Abstract
Background
The changes in the liver in nonalcoholic fatty liver disease (NAFLD) range over a wide spectrum, extending from steatosis to steatohepatitis (NASH). However it has remained difficult to differentiate between NASH and non-progressive NAFLD on the basis of the clinical findings alone.
Aims
In this study we investigated the clinical usefulness of plasma Pentraxin3 (PTX3) levels to predict NASH. Plasma PTX3 was measured in 70 patients with histologically verified NAFLD (28 with non-NASH and 42 with NASH) and 10 healthy control subjects.
Results
The plasma PTX3 level was significantly higher in the NASH cases than in the non-NASH cases (p = 0.0021) and control subjects (p = 0.045). And the plasma PTX3 level was significantly higher in the stages 3–4 NAFLD cases than in the stages 0–2 NAFLD cases (p < 0.0001). The PTX3 values were closely correlated with the stages of liver fibrosis (p < 0.0001, Kruskal-Wallis test). To detect NASH compared with non-NASH, the area under the curve for plasma PTX3 were 0.755, and to detect stages 3–4 NAFLD compared with stages 0–2 NAFLD, the area under the curve for plasma PTX3 were 0.850.
Conclusion
This is the first study to demonstrate consistent and profound elevation of plasma PTX3 levels in NASH in comparison with non-NASH. The results suggest that plasma PTX3 levels may not only be laboratory values that differentiate NASH from non-NASH, but marker of the severity of hepatic fibrosis in NASH.
Background
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver injury in many countries around the world [1,2]. It represents a spectrum of conditions that are histologically characterized by macrovesicular hepatic steatosis, and the diagnosis is made in patients who have not consumed alcohol in amounts sufficient to be considered harmful to the liver. The histological changes range over a wide spectrum, extending from simple steatosis, which is generally non-progressive, to nonalcoholic steatohepatitis (NASH), liver cirrhosis, liver failure, and sometimes even hepatocellular carcinoma [3,4]. Liver biopsy is recommended as the gold standard for both the diagnosis and staging of fibrosis in NASH patients [1,4,5], but it is invasive [6] and avoidance of liver biopsy would be desirable. Several clinical studies have attempted to identify serum markers that correlate with the severity of liver fibrosis in NASH patients. Many clinical variables have been proposed as predictors of severe fibrosis in NAFLD patients, including old age, underlying type 2 diabetes mellitus, obesity, serum transaminase level, platelet count, etc [7-9].
We have previously reported that measurement of the serum high-sensitivity C-reactive protein (CRP) level is clinically useful for the diagnosis of NASH [10]. CRP and serum amyloid P component (SAP) are well known members of the pentraxin family of proteins, which is subdivided into two subclasses according the length and structure of the molecules: long and short. The classical short PTXs, CRP and serum amyloid P, are acute-phase proteins in humans [11], that are produced in the liver in response to inflammatory mediators, most prominently IL-6. Long PTXs are characterized by an unrelated N-terminal domain coupled to a PTX-like C-terminal domain [12,13]. The prototypic long PTX3 is rapidly produced in response to Toll-like receptor engagement, tumor necrosis factor-α, and IL-1β and released by diverse cell types, including monocytes/macrophages, endothelial cells, vascular smooth muscle cells, fibroblasts, and adipocytes [14-19]. Plasma PTX3 levels have recently been found to be elevated in patients with vasculitis [20], acute myocardial infarction [21,22], and systemic inflammation or sepsis [23], however, there is no information about changes in PTX3 levels in NAFLD or NASH patients. We hypothesized that plasma PTX3 levels increase in patients with NASH, and investigated the clinical usefulness for the diagnosis and staging of liver fibrosis in NASH patients.
Methods
Patients
70 Japanese NAFLD patients (42 NASH and 28 non-NASH) and 10 healthy control subjects were recruited. All control subjects were confirmed to have normal liver function and no viral hepatitis infection or alcoholics. All of the 70 NAFLD patients were performed liver biopsy. The study was conducted with the approval of the Ethics Committee of Yokohama City University.
A detailed history was obtained and a physical examination performed on all the 70 NAFLD patients. The histological criteria for the diagnosis of NAFLD are the presence of macrovesicular fatty change in hepatocytes with displacement of the nucleus to the edge of the cell [24]. The criteria for exclusion from participation in the study: history of hepatic disease, such as chronic hepatitis C or concurrent active hepatitis B (serum positive for hepatitis B surface antigen), autoimmune hepatitis, primary biliary cirrhosis (PBC), sclerosing cholangitis, hemochromatosis, α1-antitrypsin deficiency, Wilson's disease, and current or past consumption of more than 20 g of alcohol daily. No patients had taken medicine which cause fatty liver, such as amiodarone, diltiazem, tamoxifen, steroids [24]. None of the patients had any clinical evidence of hepatic decompensation, such as hepatic encephalopathy, ascites, variceal bleeding, or elevated serum bilirubin level to more than twofold the upper limit of normal. Patients with heart failure or autoimmune rheumatic disease, which have been suggested to increase plasma PTX3 levels [25,26], and patients with a history of treatment with statins, which reduce plasma PTX3 levels [27], were also excluded.
Clinical and laboratory evaluation
Body weight and height were measured with a calibrated scale after requesting the patients to remove their shoes and any heavy clothing. Whole blood from 70 NAFLD patients and 10 healthy control subjects was immediately collected into a tube containing ethylene diaminetetraacetate (EDTA), and after centrifugation at 1500 × g for 15 min at room temperature, the plasma was frozen, and stored at -80°C until analyzed. Serum samples were obtained from 70 NAFLD patients after an overnight fast (12 hours) to measure AST, ALT, GGT, albmin, glucose, insulin, type IV collagen 7s domain, and hyaluronic acid levels. The serum insulin levels were measured by radioimmunoassay, and the other values were measured in a conventional automated analyzer.
Insulin resistance was calculated by the modified homeostasis model assessment of insulin resistance (HOMA-IR), using the following formula: HOMA-IR = fasting insulin (μU/ml) × plasma glucose (mg/dl)/405. HOMA-IR was originally reported by Matthews, and it has since been modified [28]. This index has been shown to be closely correlated with the results of the euglycemic-hyperinsulinemic clamp method to determine insulin resistance in type 2 DM patients.
Pentraxin3 measurements
Plasma PTX3 levels were measured with a sandwich ELISA based on a previously described method [26]. Briefly, the assay plate was coated with the F(ab')2 fragment of a monoclonal anti-human PTX3 antibody, PPMX0104 (Perseus Proteomics Inc., Tokyo, Japan) as the capture antibody and the F(ab')2 fragment of a monoclonal ant-human PTX3 antibody, PPMX0105 (Perseus Proteomics Inc., Tokyo, Japan) was conjugated with horseradish peroxidase as the detection antibody. Sample dilution (100 μl) was added to the wells. Each calibrator or plasma samples (10 μl) were added to the wells and incubated for 1 hour with shaking. The wells were aspirated and washed five times with washing buffer. PPMX0105-enzyme conjugate (100 μl) was added to the wells and incubated for 1 hour with shaking. The wells were aspirated and washed again, and substrate solution was added to each well. After 30 min of incubation, stop solution of the kit (100 μl) was added. The absorbance at 450 nm was measured with a microplate reader system. This assay system measured plasma PTX3 concentration linearly between 0.1 and 20 ng/ml.
Determination of the visceral and subcutaneous fat areas
The abdominal fat distribution of the subjects was determined by computed tomography (CT), conducted with the subjects in the supine position in accordance with a previously described procedure [29]. The subcutaneous fat area (SFA) and intra-abdominal visceral fat area (VFA) were measured at the level of the umbilicus in terms of the CT number, by a standardized method. In brief, a region of interest was defined in the subcutaneous fat layer by tracing its contour on each scan, and the attenuation range for fat tissue was measured in terms of the CT number (in Hounsfield units).
Pathology
The biopsy specimens of liver tissue were stained with hematoxylin-eosin, reticulin, and Masson trichrome stain, and all biopsy specimens were analyzed by one experienced pathologist blinded to the results of clinical data. Macrovesicular steatosis affecting at least 5% of the hepatocytes was observed in every case, and the cases were classified as having steatosis or steatohepatitis. In addition to steatosis, the minimal criteria for the diagnosis of steatohepatitis include the presence of lobular inflammation and either ballooning of cells or perisinusoidal/pericellular fibrosis in zone 3 of the hepatic acini [30-32]. Subjects with cirrhosis were defined as cases of NASH-associated cirrhosis according to a previously proposed clinicopathological classification [32]. The severity of fibrosis in every case was scored according to the method of Brunt [33]. The degree of steatosis was graded as follows based on the percentage of hepatocytes containing macrovesicular fat droplets: grade 0, no steatosis; grade 1, < 33% hepatocytes contain macrovesicular fat droplets; grade 2, 33% – 66% of the hepatocytes contain macrovesicular fat droplets; grade 3, > 66% of the hepatocytes contain macrovesicular fat droplets. The stages of fibrosis were expressed on the following 4-point scale, as follows: stage 0, none; stage 1, perivenular and/or perisinusoidal fibrosis in zone 3; stage 2, combined pericellular portal fibrosis; stage 3, septal/bridging fibrosis; stage 4, cirrhosis. Based on this classification, the subjects were grouped into 2 groups, a group with stages 0–2 NAFLD and a group with stages 3–4 NAFLD.
Statistical analysis
Data were expressed as means ± SD, unless indicated otherwise. The statistical analysis was performed with SPSS 12.0 software (SPSS, Inc., Chicago, IL, USA). The t-test or Wilcoxon rank sum test, as appropriate, was used for univariate comparisons between patient groups. Because many of the variables were not normally distributed, the Kruskal-Wallis test was used for comparisons of more than two independent groups. The diagnostic performance of the plasma PTX3 level was assessed by analysis of receiver operating characteristic (ROC) curves. The ROC curve is a plot of sensitivity versus 1 – specificity for all possible cutoff values. The most commonly used index of accuracy is the area under the ROC curve (AUROC), with values close to 1.0 indicating high diagnostic accuracy. Calculations of correlation coefficients and linear regression analysis were used to test for associations between the variables. Multivariate analysis was performed by using a binary logistic regression analysis. P values < 0.05 were considered significant.
Results
Patients Characteristics
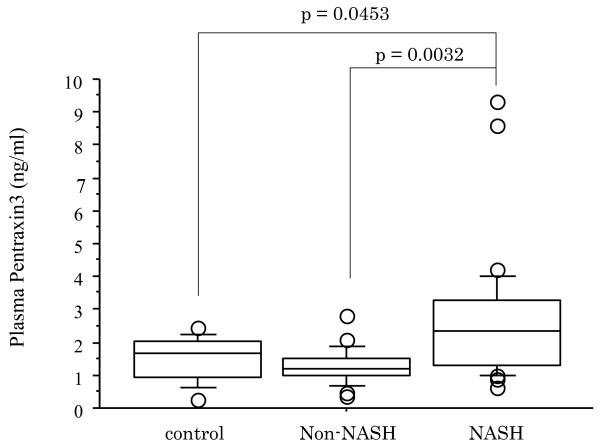
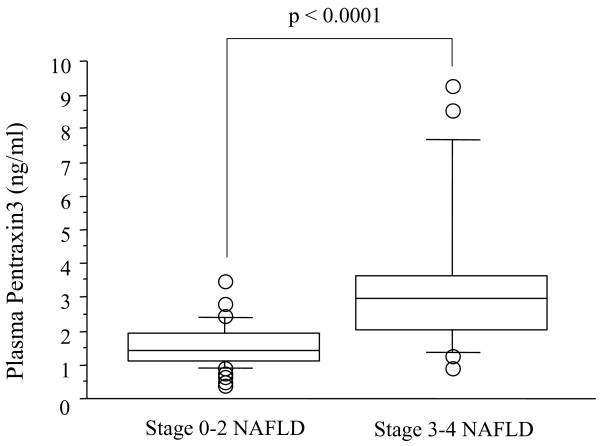
The histological findings in the liver biopsy specimens of the subjects with non-NASH (n = 28) and steatohepatitis (NASH) (n = 42) are shown in Table 1. The clinical and biochemical characteristics of the NASH patients and non-NASH patients are shown in Table 2. Marked elevation of the plasma PTX3 level was observed in the patients with NASH in comparison with the patients with non-NASH (p = 0.0021) and healthy control subjects (p = 0.0453). And there was no significance between non-NASH and control subjects in the plasma PTX3 level (p = 0.2892) (Fig. 1). Significant differences were also found in the serum AST level (p = 0.0031), ALT level (p = 0.0115), and hyaluronic acid level (p = 0.0373) between the NASH patients and those with non-NASH patients (Table 2). The clinical and biochemical characteristics of stages 0–2 NAFLD group and stages 3–4 NAFLD group are shown in Table 3. A significantly higher plasma PTX3 (p < 0.0001) was found in stages 3–4 NAFLD group in comparison with stages 0–2 NAFLD group (Fig. 2), and a markedly higher hyaluronic acid level (p = 0.0090) and type IV collagen 7s level (p = 0.0018) and markedly lower serum albumin level (p = 0.017) and platelet count (p = 0.0134) were observed between two groups (Table 3).
Table 1.
Histopathological Findings in the NASH patients and non-NASH patients
| (NASH) (n = 42) | Non-NASH (n = 28) | |
| Steatosis grade | ||
| 1 | 22 | 20 |
| 2 | 17 | 6 |
| 3 | 3 | 2 |
| Inflammatory activity | ||
| 0 | 4 | 11 |
| 1 | 27 | 17 |
| 2 | 9 | 0 |
| 3 | 2 | 0 |
| Fibrosis stage | ||
| 0 | 0 | 28 |
| 1 | 21 | 0 |
| 2 | 4 | 0 |
| 3 | 11 | 0 |
| 4 | 6 | 0 |
Table 2.
Clinical and Biochemical Characteristics of the non-NASH Patients and NASH Patients
| Non-NASH patients | NASH patients | P value | |
| Age (years) | 49.0 ± 15.3 | 52.8 ± 13.5 | 0.2995 |
| BMI (m/kg2) | 27.6 ± 5.1 | 28.9 ± 6.0 | 0.3989 |
| VFA (cm2) | 128.6 ± 55.7 | 133.3 ± 58.8 | 0.8031 |
| SFA (cm2) | 196.9 ± 61.4 | 232.5 ± 120.9 | 0.3765 |
| AST (U/ml) | 35.7 ± 17.5 | 63.4 ± 40.7 | 0.0031 |
| ALT (U/ml) | 56.8 ± 31.5 | 89.9 ± 55.7 | 0.0115 |
| FBS (mg/dl) | 118.7 ± 39.9 | 118.2 ± 30.8 | 0.9586 |
| IRI (ul/ml) | 13.6 ± 10.8 | 14.3 ± 8.2 | 0.7999 |
| HOMA-IR | 3.47 ± 2.64 | 4.05 ± 2.54 | 0.4725 |
| HDL cholesterol (mg/l) | 50.3 ± 12.6 | 49.3 ± 11.4 | 0.7463 |
| LDL cholesterol (mg/l) | 133.7 ± 27.0 | 128.6 ± 39.3 | 0.5928 |
| Triglyceride (mg/l) | 159.0 ± 48.9 | 162.8 ± 81.9 | 0.8502 |
| Albumin | 4.51 ± 0.28 | 4.45 ± 0.51 | 0.6175 |
| Platelet count | 25.8 ± 5.7 | 23.2 ± 8.0 | 0.1816 |
| Hyaluronic acid (ng/dl) | 21.6 ± 15.1 | 50.1 ± 50.2 | 0.0373 |
| Type IV collagen 7s (ng/dl) | 4.41 ± 0.98 | 5.14 ± 1.31 | 0.0611 |
Data are expressed as means ± SD. VFA: visceral fat area, SFA: subcutaneous fat area, AST: aspartate aminotransferase, ALT: alanine aminotransferase, FBS: fasting blood sugar, IRI: immunoreactive insulin, HOMA-IR: homeostasis model assessment of insulin resistance.
Figure 1.
Plasma PTX3 levels in healthy control subjects, non-NASH patients, and NASH patients. Box plots of plasma PTX3 levels showing interquartile range (box), median (thick line), range (thin lines) and outliers (circles). The length of the box represents the interquatile range within which 50% of the values were located. Plasma PTX3 levels of healthy control subjects, non-NASH and NASH patients.
Table 3.
Clinical and Biochemical Characteristics of Stages 0–2 and Stages 3–4 NAFLD
| Stages 0–2 NAFLD | Stages 3–4 NAFLD | P value | |
| Age (years) | 51.5 ± 13.4 | 52.8 ± 15.1 | 0.7564 |
| BMI (m/kg2) | 28.1 ± 4.9 | 30.2 ± 6.7 | 0.2133 |
| VFA (cm2) | 132.1 ± 63.7 | 135.7 ± 33.4 | 0.8716 |
| SFA (cm2) | 216.9 ± 95.3 | 256.8 ± 108.2 | 0.0626 |
| AST (U/ml) | 54.6 ± 41.0 | 58.1 ± 26.9 | 0.7526 |
| ALT (U/ml) | 80.3 ± 49.3 | 82.4 ± 59.5 | 0.8903 |
| FBS (mg/dl) | 122.9 ± 39.9 | 112.3 ± 17.8 | 0.3598 |
| IRI (ul/ml) | 13.0 ± 9.06 | 18.3 ± 9.10 | 0.0762 |
| HOMA-IR | 3.56 ± 2.59 | 5.16 ± 2.43 | 0.0677 |
| HDL cholesterol (mg/l) | 50.1 ± 12.5 | 47.8 ± 10.2 | 0.5277 |
| LDL cholesterol (mg/l) | 130.6 ± 33.3 | 129.8 ± 41.0 | 0.9430 |
| Albumin (mg/l) | 4.55 ± 0.27 | 4.23 ± 0.66 | 0.0170 |
| Platelet count (ng/ml) | 25.8 ± 6.6 | 20.0 ± 8.7 | 0.0134 |
| Hyaluronic acid (ng/dl) | 32.7 ± 27.8 | 72.4 ± 68.1 | 0.0090 |
| Type IV collagen 7s (ng/dl) | 4.65 ± 0.93 | 5.94 ± 1.53 | 0.0018 |
Data are expressed as means ± SD. VFA: visceral fat area, SFA: subcutaneous fat area, AST: aspartate aminotransferase, ALT: alanine aminotransferase, FBS: fasting blood sugar, IRI: immunoreactive insulin, HOMA-IR: homeostasis model assessment of insulin resistance.
Figure 2.
Plasma PTX3 levels in stages 0–2 and stages 3–4 NAFLD. Box plots of plasma PTX3 levels showing interquartile range (box), median (thick line), range (thin lines) and outliers (circles). The length of the box represents the interquatile range within which 50% of the values were located. Plasma PTX3 levels in stages 0-2 NAFLD and stages 3-4 NAFLD.
Hepatic fibrosis and plasma PTX3 levels
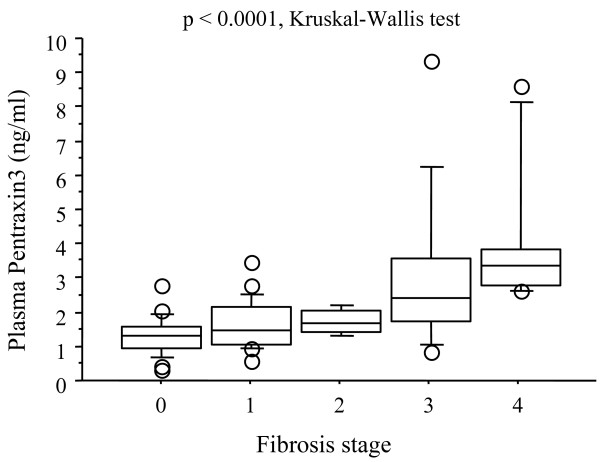
Since the plasma PTX3 level was significantly elevated in the NASH patients in comparison with the non-NASH patients, and in stages 3–4 NAFLD group in comparison with stages 0–2 NAFLD group, we investigated the relationship between stage of fibrosis and the plasma PTX3 levels in the NAFLD patients. Analysis of the plasma PTX3 levels of the NAFLD patients in relation to the histological stage of fibrosis revealed stepwise increases in the plasma PTX3 levels as the stages of hepatic fibrosis increased (p < 0.0001, Kruskal-Wallis test) (Fig. 3), and the differences between stage 0 and stage 3, stage 0 and stage 4, stage 1 and stage 3, stage 1 and stage 4, and stage 2 and stage 4 were significant (p = 0.0009, p < 0.0001, p = 0.0136, p = 0.0001, p = 0.0465, respectively).
Figure 3.
Relation between Plasma PTX3 levels and fibrosis stage. Comparison between plasma PTX3 levels in various stages of fibrosis. Box plots of plasma PTX3 levels showing interquartile range (box), median (thick line), range (thin lines) and outliers (circles). The length of the box represents the interquatile range within which 50% of the values were located. A steady stepwise increase in serum high-sensitivity CRP levels was observed with increasing severity of hepatic fibrosis (p = 0.0049).
Relation between plasma PTX3 and grade of hepatic steatosis or grade of necroinflammation
Analysis of the plasma PTX3 levels in relation to the histological grade of steatosis or grade of necroinflammation showed no relation between the plasma PTX3 levels and either of the two other parameters (p = 0.1554, p = 0.1745, respectively by Kruskal-Wallis test).
Receiver operating characteristic (ROC) curves for differentiating between NASH and non-NASH based on the plasma PTX3 level
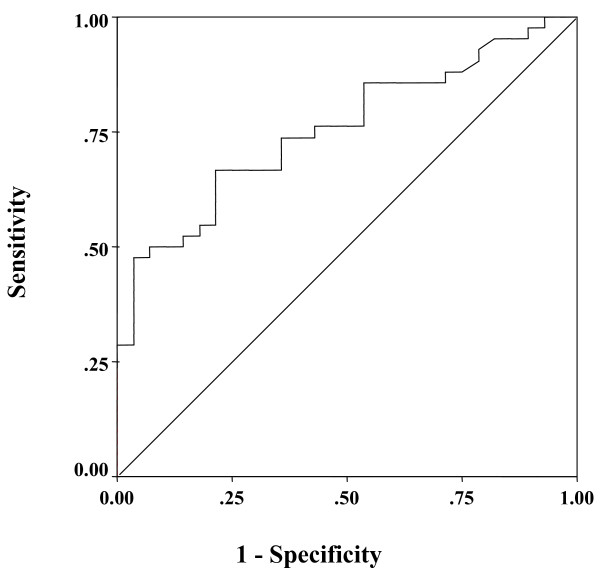
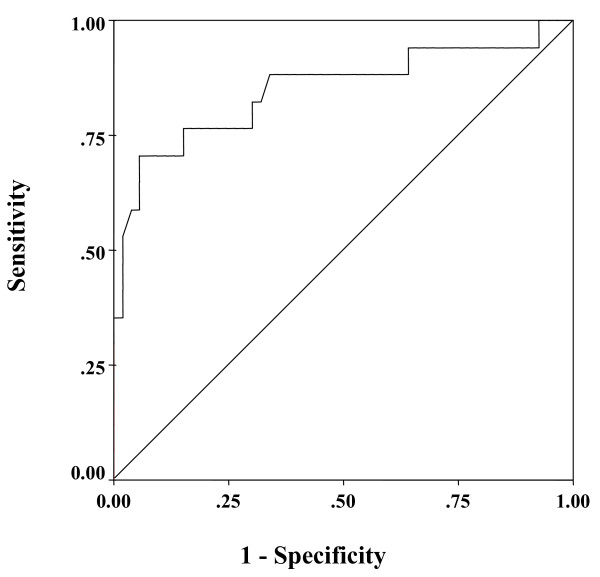
To detect NASH compared with non-NASH, the area under the curve for plasma PTX3 was 0.755 by ROC analysis (Fig. 4). The best cutoff value for diagnosis of NASH was also investigated by ROC analysis, and sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. The results showed that 1.61 ng/ml was the optimal plasma PTX3 cutoff value for diagnosis of NASH, and its sensitivity, specificity, PPV, and NPV were 66.7%, 78.6%, 82.4%, and 61.1%, respectively.
Figure 4.
ROC curve for differentiating steatosis and NASH according to the plasma PTX3 level. ROC curve for differentiating between steatosis and NASH on the basis of the plasma PTX3 level (area under the curve = 0.719).
ROC curves for differentiating between stages 0–2 NAFLD and stages 3–4 NAFLD based on the plasma PTX3 level
To detect stages 3–4 NAFLD compared with stages 0–2 NAFLD, the area under the curves for plasma PTX3 were 0.850 by ROC analysis (Fig. 5). The results of the analysis showed 2.45 ng/ml to be the optimal plasma PTX3 cutoff value for diagnosis of advanced NAFLD, and its sensitivity, specificity, PPV, and NPV were 70.6%, 94.3%, 80.8%, and 90.9%, respectively.
Figure 5.
ROC curve for differentiating between advanced NAFLD and non-advanced NAFLD on the basis of the plasma PTX3 level. ROC curve for differentiating between non-advanced NAFLD and advanced NAFLD on the basis of the plasma PTX3 level (area under the curve = 0.849).
Multiple regression analysis for demographic factors in the NASH patients
We performed the multiple logistic regression analysis by using the plasma PTX3 levels, AST, ALT, type IV collagen 7s domain, hyaluronic acid, which were significantly elevated factors in NASH patients than in non-NASH patients by the univariate analysis. Plasma PTX3 level was still significant in the NASH patients as compared with that in non-NASH patients by multiple logistic regression analysis (Table 4).
Table 4.
Multiple Logistic Regression Analysis of Factors Associated with NASH Compared to non-NASH.
| Factor | Odds ration | 95% CI | p Value |
| plasma PTX3 level (ng/ml) | 0.254 | 0.059 – 0.984 | 0.0488 |
| AST (U/ml) | 0.984 | 0.910 – 1.064 | 0.692 |
| ALT (U/ml) | 1.008 | 0.954 – 1.065 | 0.7699 |
| Type IV collagen 7s domain (ng/ml) | 1.128 | 0.407 – 3.124 | 0.8171 |
| hyaluronic acid (ng/ml) | 0.965 | 0.908 – 1.025 | 0.2499 |
Note: Intercept = -19.493, R2 for entire model = 0.310
Correlation between plasma PTX levels and serum CRP levels in the NAFLD patients
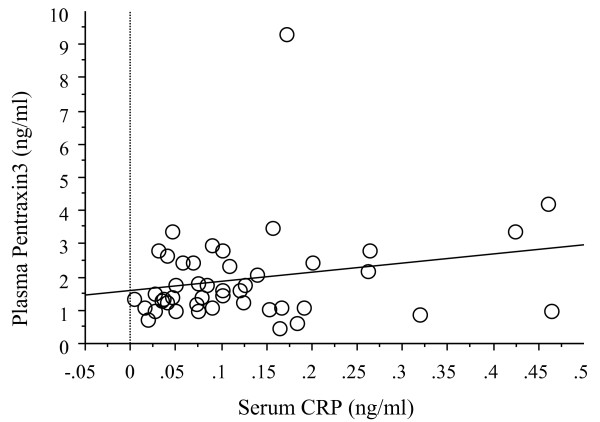
We compared the plasma PTX3 levels and serum CRP levels in 70 NAFLD patients. No correlation was found between plasma PTX3 levels and serum CRP levels (r = 0.220, p = 0.1431) (Fig. 6).
Figure 6.
Correlation between the plasma PTX3 levels and the serum CRP levels. No correlation was found between plasma PTX3 levels and serum CRP levels (r = 0.220, p = 0.1431)
Discussion
The results of this study demonstrated markedly higher plasma PTX3 levels in the NASH patients in a comparison with the non-NASH patients, and in stages 3–4 NAFLD as compared with stages 0–2 NAFLD.
A growing body of evidence has recently been collected that supports the notion that NAFLD is a feature of the metabolic syndrome. NAFLD sometimes progresses to cirrhosis, it is considered to be the most common form of chronic liver disease in obese patients. Because there are few effective treatments for NAFLD, the identification of important risk factors for disease progression should provide valuable information in regard to both risk stratification and development of risk-reduction strategies [34].
C-reactive protein (CRP) is one of the major acute-phase proteins and a marker of systemic inflammation. Some recent reports have suggested that hs-CRP may be an independent risk factor for progression of NAFLD [35-37], and we have reported the clinical usefulness of the measurement of hs-CRP for diagnosis of NASH [10]. PTX3 is a prototypic member of the long pentraxin family and a newly discovered marker of the acute phase inflammatory response. PTX3 is structurally related to, but distinct from classic members of the short pentraxin family, including from serum amyloid protein and CRP. The short pentraxins CRP and serum amyloid protein are reported to be produced by the liver as a systemic response to local inflammation, whereas expression of the long pentraxin PTX3 is rapidly induced in damaged tissue and may more directly reflect the tissue inflammatory response and its expression pattern is more tissue specific [21,38]. The gene coding PTX3 was originally described as an interleukin (IL)-1β-inducible gene in endothelial cells [14] and a tumor necrosis factor (TNF)-α inducible gene in fibroblasts [15]. PTX3 is produced by a variety of cell types, including monocytes/macrophages and endothelial cells in response to pro-inflammatory stimuli, such as TNF-α, IL-1β, and lipopolysaccharide (LPS) [39], all of which are reported to be essential factors in the pathogenesis and progression of NASH [40-42]. Patients with NAFLD are well known to have metabolic syndrome, and increasing number of metabolic syndrome risk factors is associated with increasing CRP [43,44]. Furthermore, metabolic syndrome in patients with NAFLD was shown to be associated with greater histologic severity [45]. However Inoue K et al. reported that PTX3 is an independent factor from metabolic risk factors, unlike CRP [46]. And previous report [21] and this study showed no correlation between plasma concentrations of PTX3 and CRP. Because of the similarities and differences between PTX3 and CRP, it is important to assess the usefulness of PTX3 as a novel diagnostic tool for NASH.
We therefore investigated the relationship between plasma PTX3 levels and several stages of NAFLD, including NASH. The results demonstrated that plasma PTX3 levels can be used to reliably differentiate NASH patients from non-NASH patients. A more detailed study revealed that plasma PTX3 levels can be used to differentiate between stages 3–4 NAFLD and stages 0–2 NAFLD, and that higher plasma PTX3 levels in NAFLD patients are associated with severe stages of hepatic fibrosis. Plasma PTX3 levels in healthy volunteers showed around 1.5 ng/ml, and plasma PTX3 levels in NASH patients were around 2.6 ng/ml. Furthermore in patients with stage 3–4 NAFLD patients was further elevated (around 3.8 ng/ml). Thus, we showed that the baseline plasma PTX3 level is not only a strong clinical marker of NASH, but also of the severity of liver fibrosis in NAFLD patients. PTX3 is reported to bind to apoptotic cells inhibiting their recognition by dendritic cells [47], and there is evidence for a regulatory role of PTX3 in noninfectious inflammatory reactions [48].
Measurement of plasma PTX3 levels is likely to be useful for targeted therapies for primary prevention of cardiovascular disease [49]. Similarly, plasma PTX3 may be also useful for targeted therapy against fibrosis in NASH patients, because in our study, the plasma PTX3 level was found to be more significantly elevated in the advanced stages of NAFLD patient.
Conclusion
This is the first study to demonstrate a consistent and profound elevation of plasma PTX3 levels in NASH patients whose diagnosis was based on results of liver biopsy, the gold standard for the diagnosis of NASH. Plasma PTX3 measurements may be noninvasive and appear to show promise as a means to differentiate NASH from non-NASH patients, and as a clinical marker of the severity of liver fibrosis in NASH patients.
Abbreviations
PTX3: pentraxin3; CRP: C-reactive protein; VFA: visceral fat area; BMI: body mass index; SFA: subcutaneous fat area; HOMA-IR: homeostasis model assessment for insulin resistance; IL: interleukin.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MY and TU performed the literature review, collected the clinical data, and drafted the manuscript, with contributions from MS, HA, and TK. SK and YN organized the field survey for data collection. HE, HM, HI, TK, KF, KY, HT, HK, MI, NK, KK and SS collected the clinical data. SM analyzed the liver pathology. AN was responsible for the design of the study. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
We thank Machiko Hiraga for her technical assistance. This work was supported in part by a Grant-in-Aid from the Ministry of Health, Labour and Welfare of Japan to A.N., a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KIBAN-B) to A.N., and a grant from the National Institute of Biomedical Innovation to A.N.
Contributor Information
Masato Yoneda, Email: yoneda@med.yokohama-cu.ac.jp.
Takashi Uchiyama, Email: maruru0203@yahoo.co.jp.
Shingo Kato, Email: shingo800m@hotmail.com.
Hiroki Endo, Email: t066011b@yokohama-cu.ac.jp.
Koji Fujita, Email: kfujita@yokohama-cu.ac.jp.
Kyoko Yoneda, Email: t066044g@yokohama-cu.ac.jp.
Hironori Mawatari, Email: mawatari-ykh@umin.ac.jp.
Hiroshi Iida, Email: hiro19780905@yahoo.co.jp.
Hirokazu Takahashi, Email: hirokazu@med.yokohama-cu.ac.jp.
Hiroyuki Kirikoshi, Email: hkirikos@med.yokohama-cu.ac.jp.
Masahiko Inamori, Email: inamorim@med.yokohama-cu.ac.jp.
Yuichi Nozaki, Email: nzaki-y@fukuhp.yokohama-cu.ac.jp.
Noritoshi Kobayashi, Email: norikoba@yokohama-cu.ac.jp.
Kensuke Kubota, Email: kubotak@yokohama-cu.ac.jp.
Satoru Saito, Email: ssai1423@yokohama-cu.ac.jp.
Shiro Maeyama, Email: shiromaeyama915@msg.biglobe.ne.jp.
Mina Sagara, Email: msagara@ppmx.com.
Hiroyuki Aburatani, Email: haburata-tky@umin.ac.jp.
Tatsuhiko Kodama, Email: kodama@lsbm.org.
Atsushi Nakajima, Email: nakajima-tky@umin.ac.jp.
References
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;18:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Farrell GC. Non-alcoholic steatohepatitis: what is it, and why is it important in the Asia-Pacific region? J Gastroenterol Hepatol. 2003;18:124–38. doi: 10.1046/j.1440-1746.2003.02989.x. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95:1056–62. [PubMed] [Google Scholar]
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- Cadranel JF. Good clinical practice guidelines for fine needle aspiration biopsy of the liver: past, present and future. Gastroenterol Clin Biol. 2002;26:823–824. [PubMed] [Google Scholar]
- Sakugawa H, Nakayoshi T, Kobashigawa K, Yamashiro T, Maeshiro T, Miyagi S, Shiroma J, Toyama A, Nakayoshi T, Kinjo F, Saito A. Clinical usefulness of biochemical markers of liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroenterol. 2005;11:255–9. doi: 10.3748/wjg.v11.i2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–23. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hashimoto E, Kaneda H, Noguchi S, Hayashi N. Nonalcoholic steatohepatitis: risk factors for liver fibrosis. Hepatol Res. 2002;24:429–438. doi: 10.1016/s1386-6346(02)00246-2. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, Abe Y, Kubota K, Saito S, Iwasaki T, Terauchi Y, Togo S, Maeyama S, Nakajima A. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42:573–82. doi: 10.1007/s00535-007-2060-x. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–66. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- Doni A, Michela M, Bottazzi B, Peri G, Valentino S, Polentarutti N, Garlanda C, Mantovani A. Regulation of PTX3, a key component of humoral innate immunity in human dendritic cells: stimulation by IL-10 and inhibition by IFN-gamma. J Leukoc Biol. 2006;79:797–802. doi: 10.1189/jlb.0905493. [DOI] [PubMed] [Google Scholar]
- Breviario F, d'Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–7. [PubMed] [Google Scholar]
- Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993;150:1804–12. [PubMed] [Google Scholar]
- Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, Peri G, Breviario F, Salmona M, De Gregorio L, Dragani TA, Srinivasan N, Blundell TL, Hamilton TA, Mantovani A. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–72. [PubMed] [Google Scholar]
- Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–93. [PubMed] [Google Scholar]
- Abderrahim-Ferkoune A, Bezy O, Chiellini C, Maffei M, Grimaldi P, Bonino F, Moustaid-Moussa N, Pasqualini F, Mantovani A, Ailhaud G, Amri EZ. Characterization of the long pentraxin PTX3 as a TNFalpha-induced secreted protein of adipose cells. J Lipid Res. 2003;44:994–1000. doi: 10.1194/jlr.M200382-JLR200. [DOI] [PubMed] [Google Scholar]
- Klouche M, Peri G, Knabbe C, Eckstein HH, Schmid FX, Schmitz G, Mantovani A. Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis. 2004;175:221–8. doi: 10.1016/j.atherosclerosis.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Fazzini F, Peri G, Doni A, Dell'Antonio G, Dal Cin E, Bozzolo E, D'Auria F, Praderio L, Ciboddo G, Sabbadini MG, Manfredi AA, Mantovani A, Querini PR. PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. 2001;44:2841–50. doi: 10.1002/1529-0131(200112)44:12<2841::aid-art472>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, Pizzetti F, Maggioni AP, Moccetti T, Metra M, Cas LD, Ghezzi P, Sipe JD, Re G, Olivetti G, Mantovani A, Latini R. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–41. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D, Tarli L, Schweiger C, Fresco C, Cecere R, Tognoni G, Mantovani A, Lipid Assessment Trial Italian Network (LATIN) Investigators Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–54. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- Muller B, Peri G, Doni A, Torri V, Landmann R, Bottazzi B, Mantovani A. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–7. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–25. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Takeishi Y, Niizeki T, Koyama Y, Kitahara T, Sasaki T, Sagara M, Kubota I. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am Heart J. 2008;155:75–81. doi: 10.1016/j.ahj.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Kravitz MS, Shoenfeld Y. Autoimmunity to protective molecules: is it the perpetuum mobile (vicious cycle) of autoimmune rheumatic disease? Nat Clin Pract Rheumatol. 2006;2:481–490. doi: 10.1038/ncprheum0290. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Takabe W, Mataki C, Wada Y, Izumi A, Saito Y, Hamakubo T, Kodama T. Global analysis of RNA expression profile in human vascular cells treated with statins. J Atheroscler Thromb. 2004;11:62–72. doi: 10.5551/jat.11.62. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, Arai T, Kotani K, Funahashi T, Yamashita S, Matsuzawa Y. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–9. [PubMed] [Google Scholar]
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- Hui JM, Kench JG, Chitturi S, Sud A, Farrell GC, Byth K, Hall P, Khan M, George J. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38:420–7. doi: 10.1053/jhep.2003.50320. [DOI] [PubMed] [Google Scholar]
- Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ. Treatment of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17:S385–S388. doi: 10.1046/j.1440-1746.17.s3.32.x. [DOI] [PubMed] [Google Scholar]
- Park SH, Kim BI, Yun JW, Kim JW, Park DI, Cho YK, Sung IK, Park CY, Sohn CI, Jeon WK, Kim H, Rhee EJ, Lee WY, Kim SW. Insulin resistance and C-reactive protein as independent risk factors for non-alcoholic fatty liver disease in non-obese Asian men. J Gastroenterol Hepatol. 2004;19:694–8. doi: 10.1111/j.1440-1746.2004.03362.x. [DOI] [PubMed] [Google Scholar]
- Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Keum DK, Kim BI. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138–43. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB, Jr, Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54:3140–7. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Garlanda C, Bottazzi B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine. 2003;21:S43–7. doi: 10.1016/s0264-410x(03)00199-3. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–66. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–63. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- Nozaki Y, Saibara T, Nemoto Y, Ono M, Akisawa N, Iwasaki S, Hayashi Y, Hiroi M, Enzan H, Onishi S. Polymorphisms of interleukin-1 beta and beta 3-adrenergic receptor in Japanese patients with nonalcoholic steatohepatitis. Alcohol Clin Exp Res. 2004;28:106S–110S. doi: 10.1111/j.1530-0277.2004.tb03226.x. [DOI] [PubMed] [Google Scholar]
- Kirsch R, Clarkson V, Verdonk RC, Marais AD, Shephard EG, Ryffel B, de la M, Hall P. Rodent nutritional model of steatohepatitis: effects of endotoxin (lipopolysaccharide) and tumor necrosis factor alpha deficiency. J Gastroenterol Hepatol. 2006;21:174–82. doi: 10.1111/j.1440-1746.2005.04220.x. [DOI] [PubMed] [Google Scholar]
- Patel DA, Srinivasan SR, Xu JH, Li S, Chen W, Berenson GS. Distribution and metabolic syndrome correlates of plasma C-reactive protein in biracial (black-white) younger adults: the Bogalusa Heart Study. Metabolism. 2006;55:699–705. doi: 10.1016/j.metabol.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- Kang H, Greenson JK, Omo JT, Chao C, Peterman D, Anderson L, Foess-Wood L, Sherbondy MA, Conjeevaram HS. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am J Gastroenterol. 2006;101:2247–53. doi: 10.1111/j.1572-0241.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- Inoue K, Sugiyama A, Reid PC, Ito Y, Miyauchi K, Mukai S, Sagara M, Miyamoto K, Satoh H, Kohno I, Kurata T, Ota H, Mantovani A, Hamakubo T, Daida H, Kodama T. Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arterioscler Thromb Vasc Biol. 2007;27:161–7. doi: 10.1161/01.ATV.0000252126.48375.d5. [DOI] [PubMed] [Google Scholar]
- Rovere P, Peri G, Fazzini F, Bottazzi B, Doni A, Bondanza A, Zimmermann VS, Garlanda C, Fascio U, Sabbadini MG, Rugarli C, Mantovani A, Manfredi AA. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000;96:4300–6. [PubMed] [Google Scholar]
- Souza DG, Soares AC, Pinho V, Torloni H, Reis LF, Teixeira MM, Dias AA. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol. 2002;160:1755–65. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotooka N, Inoue T, Fujimatsu D, Morooka T, Hashimoto S, Hikichi Y, Uchida T, Sugiyama A, Node K. Pentraxin3 is a novel marker for stent-induced inflammation and neointimal thickening. Atherosclerosis. 2008;197:368–74. doi: 10.1016/j.atherosclerosis.2007.05.031. [DOI] [PubMed] [Google Scholar]