P element on one chromosome and that surrounding the B P element on the homolog, thereby deleting most of the intervening DNA.
Thirty-one of the 43 recombinant lines were lethal when homozygous and also when heterozygous with Df(3)23D1. The DNA proximal to the L and distal to the B P elements appeared to be mostly intact in these lines because all 28 lines that were tested amplified a normal-sized fragment from DNA proximal to L (band 1; cf. Figure 1 and Table 1), and 27/28 produced a normal-sized fragment from DNA distal to B (product 8 obtained using primer pair 8F + 8R, cf. Figure 1 and Table 1) (3 of 31 lethal lines were embryonic lethal and were not subjected to these PCR tests). These findings strongly suggest that the vast majority of these recombinants carried deletions that were confined to the region between the two P elements.
The 31 homozygous lethal lines could be further divided into several classes on the basis of the structure of the DNA bounded by these two P elements (Table 1). The vast majority of the homozygous lethal lines produced normal-sized PCR products on the proximal side of the L P element (29 of the 31 lines produced normal-sized PCR product 2) while failing to do so on the distal side [PCR products 3 and 4 were not obtained for 30/31 and 26/28, respectively (the 3/31 embryonic lethal lines were not tested for product 4)]. In contrast, and as summarized in Table 1, there was more variation on the side associated with the B P element, with the DNA proximal to the B element being intact in many cases, and DNA distal to B being disrupted in some cases. Thus, 19/28 and 25/31 amplified normal-sized (proximal) products 5 and 6, respectively, and 25/31 amplified normal-sized (distal) fragment 7. Nevertheless 27/28 amplified normal product 8, suggesting that the DNA was disrupted only in the immediate vicinity of the B element insertion. This conclusion is supported by our limited Southern analyses (see below). Lines that showed a more complex molecular microstructure, as well as homozygous viable lines that did not show a simple PCR pattern (see above), may harbor small rearrangements in the vicinity of the P-insertion sites. In the scheme used to produce the recombinants, one P element is first combined with the P-element transposase, and the second P element is then introduced. The small rearrangements may in part be the result of recombination events between this second P element and “local hops” produced during the first step of the scheme (cf. Takaesu et al. 2006). This is consistent with the fact that the B insert was used in the first step of the scheme and is the side most often associated with a more complex molecular picture.
A subset of the homozygous lethal lines that appeared intact proximal to L and distal to B was analyzed by Southern blot to determine the gross integrity of DNA proximal to L, between L and B, and distal to B. Figure 3A summarizes the results obtained with hybridization probes 1–6 using DNA from hemizygous controls [L and B lines heterozygous with Df(3)23D1] and from lines 12, 13, 36, 38, and 41; representative examples obtained with probes 2, 4, and 6 are shown in Figure 3B. The results obtained indicate that the regions proximal to L and distal to B are grossly normal, whereas the region between the two inserts has been deleted. These findings, coupled with the lack of CCAP-IR and the PCR results interrogating the region immediately flanking the insertion sites, strongly suggest that the lesions present in these lines are limited to the DNA between the L and the B inserts in the vast majority of cases.
We used two further criteria to choose the most desirable deletions, which, for our purposes, were those in which the region between L and B insertions had been most completely deleted. First, we limited our search to lines that had completely lost the white eye color marker gene present on the P elements. Of these, we chose for further characterization recombinant chromosomes in which the putative deletion could be spanned by PCR. For this we used the 2F + 7R primer pair; these primers are, respectively, 896 bp proximal to L and 673 bp distal to B (1569 bp, total; see Figure 1). As shown in Figure 2A, 3 lines (nos. 12, 13, and 38) amplified products of ∼7, ∼4, and ∼1.5 kb using this primer pair; animals from the remaining 25 larval lethal lines produced products >10 kb and were not analyzed further (data not shown). Since the L (P{LacW}) and the B (P{GT1}) are 10.7 and 8.5 kb in length, respectively, our PCR results suggest that the majority of the intervening genomic DNA as well as the P-element DNA was likely deleted in these 3 lines. Sequencing ∼1.2 kb from either end of the 2F + 7R PCR products for these 3 lines showed that the genomic DNA flanking the P elements was intact up to the insertion site, beyond which it matched P-element sequences. These sequence analyses are summarized in Figure 4 and supplemental Figure 1. For Line 12 (Figure 4B, supplemental Figure 1A) the ∼400 bp of P-element DNA that were sequenced from either end of the 2F + 7R amplification product, and that are closest to the insertion sites, corresponded exactly with the ends of an intact P{GT1} in the original (inverted) orientation found in the parental B strain, but on both the proximal side of the deletion (which corresponds to the site of insertion of the P{LacW} found in the L strain) as well as the distal sides of the deletion. In Line 13 (Figure 4C, supplemental Figure 1B) these P-element sequences corresponded exactly with the 5′ end of an intact P{LacW} element on the proximal side of the deletion (as found in the parental L) and with the 5′ end of an intact P{GT1} (in the inverted orientation) on the distal side (as found in the parental B element). Finally, in line 38 (Figure 4D, supplemental Figure 1C) the genomic sequences corresponding to the proximal and distal boundaries of the deletion were separated by a stretch of only 30 bp of P-element sequence. This 30-bp fragment consisted of a 15-bp inverted repeat identical to one-half of the P-element terminal repeat sequence (solid arrows in Figure 4). This product likely arose by hybrid excision repair (HER) (cf. Beall and Rio 1997), by nonhomologous end joining (NHEJ) of the 17-bp single-stranded ends that are created when P elements excise (C. Flores, personal communication).
Figure 4.—
Sequence analyses of deletions 12, 13, and 38: summary of the structure of the parental chromosomes “L” (top) and “B” (bottom) (A) and of lines 12 (B), 13 (C), and 38 (D) based on analyses of the sequences of boundaries between genomic and P-element DNA. Open and solid bars represent proximal and distal genomic DNA (relative to the centromere), respectively, and dotted lines indicate genomic DNA between the P-element insertions, which are depicted by inverted triangles in A [L is a placW element inserted in the (+) strand and is indicated by a solid triangle; B is a pGT1 element inserted in the (−) strand and is indicated by an open triangle]. Inverted repeats at the ends of the P elements are represented by a solid arrow plus an open arrow because of the features of the structure of line 38 (indicated in D). In B and C, the question marks (“?”) reflect the fact that only ∼400 bp of P-element DNA was sequenced from the proximal and distal directions, so the complete sequence of remaining P-element DNA is not known.
DISCUSSION
Use of P-element-induced recombination between P elements in trans for producing deletions with defined end points:
Here we show that placing two P elements in trans in the presence of transposase and screening for male recombination resulted in the isolation of genetic deletions that were bracketed by the two P-element insertion sites. These deletions were ∼40 kb in size, which is far greater than the size that is typical for deletions created by imprecise excision of P elements (e.g., Xu et al. 2006 and our initial screens using each P element singly). Several lines of evidence support our hypothesis that the deletions obtained here are roughly bounded by the P elements used:
Of the 31 lethal recombinant lines obtained (of 43 lines isolated, which included 12 homozygous viable lines), PCR tests suggested that at least 28 of these were lacking most of the DNA bounded by the P elements, whereas the DNA outside of this region seemed intact.
Animals homozygous for these 28 lethal recombinants expressed the same terminal phenotype at the third instar larval stage and lacked immunoreactivity to CCAP, a gene located 15 kb distal and 25 kb proximal to the P elements, respectively.
Southern analyses of 5 lethal recombinants and direct sequencing of 3 of these further suggested that the region between the P-element insertion points was deleted whereas the regions outside it were intact.
Nevertheless, our tests could not exhaustively test the integrity of the whole genetic region [defined by Df(3R)23D1], so it is possible that additional lesions were induced on the lethal recombinant chromosomes. The fact that 3/31 lines were embryonic lethal suggests that such lesions may have occurred in other lines. In addition, some of the homozygous lethal recombinant chromosomes that were embryonic viable when homozygous or hemizygous harbored small rearrangements in the vicinity of the P-insertion sites (see Table 1), which emphasizes the importance of molecularly characterizing the end points of desirable deletions.
A previous study by Cooley et al. (1990) showed that the excision of P elements placed in cis could result in the deletion of the intervening DNA. In contrast, no such deletions were obtained after screening ∼4000 progeny resulting from the mobilization of the same two P elements in trans. We do not know why we succeeded where Cooley et al. (1990) failed, but it is likely to be due to low recovery frequency. We recovered P-element-induced recombinants at a frequency of 0.1–0.5%, and of these ∼3/4 were deletions. A conservative estimate would therefore predict that at least three lethals should have been recovered among 4000 flies. However, it is not improbable for the expected number to be zero due to variable excision frequencies of P elements inserted at different genomic locations.
Although the deletions obtained by Cooley et al. (1990) were produced at the higher rate of ∼1%, placing the two P elements in cis can be a challenge, especially if one wishes to produce small deletions. For this reason, we favor the approach described here. Ongoing comprehensive efforts by the Bloomington Drosophila Stock Center have shown that this approach is generally applicable for producing genetic deletions using combinations of different P-element constructs (Parks et al. 2004). At the cytological level, the resulting deletions appear to be limited to the region between the insertion sites, are much larger than those produced here (indicating that proximity is not a limiting factor), and have been recovered at frequencies similar to that reported here (K. Cook, personal communication); in a few cases, the molecular limits of the resulting deletions have been broadly confirmed (Stathopoulos et al. 2004; Takaesu et al. 2006). This method has also been successfully used by others to produce deletions that include the MICAL locus (Terman et al. 2002). Although the generation of genetic deletions using P elements in trans is included in the arsenal of tools used in Drosophila research (reviewed in Venken and Bellen 2005), to our knowledge this study is the first to molecularly characterize the lesions present in a number of the resulting recombinant chromosomes.
Our findings invite a comparison of the use of standard P elements vs. FRT-containing mobile elements for making deletions with defined end points (cf. Parks et al. 2004; Ryder et al. 2004, 2007). The number of FRT-containing inserts can be accurately estimated to be in the vicinity of 20,259 distinct insertions (supplemental Table 3; K. Cook, personal communication). By contrast, the number of “conventional” P-element insertions is much more difficult to estimate, as it is made up of a number of separate collections of unknown overlap, which in many cases contain stocks for which the insertion site has not been molecularly mapped. If we consider only the highly curated set of insertions that have been mapped by flanking sequence and are held at the Bloomington Drosophila Stock Center (cf. Bellen et al. 2004), the number of distinct inserts is 8346 (supplemental Table 3; R. Levis, personal communication; note that FRT-containing XP elements were not included in the census for P-element insertions). Nevertheless, the true total number of inserts in public collections could be as high as three times this number. In addition, deletions using FRT-containing elements can be produced only when the two FRT sites are in the same orientation, potentially reducing the number of usable inserts to one-half of the total number flanking a region of interest, whereas conventional P's can be used in trans for making genetic deletions in either orientation (Parks et al. 2004). Finally, the insertional preference of FRT-containing piggyBac elements (Thibault et al. 2004) is different from that of P's (Spradling et al. 1999), making the potential utility of the FRT- vs. non-FRT-containing insert collections difficult to evaluate for a given target region. Thus, we recommend that both collections be considered for creating a custom genetic deletion, as it is very likely that the desired deletion can best be induced using two conventional P-element insertions.
Scheme for producing deletions using P elements in trans:
Our analyses suggest a straightforward scheme for identifying recombinants bearing a desired deletion. If essential genes exist in the interval between the two P elements, the recombinant chromosomes could first be screened for homozygous lethality. If either P element is located immediately next to a vital gene, however, false positives may be produced by a “simple” P-induced male recombination event in which the recombinant chromosome is essentially intact except for the deletion of DNA immediately flanking the P element (Preston et al. 1996). Therefore, it is advisable to carry out a complementation test against a gene located well within the region to be deleted. In our study, CCAP-IR served a similar purpose to a complementation assay, as the CCAP gene lies ∼15 kb distal to the L element and 25 kb proximal to the B element. This assay was sufficient to confirm the utility of 28 of the 31 homozygous lethal lines, of the 43 recombinant lines generated.
The next step depends on whether one wishes the deletion to still carry one (or both) of the markers associated with the P element (mini-white for this study). Retaining such a marker (or markers) might be desirable for some applications, such as uses in which the marker would serve to follow the segregation of this chromosome, etc. If this were desirable, the best prescription would be to determine which of the lines that still carry this eye color marker fail to amplify PCR products from genomic DNA located between the two P elements (e.g., products 4 and 5 in Figure 1 and Table 1) but produce normal amplification products immediately outside of the P elements (e.g., products 1 and 8 in Figure 1 and Table 1). These tests would select for deletions that lack the DNA between the P elements but retain sequences outside of this region. Here, it would have produced 7 candidates from the 31 homozygous lethal lines (line nos. 15, 25, 24, 25, 32, 34, and 36; see Table 1). Further tests would then be done to ensure that the intervening DNA has indeed been deleted (e.g., PCR, Southern analyses, and DNA sequencing).
If, on the other hand, retaining the P-element marker were immaterial, the simplest test would be to carry out a PCR using a primer proximal to the proximal insert in combination with a primer distal to the distal insert (e.g., primers 2F + 7R, Figure 1). Obtaining a product would imply that most of the DNA between the two primers has been deleted. Note that this test can be done on flies heterozygous for a wild-type chromosome (e.g., over a balancer chromosome), as the DNA from the latter chromosome will amplify a product of much larger size (if any were produced; in our case it was not because the amplification procedure could not amplify a 40-kb product). Deletions that produce a small amplification product are likely to be lacking the markers of both P elements (in our study, 3/3 of the lines containing small amplification products were white eyed), so prescreening for the absence of these markers would reduce the list of candidates to be tested.
The key feature of the screen used here is that it was based on the identification of P-element-induced recombinants. If, by contrast, the screen were based on the loss of the markers present on the P elements, the majority of the candidates would be simple excisions (precise or imprecise) of one or both of the P elements. Indeed, single excisions occur at a frequency of ∼10–15% (e.g., Engels et al. 1990), which is a frequency 10–100 times higher than that of male recombination events involving either a single P element (1%; Preston et al. 1996) or two P elements in trans (0.1–0.5%; this study). Even in our case, screening among the recombinants for the loss of both markers would not be recommended, unless deletions lacking these markers were desired (see above). Although such a criterion would have enriched for deletions, many potential deletions would have been discarded. Indeed, 78% (14/18) of recombinants that had lost both markers were deletions, but only 45% (14/31) of the deletions had lost both markers.
The deletions produced here removed the gene encoding the neuropeptide crustacean cardioactive peptide. On the basis of results obtained in the moth, Manduca sexta, this neuropeptide was believed to be critical for turning on ecdysis, the behavior used by insects to shed their old cuticle at the end of the molt (Ewer and Reynolds 2002). However, we have produced Drosophila bearing targeted ablations of these neurons and found that they express essentially normal ecdysis in the larva but fail at pupal ecdysis (Park et al. 2003). Larvae homozygous for the deletions produced here did not die until the end of the third instar, consistent with our finding that CCAP is not essential for turning on the behavior in the larva. Since these deletions removed 14 predicted genes in addition to that encoding CCAP, the exact function of CCAP will necessitate the production of smaller deletions or of point mutations. The deletions produced here will be of great utility for aiding in the isolation of mutations in the ∼1-kb gene that encodes the nine-amino-acid CCAP neuropeptide. For example, the relatively small number of genes included in the deletions generated here means that a heavily mutagenized chromosome can realistically be tested using complementation tests for hits within this interval. Mutations within the resulting complementation groups can then be tested for mutations within CCAP using CCAP immunohistochemistry.
Acknowledgments
We are very grateful to Kevin Cook for his support and encouragement during this project and for his comments on the manuscript. We thank Kevin Cook and Robert Levis for providing unpublished information used for supplemental Table 3. We thank Carlos Flores for additional comments on the manuscript. We appreciate the help from Tashana Williams, Gunisha Singh, and Steven Baker with PCR, fly scoring, and CCAP staining and Hans-Jürgen Agricola (University of Jena, Germany) for the gift of anti-CCAP antibody. We acknowledge the Bloomington Drosophila Stock Center for stocks and the Berkeley Drosophila Genome Project for genomic information and resources. This work was supported by grants to J.E. from the U.S. Department of Agriculture and the National Science Foundation and by Fondo Nacional de Desarrollo Científico y Tecnológico grant 1071079.
References
- Ballinger, D. G., and S. Benzer, 1989. Targeted gene mutations in Drosophila. Proc. Natl. Acad. Sci. USA 86 9402–9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall, E. L., and D. C. Rio, 1997. Drosophila P-element transposase is a novel site-specific endonuclease. Genes Dev. 11 2137–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen, H. J., R. W. Levis, G. Liao, Y. He, J. W. Carlson et al., 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167 761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley, L., R. Kelley and A. Spradling, 1988. Insertional mutagenesis of the Drosophila genome with single P elements. Science 239 1121–1128. [DOI] [PubMed] [Google Scholar]
- Cooley, L., D. Thompson and A. C. Spradling, 1990. Constructing deletions with defined endpoints in Drosophila. Proc. Natl. Acad. Sci. USA 87 3170–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak, P., M. M. Omar, R. D. Saunders, M. Pal, O. Komonyi et al., 1997. P-element insertion alleles of essential genes on the third chromosome of Drosophila melanogaster: correlation of physical and cytogenetic maps in chromosomal region 86E–87F. Genetics 147 1697–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels, W. R., D. M. Johnson-Schlitz, W. B. Eggleston and J. Sved, 1990. High-frequency P element loss in Drosophila is homolog dependent. Cell 62 515–525. [DOI] [PubMed] [Google Scholar]
- Ewer, J., and S. Reynolds, 2002. Neuropeptide control of molting in insects, pp. 1–92 in Hormones, Brain and Behavior, edited by D. W. Pfaff, A. P. Arnold, S. E. Fahrbach, A. M. Etgen and R. T. Rubin. Academic Press, San Diego.
- FlyBase, 2000. A database of the Drosophila genome. (http://flybase.bio.indiana.edu/).
- Gong, W. J., and K. G. Golic, 2003. Ends-out, or replacement, gene targeting in Drosophila. Proc. Natl. Acad. Sci. USA 100 2556–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granderath, S., A. Stollewerk, S. Greig, C. S. Goodman, C. J. O'Kane et al., 1999. loco encodes an RGS protein required for Drosophila glial differentiation. Development 126 1781–1791. [DOI] [PubMed] [Google Scholar]
- Grigliatti, T. A., 1998. Transposons—gene tagging and mutagenesis, pp. 85–107 in Drosophila: A Practical Approach, edited by D. B. Roberts. IRL Press/Oxford University Press, New York.
- Huet, F., J. T. Lu, K. V. Myrick, L. R. Baugh, M. A. Crosby et al., 2002. A deletion-generator compound element allows deletion saturation analysis for genomewide phenotypic annotation. Proc. Natl. Acad. Sci. USA 99 9948–9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, K., and S. F. Goodwin, 1990. “Site-selected” transposon mutagenesis of Drosophila. Proc. Natl. Acad. Sci. USA 87 1686–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacsovich, T., Z. Asztalos, W. Awano, K. Baba, S. Kondo et al., 2001. Dual-tagging gene trap of novel genes in Drosophila melanogaster. Genetics 157 727–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J., A. J. Schroeder, C. Helfrich-Förster, F. R. Jackson and J. Ewer, 2003. Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of behavior. Development 130 2645–2656. [DOI] [PubMed] [Google Scholar]
- Parks, A. L., K. R. Cook, M. Belvin, N. A. Dompe, R. Fawcett et al., 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36 288–292. [DOI] [PubMed] [Google Scholar]
- Preston, C. R., and W. R. Engels, 1996. P-element-induced male recombination and gene conversion in Drosophila. Genetics 144 1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston, C. R., J. A. Sved and W. R. Engels, 1996. Flanking duplications and deletions associated with P-induced male recombination in Drosophila. Genetics 144 1623–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart, J. M., and D. Ferrandon, 1998. Green balancers. Dros. Inf. Serv. 81 201–202. [Google Scholar]
- Rong, Y. S., 2002. Gene targeting by homologous recombination: a powerful addition to the genetic arsenal for Drosophila geneticists. Biochem. Biophys. Res. Commun. 297 1–5. [DOI] [PubMed] [Google Scholar]
- Rørth, P., 1996. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc. Natl. Acad. Sci. USA 93 12418–12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder, E., F. Blows, M. Ashburner, R. Bautista-Llacer, D. Coulson et al., 2004. The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics. 167 797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder, E., M. Ashburner, R. Bautista-Llacer, J. Drummond, J. Webster et al., 2007. The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics. 177 615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz, H. K., T. W. Cline and P. Schedl, 1987. Functional changes associated with structural alterations induced by mobilization of a P element inserted in the Sex-lethal gene of Drosophila. Genetics 117 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., and D. W. Russell, 2001. Molecular Cloning: A Laboratory Manual, Ed. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Spradling, A. C., D. Stern, A. Beaton, E. J. Rhem, T. Laverty et al., 1999. The Berkeley Drosophila Genome Project gene disruption project: single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153 135–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos, A., B. Tam, M. Ronshaugen, M. Frasch and M. Levine, 2004. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 18(6): 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaesu, N. T., C. Hyman-Walsh, Y. Ye, R. G. Wisotzkey, M. J. Stinchfield et al., 2006. dSno facilitates baboon signaling in the Drosophila brain by switching the affinity of Medea away from Mad and toward dSmad2. Genetics. 174 1299–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman, J. R., T. Mao, R. J. Pasterkamp, H. H. Yu and A. L. Kolodkin, 2002. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 109 887–900. [DOI] [PubMed] [Google Scholar]
- Thibault, S. T., M. A. Singer, W. Y. Miyazaki, B. Milash, N. A. Dompe et al., 2004. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 36 283–287. [DOI] [PubMed] [Google Scholar]
- Tsubota, S., and P. Schedl, 1986. Hybrid dysgenesis-induced revertants of insertions at the 5′ end of the rudimentary gene in Drosophila melanogaster: transposon-induced control mutations. Genetics 114 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken, K. J., and H. J. Bellen, 2005. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat. Rev. Genet. 6 167–178. [DOI] [PubMed] [Google Scholar]
- Xu, Y., M. Condell, H. Plesken, I. Edelman-Novemsky, J. Ma et al., 2006. A Drosophila model for Barth syndrome. Proc. Natl. Acad. Sci. USA 103 11584–11588. [DOI] [PMC free article] [PubMed] [Google Scholar]