Abstract
Phytoplasmas are unculturable, insect-transmissible plant pathogens belonging to the class Mollicutes. To be transmitted, the phytoplasmas replicate in the insect body and are delivered to the insect's salivary glands, from where they are injected into the recipient plant. Because phytoplasmas cannot be cultured, any attempt to recover phytoplasmal DNA from infected plants or insects has resulted in preparations with a large background of host DNA. Thus, studies of the phytoplasmal genome have been greatly hampered, and aside from the rRNA genes, only a few genes have hitherto been isolated and characterized. We developed a unique method to obtain host-free phytoplasmal genomic DNA from the insect vector's saliva, and we demonstrated the feasibility of this method by isolating and characterizing 78 new putative phytoplasmal open reading frames and their deduced proteins. Based on the newly accumulated information on phytoplasmal genes, preliminary characteristics of the phytoplasmal genome are discussed.
Phytoplasmas are a group of plant-pathogenic, phloem-restricted bacteria belonging to the class Mollicutes which represents the smallest self-replicating life forms on earth and are characterized by a lack of firm cell walls and remarkably small (580- to 1,350-kb), AT-rich genomes (40). Phytoplasmas are the causal agents of diseases in hundreds of plants (1, 22, 31) and are transmitted from plant to plant by grafting and other vegetative propagation techniques and by specific phloem-feeding insects, especially leafhoppers, planthoppers, and psyllids (31). Many diseases caused by phytoplasmas are of great economic importance, in particular those of trees, perennials, and high-value vegetable crops (1).
Since the phytoplasma is restricted to the phloem tissue, its concentration in infected plants is very low. Furthermore, in contrast to mycoplasmas, phytoplasmas cannot be cultured in vitro. Therefore, phytoplasmal DNA is obtained with a high host (plant or insect) background, hampering attempts to study structural features and genome organization. In the last 15 years, some progress has been made towards enabling the enrichment of phytoplasmal fractions from extracts of infected plants and insects (4, 20, 21, 42, 47, 48). In some cases, it became possible to produce phytoplasma-specific polyclonal and monoclonal antibodies, greatly facilitating diagnosis (6, 9). However, as detailed below, only a few phytoplasmal genes have been characterized. Currently, phytoplasma detection and characterization are based predominantly on PCR amplification of rRNA genes (rDNAs), especially the 16S rDNA (1, 11, 15, 19, 25, 48, 51).
The preferred methods for phytoplasma classification have become restriction fragment length polymorphism (RFLP) analysis and sequence analysis of rDNA. Sequence analysis of 16S rDNA revealed that phytoplasmas form a monophyletic clade within the mycoplasma phylogenetic tree. This method also allowed, for the first time, a comprehensive phytoplasma classification as the basis for an emerging taxonomy. Under the provisional status Candidatus, six species have been defined to date (16, 19, 26, 28, 49, 50, 55).
Extraction and purification of phytoplasmal DNA are critical and essential steps towards understanding the molecular biology of these unique microorganisms. Since the source of phytoplasmal genomic DNA is an infected plant or insect (and not cultured bacterial cells), resulting in a high background of host DNA, a preparation containing sufficient amount of phytoplasma DNA representing the entire phytoplasma genome was critically sought. At least two groups have made considerable, yet unpublished, progress in isolating and characterizing the phytoplasmal genome of the Western X phytoplasma (L. Leifting and B. Kirkpatrick, Abstr. 14th Int. Congr. Int. Org. Mycoplasmol., abstr. 29, p. 27, 2002) and the apple proliferation phytoplasma (E. Seemuller, personal communication). However, only a few phytoplasmal non-tRNA or non-rRNA genes have been hitherto described in published papers (3-5, 10, 16, 18-20, 27, 28, 30, 33, 34, 37, 46, 54, 57); additional, as-yet-unpublished genes can be found in the GenBank database.
Recently, for the purposes of demonstrating the vector's ability to transmit phytoplasma, we devised a method of collecting insect saliva and showed, by PCR analysis of rRNA genes, that phytoplasmal DNA can be easily detected in that saliva (53). Based on those findings, we describe a novel approach to isolating phytoplasmas and their genomic DNAs and validate it by isolating and characterizing 78 new putative phytoplasmal open reading frames (ORFs).
MATERIALS AND METHODS
Insects as a source of phytoplasmas.
Leafhoppers (Orosius albicinctus) were collected from and around phytoplasma-infested vineyards and were characterized as carrying the aster yellows (AY)-type phytoplasma by PCR analysis of 16S rDNA sequences (25, 51). Samples of phytoplasma-carrying and phytoplasma-free leafhoppers were kept frozen for future validation assays.
Isolation of phytoplasmal fractions from insect saliva.
We adapted a method of phytoplasma isolation from artificial feeding medium (J. Zhang, S. Miller, C. Hoy, X. Zhou, and L. Nault, Phytopathology 88, abstr. S84, 1998), described by Tanne et al. (53), whereby phytoplasmas are isolated from the insect vector's saliva and their genomic DNA is extracted. White microcentrifuge tubes (1.5 ml) were used as insect chambers. The white tube caps were removed and replaced with insect-attracting yellow ones. The cylindrical cups in the yellow caps were filled with 200 μl of 5% sucrose in TE (10 mM Tris [pH 8.0], 1 mM EDTA) and sealed with Parafilm. The microcentrifuge tubes were then capped with the yellow caps carrying the sucrose-TE feeding medium. The bottom ends of the microcentrifuge tubes were cut, an individual insect was placed in each, and the cut end was sealed with cotton wool. Each tube, containing an individual leafhopper, was kept at 23 to 25°C for 48 to 72 h in a horizontal position with the cap facing a light source to attract the insects to the feeding medium.
Isolation of phytoplasmal genomic DNA.
Phytoplasma particles were pelleted out of the feeding solution by centrifugation at 12,000 × g for 15 min. Genomic DNA was extracted by adding 10 μl of 0.5 M NaOH, followed by the addition of 20 μl of 1 M Tris-HCl (pH 8.0) containing 1% sodium dodecyl sulfate and 20 mM EDTA. The mixture was incubated at 65°C for 15 min, precipitated with 2 volumes of ethanol, redissolved in 30 μl of TE, and kept at −80°C.
Molecular typing of phytoplasmal DNA.
A sample of the isolated phytoplasmal DNA was subjected to PCR analysis with various type-specific 16S rDNA primers. The DNA reacted positively only with the AY-specific primers (data not shown). Phytoplasma typing results were corroborated by RFLP of the PCR amplicon with four distinct restriction enzymes (26, 36) (data not shown).
Construction of a phytoplasmal genomic library.
DNAs from 100 individual feeding tubes were pooled for the construction of a genomic library. The DNA was cleaved with the restriction enzyme EcoRI and ligated into the phagemid Lambda-Zap Express (Stratagene). Ligation and packaging were performed according to the manufacturer's protocol. About 5,000 plaques were obtained in this process, and blue-white selection indicated about 70% insert-carrying recombinant clones. Plasmids were excised from the phagemids by the addition of a helper phage as directed by the manufacturer. The recombinant plasmids were purified, and the nucleotide sequences of their inserts were determined by using the dideoxy chain termination method (44) with the automatic dye-terminator cycle sequencing model ABI PRISMA 377 sequencer (Perkin-Elmer, Foster City, Calif.). Sequence data were analyzed by using the BlastN, BlastX, and ORF finder programs from the National Center for Biotechnology Information and the FASTA program from the Genetics Computer Group.
Southern and Northern blot analyses.
Total genomic DNA was extracted from plants as described by Maixner et al. (29). Leaf tissue was ground in extraction buffer (100 mM Tris-HCl [pH 8.0], 3% cetyl trimethyl ammonium bromide, 1.4 M NaCl, 20 mM EDTA, 0.2% 2-mercaptoethanol) at a tissue/buffer ratio of 1:5 (wt/vol). The slurry was incubated for 20 min at 65°C and then centrifuged for 10 min at 3,000 × g. The supernatant fluid was collected and extracted with an equal volume of chloroform-isoamylalcohol (24:1, vol/vol), followed by centrifugation, collection of the aqueous phase, and precipitation with 1 volume of isopropanol. The DNA pellet was washed with 70% ethanol, resuspended in 50 μl of 10 mM Tris-HCl (pH 8.0)-1 mM EDTA. Total cell RNA was extracted from plant leaves with Tri reagent (Sigma) according to the manufacturer's protocol.
DNA was cleaved with the restriction enzyme HindIII, electrophoresed, and blotted onto nitrocellulose membranes. RNA was electrophoresed in formamide-formaldehyde gels and blotted onto membranes. Southern and Northern blot procedures were carried out by standard protocols (43).
Probes for the Southern and Northern blot hybridizations were prepared from selected clones with the Prime a Gene labeling kit (Promega). Due to the high A-T content of the phytoplasmal DNA, hybridization was carried out at 56°C, and the membranes were washed at medium stringency, i.e., at 56°C in 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate [pH 7.2]).
PCR.
Primers were designed from the nucleotide sequences of clones A10, A182, A244, and A380. The nucleotide sequences of the primers are shown in Table 1. Plant or leafhopper DNA (100 ng) served as a template. The reaction mixture was incubated at 95°C for 10 min and then subjected to 40 PCR cycles (denaturation at 92°C for 30 s, annealing for 30 s, and elongation and 72°C for 30 s), followed by 7 min of incubation at 72°C. The available amount of leafhopper DNA extracted from a single insect was scarce. To increase sensitivity and specificity, a nested PCR step was added to the analysis of DNA from several insects. For nested PCR, a sample from the primary PCR was diluted 1:100 and 2 μl of it served as a template for the nested PCR stage. PCR products were electrophoresed in 1.2% agarose gels and stained with ethidium bromide.
TABLE 1.
Primers used for PCR in this study.
| Clone | Primer sequence (5′ to 3′) | Position | Annealing temp (°C) | Product length (bp) |
|---|---|---|---|---|
| A380 | TCAACCCAATGTCGTATAATCG | 373-394 | 62 | 500 |
| AACGGAGTTGAACCGCTTAAGG | 873-852 | 62 | ||
| A182 | GGGGAATACTTTAATTTCTGCA | 169-190 | 60 | |
| TCCAACCGCTTCTAAAACTG | 775-756 | 60 | ||
| A182, nested | GGAACCTCTTAGGGAAGATAA | 269-290 | 58 | 416 |
| GTGCTCCACAATATTTGGCT | 695-676 | 58 | ||
| A10 | TACAGCTGGACCTTCTTCTT TA | 360-381 | 56 | |
| TGCACAAGGTGTAGCATATTC | 1245-1225 | 56 | ||
| A10, nested | CAGCACGTTGAGGAAATCTAA | 461-481 | 58 | 727 |
| GCCTATAACTACTCCCAAACAA | 1188-1167 | 58 | ||
| A244 | AGAAGATGCTGGACGTACAGAA | 136-157 | 54 | |
| CATTACCATAGCGACCTGAC | 1330-1311 | 54 | ||
| A244, nested | ATAAAAGCAGGGGCAACAGTAA | 191-212 | 56 | 1,034 |
| GTAATTCCAAGTTCAGGACCAA | 1224-1203 | 56 |
RESULTS
Characterization of phytoplasmal clones.
A collection of 32 recombinant phytoplasmal clones, carrying inserts of about 300 to 4,000 kb in size, were subjected to sequence analysis. The information obtained is summarized in Table 2. These clones carry 78 putative ORFs. All but one (the gene for the elongation factor Tu) of the presently reported ORFs are new and have not hitherto been reported for phytoplasmas. The full nucleotide sequences of the various clones have been submitted to the GenBank database, and their accession numbers are given in Table 2.
TABLE 2.
Summary of the characterized phytoplasmal (AY) clonesa
| Clone information | Blast results
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Clone | GenBank accession no. | % GC | ORF no. | Base position and orientationb | Homologous protein | Organism | % Identity | % Similarity |
| A1 | AY191277 | 54 | 1 | 89-988 + | Hypothetical proteinc | |||
| 2 | 153-1067 + | Hypothetical protein | ||||||
| 3 | 982-350 − | Hypothetical protein | ||||||
| A2 | AY191278 | 36 | 1 | 605-315 − | Conserved hypothetical protein | Helicobacter pylori 26695 | 26 | 47 |
| 2 | 648-818 + | Hypothetical protein | ||||||
| 3 | 1309-1127 − | Hypothetical protein | ||||||
| 4 | 1592-1443 − | gtf2ird2 | Mus musculus | 40 | 59 | |||
| 5 | 1640-1822 + | Hypothetical protein | Plasmodium falciparum 3D7 | 46 | 64 | |||
| 6 | 2248-1901 − | IpaD | Shigella sonnei | 31 | 58 | |||
| 7 | 2428-2261 − | Hypothetical protein | ||||||
| 8 | 2460-2636 + | Similar to hornerin | Mus musculus | 40 | 52 | |||
| 9 | 2850-3200 + | Putative dipeptidase protein | Sinorhizobium meliloti | 50 | 56 | |||
| 10 | 3172-2996 − | Hypothetical protein | ||||||
| 11 | 3107-3370 + | Hypothetical protein | ||||||
| 12 | 3497-3823 + | agCP3554 | Anopheles gambiae | 51 | 69 | |||
| A10 | AY191279 | 24 | 1 | 2-238 + | rRNA methylase | Thermoanaerobacter tengcongensis | 40 | 60 |
| 2 | 1502-240 − | Threonine dehydratase | Bacillus halodurans | 56 | 75 | |||
| A93 | AY191280 | 25 | 1 | 214-1 − | NADH dehydrogenase 1 | Mitochondrion of Flexarida chaotica | 79 | 91 |
| A115 | AY191281 | 23 | 1 | 268-1 − | Hypothetical protein | |||
| 2 | 69-350 + | EF-G | Porphyromonas gingivalis | 58 | 83 | |||
| 3 | 326-628 + | rpS10 | Leptospira interrogans | 59 | 79 | |||
| 4 | 908-661 − | Hypothetical protein | ||||||
| 5 | 665-909 + | rpL3 | Thermoanaerobacter tengcongensis | 59 | 78 | |||
| A167 | AY191282 | 38 | 1 | 725-1 − | DNA polymerase | Mitochondrion of Neurospora intermedia | 32 | 48 |
| 2 | 774-451 − | Putative DNA polymerase | Mitochondrion of Porphyra sp. | 30 | 55 | |||
| A182 | AY191283 | 26 | 1 | 56-400 + | rpL18 | Bacillus stremophilus | 45 | 57 |
| 2 | 413-934 + | rpS5 | Shewanella oneidensis MR-1 | 46 | 64 | |||
| 3 | 939-1105 + | rpL15 | Neisseria meningitidis MC58 | 76 | 85 | |||
| A226 | AY191284 | 29 | 1 | 230-1 − | Cytochrome c oxidase polypeptide III | Mitochondrion of Spodoptera frugiperda | 67 | 77 |
| A239 | AY191285 | 24 | 1 | 8-361 + | Acetohydroxy acid synthase II | Escherichia coli O157:H7 EDL933 | 35 | 48 |
| 2 | 349-609 + | Hypothetical protein | ||||||
| 3 | 600-1634 + | Ketol-acid reductoisomerase, mito- hondrial precursor | Neurospora crassa | 49 | 66 | |||
| A244 | AY191286 | 27 | 1 | 2-871 + | 2-Isopropylmalate synthase | Aquifex aeolicus | 53 | 71 |
| 2 | 872-1885 + | 3-Isopropylmalate dehydratase large subunit | Oceanobacillus iheyensis | 49 | 68 | |||
| A276 | AY191287 | 34 | 1 | 78-164 + | Surface protein, putative | Shewanella oneidensis MR-1 | 39 | 58 |
| A290 | AY191288 | 18 | 1 | 95-493 + | Signal peptidase 1 | Chlorobium tepidume TLS | 38 | 57 |
| 2 | 450-647 + | rpS21 | Myxococcus xanthus | 44 | 68 | |||
| 3 | 651-963 + | Shikimate 5-dehydrogenase | Clostridium perfringens | 36 | 63 | |||
| A293 | AY217338 | 33 | 1 | 617-378 − | Hypothetical protein | |||
| 2 | 629-883 + | Hypothetical protein | ||||||
| 3 | 1214-702 − | RNA polymerase I | Plasmodium falciparum 3D7 | 44 | 67 | |||
| A294 | AY191289 | 30 | 1 | 133-2 − | Putative transport protein | Escherichia coli | 39 | 51 |
| A295 | AY1912290 | 34 | 1 | 188-1 − | Hypothetical protein | Leptospira interrogans serovar Lai strain 56601 | 29 | 52 |
| 2 | 13-210 + | Putative N-acetyltransferase Camello 4 | Mus musculus | 31 | 54 | |||
| A314 | AY191291 | 25 | 1 | 5-981+ | NADH dehydrogenase subunit 5 | Mitochondrion of Pyrocoelia rufa | 51 | 66 |
| A315 | AY191292 | 48 | 1 | 128-2 − | Integrase protein | Salmonella enterica serovar Typhimurium | 54 | 62 |
| A328 | AY191293 | 30 | 1 | 106-1197 + | Hypothetical protein | |||
| A352 | AY217339 | 33 | 1 | 1314-1084 − | Caspase-9 long chain | Mouse | 50 | 62 |
| 2 | 1231-1551 + | Outer capsid protein | Porcine group C rotavirus | 40 | 65 | |||
| 3 | 1343-1510 + | Hypothetical protein | ||||||
| 4 | 1886-2071 + | Hypothetical protein | ||||||
| 5 | 2664-2825 + | Hypothetical protein | ||||||
| 3 | 508-359 − | Hypothetical protein | /PICK> | |||||
| A370 | AY217340 | 40 | 1 | 534-1 − | Reverse transcriptase | Picea glauca | 49 | 69 |
| 2 | 1250-375 − | Putative Gag-Pol polyprotein | Oryza sativa | 37 | 56 | |||
| 3 | 1639-1328 − | Putative copia-type polyprotein | Oryza sativa | 51 | 64 | |||
| A380 | AY191294 | 23 | 1 | 2-172 + | Cytochrome oxidase subunit III | Mitochondrion of Manis tetradactyla | 45 | 60 |
| 2 | 225-599 (+) | NADH dehydrogenase subunit 3 | Anopheles gambiae | 64 | 85 | |||
| 3 | 1302-709(−) | Cyclophilin D | Dictyostelium discoideum | 40 | 56 | |||
| A424 | AY191295 | 29 | 1 | 309-476 + | Hypothetical protein | |||
| 2 | 322-471 + | Hypothetical protein | ||||||
| A427 | AY191296 | 22 | 1 | 2-160 + | Threonyl-tRNA synthetase | Buchnera aphidicola strain sg | 92 | 94 |
| A440 | AY191297 | 29 | 1 | 4-429 + | Elongation factor Tu | Cellulophaga lytica | 76 | 88 |
| 2 | 652-891 + | rpL11 | Chlamydia muridarum | 63 | 86 | |||
| A448 | AY191298 | 22 | 1 | 194-1 − | rpS8 | Listeria innocuus | 62 | 81 |
| 2 | 200-723 + | rpL6 | Oceanobacillus iheyensis | 43 | 60 | |||
| A449 | AY191299 | 31 | 1 | 743-1012 + | Hypothetical protein | |||
| A450 | AY191300 | 39 | 1 | 175-2(−) | Conserved hypothetical protein | Agrobacterium tumefaciens | 33 | 53 |
| 2 | 215-18 − | Nef attachable protein | Homo sapiens | 38 | 58 | |||
| A458 | AY191301 | 45 | 1 | 222-1 − | Yjcl protein | Bacillus halodurans | 33 | 52 |
| A463 | AY191302 | 32 | 1 | 357-37 − | DNA-adenine methyltransferase | Actinobacillus actinomycetemcomitans | 28 | 46 |
| 2 | 269-436 + | Hypothetical protein | ||||||
| 3 | 542-282 − | Hypothetical protein | ||||||
| 4 | 530-781 + | Retroelement | Tricholoma matsutake | 27 | 44 | |||
| A526 | AY217341 | 29 | 1 | 129-28 − | Fusolin | Nucleopolyhedrovirus of Autographia californica | 48 | 68 |
| A533 | AY217342 | 34 | 1 | 655-825 + | Hypothetical protein | |||
| 2 | 855-1061− | ATPase subunit 6 | Kinetoplast of Trypanosoma brucei | 31 | 45 | |||
| A540 | AY217343 | 41 | 1 | 1-256 + | agCP1945 | Anopheles gambiae | 34 | 49 |
Sequences of over 50 amino acids lacking an initial methionine or a stop codon (usually ORFs at the ends of the clones) are also included.
The orientation from the T7 promoter end of the clone is arbitrarily designated +.
A sequence of at least 50 amino acids with no match by BlastX is termed a hypothetical protein.
Codon usage table.
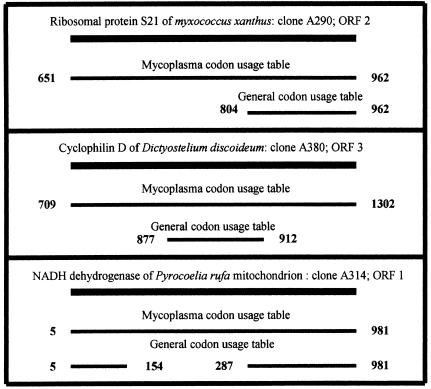
Phytoplasmas were designated mycoplasma-like organisms due to their comparatively small size, lack of a cell wall, and being intracellular parasites. On the other hand, it was suggested that phytoplasmas are closer to acholeplasmas than to mycoplasmas. This designation was made on the basis of analysis of only a single phytoplasmal operon (28) and two ribosomal protein genes (33). Acholeplasmas use the general codon usage table, while mycoplasmas use the mitochondrial codon usage table, in which the stop codon UGA is read as tryptophan (41). We have translated the obtained phytoplasmal sequences according to both codon usage tables. Applying the criteria for ORF definition as described below, we concluded that phytoplasmas read UGA as tryptophan. This was based on the following observations. (i) The general codon usage table generated 62 ORFs, compared to 78 ORFs generated by the mycoplasmal codon usage table. For example, ORF 9 of clone A2, a homologue of a bacterial gene, would have disappeared if translated according to the general codon usage table, due to insertion of four stop codons. (ii) In several cases, alignments made with the homologous genes resulted in continuous stretches of aligned sequences when translated with the mycoplasma codon usage table, while the translation according to the general codon usage table resulted in only fragmented alignments (Fig. 1, top panel). (iii) Similarly, ORFs obtained by using the general codon usage table were shorter than those obtained by using the mycoplasma codon usage table. The latter continued to align with the same homologous gene, indicating that the reading of UGA as a stop codon interrupted the reading of the gene within its coding region (Fig. 1, bottom panel). Phytoplasmal ORFs were thus defined throughout this work according to the codon usage table of mycoplasmas-mitochondria. It should be noted that all of the phytoplasmal ribosomal protein genes reported above did not carry in-frame UGA codons, and therefore translation by either codon usage table resulted in the same deduced protein sequences.
FIG. 1.
Schematic illustration of the translation of three phytoplasmal clones according to the mycoplasma-mitochondrion codon usage table and to the general codon usage table.
Homology search.
Initially, a BlastX homology search was performed for each cloned insert to find similarity to proteins in the GenBank database. At a later stage, ORFs were defined by ORF Finder, and each individual ORF was searched for homologies by using Blast and BlastX. Homology of the phytoplasma ORFs to known proteins is indicated in Table 2, provided that the level of identity is over 25%. ORFs with no homology to any known protein, or with indicated similarity below 25%, are included in Table 2 only if they represent a string of at least 50 continuous amino acids. These ORFs were designated as coding for hypothetical proteins. If the highest BlastX score was for a putative or uncharacterized gene while similarity to a defined gene was noted at a somewhat lesser score, the ORF was designated as being similar to that of the defined gene.
Analysis of the phytoplasmal ORFs revealed that 12 ORFs code for ribosomal proteins, enzymes, and factors involved in protein synthesis, rRNA processing, and tRNA acylation. Eight ORFs putatively code for enzymes involved in energy metabolism (hydrogen transfer and oxidative phosphorylation). Five ORFs code for components of RNA and DNA polymerases and for enzymes involved in DNA modification and integration. Three ORFs code for retroelements (either retrotransposons or retroviruses) and for a protein reacting with a product of the nef retrogene. The other reported putative phytoplasmal ORFs code for metabolic enzymes, surface and signal peptides, and various other proteins; some of these are discussed below. Notably, 25 of the 78 ORFs have no match in the GenBank database and are designated hypothetical proteins (Table 2). Four other ORFs putatively code for proteins similar to those found in other organisms but with no as-yet-defined function.
Although it is premature to draw conclusions about the phytoplasmal genome organization, it should be noted that, based on the presently reported sequences, the gene density is very high: 27,407 bases, out of a total of 32,753 bases sequenced (83.7%), are ORFs. Several clones carry partially overlapping ORFs at different frames, some of which are positioned on opposite strands.
Specificity of the phytoplasma clones.
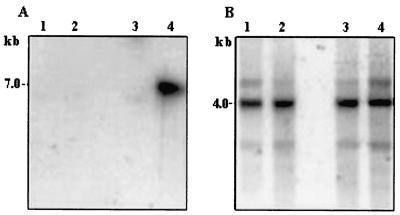
The following tests were carried out in order to ascertain that the above-described clones were of phytoplasmal origin and did not represent host sequences of plants, insects, or other microorganisms. Genomic DNAs or RNAs of noninfected, Stolbur-infected, and AY-phytoplasma-infected plants [periwinkle; Catharantus roseus (L.) G. Don] were subjected to Southern or Northern blot analysis with several inserts of selected clones as probes. An example of a Southern blot analysis is given in Fig. 2, and an example of a Northern analysis is given in Fig. 3. In both analyses, the probes reacted with DNA (Fig. 2A) or with total RNA (Fig. 3A) from AY-infected plants and not with that from noninfected plants. In the Southern analysis, the A1 clone also reacted faintly with DNA from plants infected with Stolbur (a phytoplasma related to AY). When the insert of clone A2 served as the probe in the Northern blot analysis, a complex hybridization pattern was observed, which could be attributed to the fact that 12 putative ORFs (Table 2) were identified on that clone. In order to verify equal loading of the various lanes, hybridization with the housekeeping β-tubulin gene is shown for the Southern analysis, and an ethidium bromide-stained total RNA gel (prior to hybridization), showing the rRNA bands, is shown for the Northern analysis.
FIG. 2.
Southern blot analysis of periwinkle DNA. Chromosomal periwinkle DNA (12 μg) was digested with HindIII restriction enzyme, subjected to Southern blot hybridization, and probed with 32P-labeled clone A1 (A) or with a 670-bp PCR product representing a conserved region of the tubulin gene (B). The size of the hybridization band is indicated on the left. The exposure time was 3 weeks (A) or 3 days (B). DNA was from healthy plants (lanes 1 and 2), from a Stolbur-infected plant (lanes 3), or from an AY-infected plant (lanes 4).
FIG. 3.
Northern blot analysis of periwinkle RNA. Total cellular RNAs were extracted, electrophoresed (15 μg per lane), subjected to Northern blot hybridization, and probed with 32P-labeled clone A2 (A) or stained with ethidium bromide (B). RNA was from healthy plants (lanes 1) or from an AY-infected plant (lanes 2). The base lengths of RNA are indicated on the left.
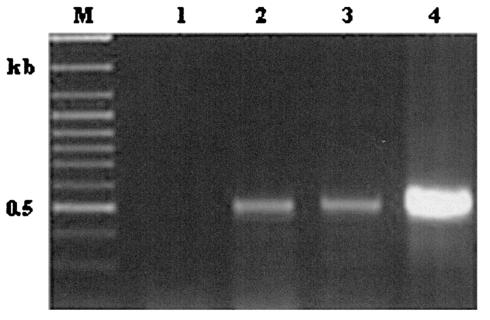
To further assess the specificity of the phytoplasmal DNA inserts, primers were generated from clone A380 (Table 1) and used for PCR analysis of genomic DNA obtained from healthy or infected plants (Fig. 4). A PCR product was obtained only with DNA extracted from phytoplasma-infected plants (Fig. 4, lanes 2 and 3). The specific PCR product was obtained with DNA from AY- and Stolbur-infected plants. Healthy plants did not produce any band upon PCR (Fig. 4, lane 1).
FIG. 4.
PCR analysis of periwinkle chromosomal DNA (100 ng per reaction). PCR primers were derived from clone A380 (Tables 1 and 2), amplifying a 500-bp region. Template DNA was from a healthy plant (lane 1), from an AY-infected plant (lane 2), from a Stolbur-infected plant (lane 3), or from clone A380 as a positive control (lane 4), Lane M, molecular size markers.
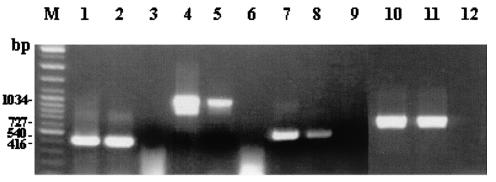
Phytoplasmal DNA was collected from insect saliva. Therefore, the possibility that the described clones were derived from insect cells which were deposited in the saliva or from other insect microorganisms (viruses or bacteria) needed to be ruled out. To address this issue, we selected phytoplasma-carrying and phytoplasma-free insects and carried out four PCR analyses on both types of insects. The PCRs were based on four distinct cloned phytoplasma-like sequences obtained in our study. (Fig. 5). In order to increase sensitivity and specificity, nested PCR was performed. Primers for these PCR assays were designed from clones A10, A182, A244, and A380 (Table 1). In all cases, PCR products were obtained from phytoplasma-infested insects (Fig. 5, lanes 2, 5, 8, and 11) and not from phytoplasma-free insects (Fig. 5, lanes 3, 6, 9, and 12). The lack of the tested sequences in the saliva of phytoplasma-free leafhoppers minimizes the possibility of contamination by microorganisms that were injected into the sucrose upon feeding. Collectively, the data suggest that the clone inserts represent authentic phytoplasma genomic fragments
FIG. 5.
PCR analysis of leafhopper DNA (100 ng per reaction). PCR primers derived from clones A10, A182, A244, and A380 (A380 is the same primer as was used for the plant PCR assay for Fig. 4) were used to amplify a segment of DNA extracted from leafhoppers. All reactions except that with A380 primers were nested PCR. Lanes 2, 5, 8, and 11, PCRs with template DNAs from phytoplasma-carrying leafhoppers. Lanes 3, 6, 9, and 12, PCRs with template DNAs from phytoplasma-free leafhoppers. Lanes 1, 4, 7, and 10, positive controls (with the cloned DNAs serving as templates) for PCRs with A182, A244, A380, and A10, respectively.
Homology of phytoplasma clones to eukaryotic genes.
Most of the listed ORFs bore homology to prokaryotic and mitochondrial genes, which are considered to be of prokaryotic origin. Ten ORFs, however, were found to be homologous, at a reasonable level, to nonmitochondrial eukaryotic genes. Eight of these eukaryotic genes were found to be located on the same physical unit with prokaryotic genes (clones A2, A239, A295, and A450). Interestingly, three ORFs were homologous to retrovirus-like sequences (clone A370; clone A463, ORF 4; and clone A450, ORF 2 [which codes for a protein that is attachable to the retroviral nef gene]), indicating the possible occurrence of retrotransposons in the phytoplasmal genome. Notably, however, all of the eukaryote-homologous clones (clone A2, ORFs 4, 5, 8, and 12; clone A239, ORF 3; clone A295, ORF 2; clone A380, ORFs 2 and 3; clone A450, ORF 2; and clone A540) exhibited a low GC content (23 to 39%), and most of them were located on the same genomic fragment adjacent to prokaryotic genes. The implications of such homologies are discussed below.
DISCUSSION
Until now, it had not been possible to obtain phytoplasmal DNA that was sufficiently free of its respective host DNA. Therefore, the study of the phytoplasmal genome was hampered, and detection and characterization of phytoplasmal genes were performed randomly. Phytoplasmas were diagnosed almost solely on the basis of 16S rDNA sequences. Aside from the presently reported sequences, only 33 phytoplasmal genes, or parts thereof, are currently listed in the GenBank database. Extrachromosal (plasmid) phytoplasma sequences have also been reported (35, 41). The presently reported method for isolating background-free phytoplasmal DNA enabled the construction of a phytoplasmal genomic library and demonstrates the ability to systematically analyze phytoplasmal genes. The usefulness of the method is demonstrated by its almost tripling the amount of phytoplasmal gene information in a short time.
Sequence analysis of the phytoplasmal clones indicated some unique mycoplasma-like features: they have a low GC content (less than 35% in most cases), and the best ORF designation was obtained through the mycoplasma-mitochondrion codon usage table. These features, in addition to the lack of a cell wall, small genome size, and intracellular location in the host tissues, indicate that phytoplasmas might be evolutionarily related to mycoplasmas. The entire genomes of five mycoplasma species are known (8, 12, 13, 17, 45). Surprisingly, however, only one of the presently reported phytoplasmal ORFs was found to be homologous, at a reasonable score, to a mycoplasmal gene (clone A448, ORF 1, is homologous to the mycoplasmal ribosomal protein S8 [rpS8] gene, albeit at a lesser score than to the same gene from Listeria innocuus). Furthermore, none of the phytoplasmal ORFs reported by others is homologous to mycoplasmal genes. It was also reported that phytoplasmas are phylogenetically closer to acholeplasmas than to mycoplasmas (33). The organization of the highly conserved ribosomal protein genes in these species also adds to the confusion. The gene order in clone A182 (rpL18, rpS5, rpL15) and in A448 (rpS8, rpL6) is similar to the gene order in Mycoplasma genitalium. On the other hand, the gene order in clone A115 (EF-G, rpS10, rpL3) resembles that of Bacillus (EF-G, EF-Tu, rpS10, rpL3) rather than those of mycoplasmas (M. genitalium and Mycoplasma pneumoniae, EF-G, rpS6, ssb, rpS18; Mycoplasma pulmonis, EF-G, EF-Tu, hypothetical protein, hypothetical protein). Analysis of the complete S10-spc operon of the onion yellows phytoplasma (33) also led to the conclusion that phytoplasmal genes resemble those of acholeplasmas and Bacillus more than those of other Mollicutes. These data indicate that the phytoplasma phylogenetic association is still an unresolved matter, and the question of the relatedness of phytoplasmas to mycoplasmas remains open.
Previous papers (see, for instance, reference 28) have indicated that phytoplasmas employ the general codon usage table. These findings were based on analysis of only a few ribosomal protein genes and the tuf gene. We analyzed 78 ORFs, and in about half of them (including all ribosomal protein genes) it did not matter which codon usage table was employed for translation. This may explain the previous reports of the use of the general codon table by phytoplasmas. However, in 16 out of the 32 reported clones (30 out of the reported 78 ORFs) a better translation was obtained with the mitochondrion-mycoplasma codon usage, while in the rest of the clones it did not matter which codon usage table was employed. This indicated that phytoplasmas use the mitochondrion-mycoplasma codon usage table, where UGA is read as tryptophan (three such cases are shown in Fig. 1). This is a large enough sample to allow a preliminary conclusion that at least the AY phytoplasma reads UGA as tryptophan. However, it uses this triplet infrequently, so that in many cases it does not matter which codon usage table is in effect.
Ten of the aforementioned ORFs are homologous to nonmitochondrial eukaryotic genes, and two ORFs (clones A352 and A526) are homologous to viral genes. It is worth noting that in addition to the eight eukaryotic genes mentioned above, four of the eukaryote-homologous ORFs are retroelements, implying the presence of transposons, or retrovirus infection, in phytoplasmas.
The eukaryote-homologous ORFs are not contaminants brought into the system from insect cells, as they are of low GC content and some are positioned on the same clone with prokaryote-homologous ORFs (for example, clones A2, A239, and A450). The close intracellular relationship between phytoplasmas and their host cells might have resulted from exchanges of genetic material (perhaps due to recombination) between the host and the pathogen. The host-homologous genes were adapted by the pathogen and underwent modifications (for instance, although they remain homologous to eukaryotic genes, they exhibit a low GC content in the phytoplasma). In this regard, it is worth mentioning that several ORFs are homologous to insect genes (mitochondrial or unassigned genes), and none (except for clone A340, which carries transposable elements) is of plant origin. The baculovirus-homologous ORF (clone A526) is also of special interest. This ORF is homologous to fusolin (also known as spindle-body protein or GP37 [38]). Fusolin has been found in baculoviruses and in insect-infecting poxviruses and has been shown to enhance infection by a factor of 104. Fusolins are missing from poxviruses that do not infect insects (32, 52). It is of special interest that some fusolins bear distant homology to chitin- and cellulose-binding proteins (38). The insect's peritrophic membrane is composed of protein and chitin, and a major component of plant cell walls is cellulose. Phytoplasmas are pathogens of both insects and plants, and it is possible that a fusolin-like protein is required for establishing phytoplasmal infection. It is also worth noting that a gene for a caspase (caspases are cysteine proteases involved in apoptosis following mitochondrial signaling [7, 24]) was found in phytoplasma (clone A352). In eukaryotic cells caspases are recruited as antibacterial agents (14); however, their origin is believed to be prokaryotic (2, 23).
Currently, phylogenetic analyses of phytoplasmas and their assignment to taxons and clades are based on the similarity or diversity found in their rDNA sequences, the gene for elongation factor Tu (tuf gene), and some ribosomal protein genes (16, 26, 28, 30, 49). For better reliability, taxonomy should rely on similarities and diversities in more than a single operon. In this respect, it is noteworthy that none of the sequences found for the isolated genes of the presently reported AY phytoplasma are homologous at their highest score to other phytoplasmal genes. Similar observations, which might challenge the present rDNA-based classification, were also made with other systems. The immunodominant membrane proteins from two phytoplasmas belonging to the same clade (as determined by 16S rDNA analysis) were found to be highly divergent in their hydrophilic region (3), and phytoplasmas diagnosed by 16S rDNA analysis as various types of apple proliferation were also found to be quite variable when PCR-RFLP analysis was performed on a nonribosomal fragment (18).
To validate the results, a determination of gene order for some other clones is also important. For example, all three ORFs in clone A380 are of mitochondrial origin (cyclophilin-D is a mitochondrial protein [56]).
Phytoplasma diagnoses are currently based on rDNA analyses. In this report, we provide PCR analyses, specific to AY, that are based on non-rDNA sequences. The information gathered here, and more sequences to come, will allow a better classification of phytoplasmas.
Many phytoplasmal clones carry ORFs in different frames and even in opposite orientations. This is to be expected when a relatively large amount of information is condensed into a small genome. This is consistent with the situation in M. genitalium. Almost every clone (accession numbers U39679 to U39729) carries overlapping ORFs in different frames, and short overlaps in opposite orientations are quite common. In some cases, longer opposite overlaps are also found; for example, the gene for O-sialoglycoprotein endopeptidase and that for signal recognition particle protein (accession no. U39684) overlap for 230 bp in opposite orientations. When two ORFs are partially overlapping and are located in opposite orientations, a sense-antisense silencing of expression, due to the formation of double-stranded RNA (39), probably takes place, unless the expression of each ORF is regulated at a different time.
The expected size of the phytoplasma genome is only 500 to 1,200 kb (50). Therefore, the above-described genomic library (about 5,000 clones with an average insert size of approximately 2,000 bp) seems to be representative of the entire phytoplasmal genome, allowing the launch of a project to decipher the entire phytoplasmal genome.
REFERENCES
- 1.Ahrens, U., and E. Seemüller. 1992. Detection of DNA of plant pathogenic mycoplasmalike organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology 82:828-832. [Google Scholar]
- 2.Aravind, L., and E. V. Koonin. 2002. Classification of the caspase-hemoglobinase fold: detection of new families and implications for the origin of the eukaryotic separins. Protein Struct. Funct. Genet. 46:355-367. [DOI] [PubMed] [Google Scholar]
- 3.Barbara, D. J., A. Morton, M. F. Clarck, and D. L. Davies. 2002. Immunodominant membrane proteins from two phytoplasmas in the aster yellows clade (chlorante aster yellows and clover phyllody) are highly divergent in their major hydrophilic region. Microbiology 148:157-167. [DOI] [PubMed] [Google Scholar]
- 4.Berg, M., D. L., Davies, M. F. Clark, H. J. Vetten, G. Maier, C. Marcone, and E. Seemuller. 1999. Isolation of the gene encoding an immunodominant membrane protein of the apple proliferation phytoplasma, and expression and characterization of the gene product. Microbiology 145:1937-1943. [DOI] [PubMed] [Google Scholar]
- 5.Blomquist, C. L., D. J. Barbara, D. L. Davies, M. F. Clark, and B. C. Kirkpatrick. 2001. An immunodominant membrane protein gene from western X-disease phytoplasma is distinct from those of other phytoplasmas. Microbiology 147:571-580. [DOI] [PubMed] [Google Scholar]
- 6.Boudon-Padieu, E., J. Larrue, and A. Caudwell. 1989. ELISA and dot blot detection of Flavescence dorée-MLO in individual leafhopper vectors during latency and inoculative state. Curr. Microbiol. 19:357-364. [Google Scholar]
- 7.Chai, J., C., Du, J.-W. Wu, S. Kyin, X. Wang, and Y. Shi. 2000. Structural and biochemical basis of apoptosis activation by Smac/DIABOLO. Nature 406:855-862. [DOI] [PubMed] [Google Scholar]
- 8.Chambaud, I., R. Heilig, S. Ferris, V. Barbe, V. Samson, F. Galisson, I. Moszer, K. Dybvig, H. Wroblewski, A. Viari, E. P. C. Rocha, and A. Blanchard. 2001. The complete genome sequence of the murine respiratory pathogen Mycoplasma pulmonis. Nucleic Acids Res. 29:2145-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, K. H., J. R. Guo, X. Y. Wu, N. Loi, L. Carraro, Y. H. Guo, Y. D. Chen, R. Osler, R. Pearson, and T. A. Chen. 1993. Comparison of monoclonal antibodies, DNA probes and PCR for detection of the grapevine yellows disease agent. Phytopathology 83:915-922. [Google Scholar]
- 10.Chu, Y. R., and C. P. Lin. 1997. Cloning and characterization of RecA gene of phytoplasma associated with peanut witches' broom. Zhiwu Binglixue Huikan 6:207-208. [Google Scholar]
- 11.Davis, R. I., B. Schneider, and K. S. Gibb. 1997. Detection and differentiation of phytoplasmas in Australia. Aust. J. Agric. Res. 48:535-544. [Google Scholar]
- 12.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, J. D. Fritchman, J. F. Weidman, K. V. Small, M. Sandusky, J. Fuhrmann, and D. Nguyen. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 13.Glass, J. I., E. J. Lefkowitz, J. S. Glass, C. R. Heiner, E. Y. Chen, and G. H. Cassell. 2000. The complete sequence of the mucosal pathogen Ureaplasma urealiticum. Nature 407:757-762. [DOI] [PubMed] [Google Scholar]
- 14.Gramsse, H., V. Jendrossek, and E. Glubins. 2001. Molecular mechanisms of bacteria induced apoptosis. Apoptosis 6:441-445. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths, H. M., W. A. Sinclair, C. D. Smart, and R. E. Davis. 1999. The phytoplasma associated with ash yellows and lilac witches'-broom: “Candidatus Phytoplasma fraxini.” Int. J. Syst. Bacteriol. 49:1605-1614. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson, D. E., I. M. Lee, S. A. Rehner, R. F. Davis, and D. T. Kingsbury. 1994. Phylogeny of mycoplasmalike organisms (phytoplasma): a basis for their classification. J. Bacteriol. 176:5244-5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Himmelreich, R., H. Hilbert, H. Plagens, E. Pirkl, B. C. Li, and R. Herrmann. 1996. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 24:4420-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarausch, W., C. Saillard, B. Helliot, M. Garnier, and F. Dosba. 2000. Genetic variability of apple proliferation phytoplasmas as determined by PCR-RFLP and sequencing of a non-ribosomal fragment. Mol. Cell. Probes 14:17-24. [DOI] [PubMed] [Google Scholar]
- 19.Jomantiene, R., R. E. Davis, J. Maas, and E. L. Dally. 1998. Classification of new phytoplasma associated with diseases of strawberry in Florida, based on analysis of 16S rRNA and ribosomal protein gene operon sequences. Int. J. Syst. Bacteriol. 48:269-277. [DOI] [PubMed] [Google Scholar]
- 20.Kakizawa, S., K. Oshima, T. Kuboyama, H. Nishigawa, H. Jung, T. Sawayanagi, T. Tsuchizaki, S. Miyata, M. Ugaki, and S. Namba. 2001. Cloning and expression analysis of phytoplasma protein translocation genes. Mol. Plant-Microbe Interact. 14:1043-1050. [DOI] [PubMed] [Google Scholar]
- 21.Kirkpatrick, B. C., D. C. Stenger, T. J. Morris, and A. H. Purcell. 1987. Cloning and detection of DNA from a nonculturable plant pathogenic mycoplasma-like organism. Science 238:197-200. [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick, B. C. 1991. Mycoplasma-like organisms: plant and invertebrate pathogens, p. 4050-4067. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K. H. Schliefer (ed.), The prokaryotes. Springer-Verlag, New York, N.Y.
- 23.Koonin, E. V., and L. Aravind. 2002. Origin and evolution of eukaryotic apoptosis: the bacterial connection. Cell Death Differ. 9:394-404. [DOI] [PubMed] [Google Scholar]
- 24.Kroemer, G. 1997. Mitochondrial implication in apoptosis. Towards an endosymbiont hypothesis of apoptosis evolution. Cell Death Differ. 4:443-456. [DOI] [PubMed] [Google Scholar]
- 25.Lee, I.-M., R. W. Hammond, R. E. Davis, and D. E. Gundersen. 1993. Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasmalike organisms. Phytopathology 83:834-842. [Google Scholar]
- 26.Lee, I.-M., D. E. Gundersen-Rindal, R. E. Davis, and I. M. Bartoszyk. 1998. Revised classification scheme of phytoplasmas based an RFLP analyses of 16S rRNA and ribosomal protein gene sequences. Int. J. Syst. Bacteriol. 48:1153-1169. [DOI] [PubMed] [Google Scholar]
- 27.Lim, P. O., and B. B. Sears. 1991. DNA sequence of the ribosomal protein genes rpl2 and rpl9, from a plant-pathogenic mycoplasma-like organism. FEMS Microb. Lett. 84:71-74. [DOI] [PubMed] [Google Scholar]
- 28.Lim, P. O., and B. B. Sears. 1992. Evolutionary relationship of a plant pathogenic mycoplasmalike organism and Acholeplasma laidlawii deduced from two ribosomal protein gene sequences. J. Bacteriol. 174:2606-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maixner, M., U. Ahrens, and E. Seemuller. 1995. Detection of German grapevine yellows (Vergilbungs krankheit) MLO in grapevine, alternative hosts and a vector by a specific PCR procedure. Eur. J. Plant Pathol. 101:241-250. [Google Scholar]
- 30.Marcone, C., I. M. Lee, R. E. Davis, A. Ragozzino, and E. Seemuller. 2000. Classification of aster yellows-group phytoplasmas based on combined analysis of rRNA and tuf gene sequences. Int. J. Syst. E vol. Microbiol. 50:1703-1713. [DOI] [PubMed] [Google Scholar]
- 31.McCoy, R. E., A. Caudwell, C. J. Chang, T. A. Chen, L. N. Chiykowski, M. T. Cousin, J. L. Dale, G. T. N. de Leeuw, D. A. Golino, K. J. Hacket, B. C. Kirkpatrick, R. Marvitz, H. Petzold, R. C. Sinha, M. Sugiura, R. F. Whitcomb, I. L. Yang, B. M. Zhu, and E. Seemüller. 1989. Plant diseases associated with mycoplasma-like organisms, p. 545-640. In R. F. Whitcomb and J. C. Tully (ed.), The mycoplasmas, vol. 5. Spiroplasmas, acholeplasmas, and mycoplasmas of plants and arthropods. Academic Press Inc., San Diego, Calif.
- 32.Mitsuhashi, W., Y. Furuta, and M. Sato. 1997. Complete nucleotide sequence of the fusolin gene of an entomopoxvirus in the cupreous chafer, Anomala cuprea Hope (Coleoptera, Scarabaeidae). Insect Biochem. Mol. Biol. 27:869-876. [DOI] [PubMed] [Google Scholar]
- 33.Miyata, S. I., K. I. Furuki, K. Oshima, T. Sawayanagi, H. Nishigawa, S. Kakizawa, H. Y. Jung, M. Ugaki, and S. Namba. 2002. Complete nucleotide sequence of the S10-spc operon of phytoplasma: gene organization and genetic code resemble those of Bacillus subtilis. DNA Cell Biol. 21:527-534. [DOI] [PubMed] [Google Scholar]
- 34.Montano, H. G., R. E. Davis, E. L. Dally, S. Hogenhout, J. P. Pimentel, and P. S. T. Brioso. 2001. “Candidatus Phytoplasma brasiliense”, a new phytoplasma taxon associated with hibiscus witches' broom disease. Int. J. Syst. E vol. Microbiol. 51:1109-1118. [DOI] [PubMed] [Google Scholar]
- 35.Nishigawa, H., K. Oshima, S. Kakizawa, H. Y. Jung, T. Kuboyama, S. Miyata, M. Ugaki, and S. Namba. 2002. A plasmid from a non-insect transmissible line of phytoplasma lacks two open reading frames that exist in the plasmid from the wild-type line. Gene 298:195-201. [DOI] [PubMed] [Google Scholar]
- 36.Orenstein, S., A. Franck, L. Kuznetzova, I. Sela, and E. Tanne. 1999. Association of phytoplasmas with yellows diseases of carrot in Israel. J. Plant Pathol. 81:193-199. [Google Scholar]
- 37.Padovan, A. C., G. Firrao, B. Schneider, and K. S. Gibb. 2000. Chromosome mapping of the sweet potato little leaf phytoplasma reveals genome heterology within the phytoplasmas. Microbiology 146:893-902. [DOI] [PubMed] [Google Scholar]
- 38.Phanis, C. G., D. P. Miller, S. C. Cassar, M. Tristem, S. M. Thiem, and D. R. O'Reilly. 1999. Identification and expression of two baculovirus gp37 genes. J. Gen. Virol. 80:1823-1831. [DOI] [PubMed] [Google Scholar]
- 39.Plasterk, H. A. 2002. RNA silencing: the genome's immune system. Science 296:1263-1265. [DOI] [PubMed] [Google Scholar]
- 40.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rekab, D., L. Carraro, B. Schneider, E. Seemuller, J. Chen, C. J. Chang, R. Loci, and G. Firrao. 1999. Gemini virus-related extrachromosomal DNAs of the X-clade phytoplasmas share high sequence similarity. Microbiology 145:1453-1459. [DOI] [PubMed] [Google Scholar]
- 42.Saillard, C., and J. M. Bové. 1990. Isolation of the DNA of various plant pathogenic mycoplasmalike organisms from infected plants. Phytopathology 80:233-237. [Google Scholar]
- 43.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki, Y., J. Ishikawa, A. Yamashita, K. Oshima, T. Kenri, K. Furuya, C. Yoshino, A. Horino, T. Shiba, T. Sasaki, and M. Hattori. 2002. The complete genomic sequence of Mycoplasma penetrans, an intracellular bacterial pathogen in humans. Nucleic Acids Res. 30:5293-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawayanagi, T., N. Horikoshi, T. Kanehira, M. Shinohara, A. Bertaccini, M. T. Cousin, C. Hiruki, and S. Namba. 1999. “Candidatus phytoplasma japonicum,” a new phytoplasma taxon associated with Japanese hydrangea phyllody. Int. J. Syst. Bacteriol. 49:1275-1285. [DOI] [PubMed] [Google Scholar]
- 47.Sears, B. B., P.-O. Lim, N. Holland, B. C. Kirkpatrick, and K. L. Klomparens. 1989. Isolation and characterization of DNA from a mycoplasmalike organism. Mol. Plant-Microbe Interact. 2:175-180. [Google Scholar]
- 48.Seed, E., E. Seemuller, B. J. Schneider, C. Saillard, B. Blanchard, Y. Bertheau, and M. T. Cousin. 1994. Molecular cloning, detection of chromosomal DNA of the MLO associated with Faba bean (Vicia faba L.) phyllody by Southern blot hybridization and the polymerase chain reaction (PCR). J. Phytopathol. 144:97-106. [Google Scholar]
- 49.Seemüller, E., C. Marcone, U. Lauer, A. Ragozzino, and M. Göschl. 1998. Current status of molecular classification of the phytoplasmas. J. Plant Pathol. 80:3-26. [Google Scholar]
- 50.Seemüller, E., M. Garnier, and B. Schneider. 2002. Mycoplasmas of plants and insects, p. 91-116. In S. Razin and R. Herrmann (ed.), Molecular biology and pathology of mycoplasmas. Kluwer Academic/Plenum Publishers, London, United Kingdom.
- 51.Smart, C. D., B. Schneider, C. L. Blomquist, L. J. Guerra, N. A. Harrison, U. Ahrens, K.-H. Lorenz, E. Seemuller, and B. C. Kirkpatrick. 1996. Phytoplasma-specific PCR primers based on sequences of the 16S-23S rRNA spacer region. Appl. Environ. Microbiol. 62:2988-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sriskantha, A., R. J. Osborne, and D. J. Dall. 1997. Mapping of the Heliothis armigera entomopoxvirus (HaEPV) genome, and analysis of genes encoding the HaEPV spheroidin and nucleoside triphosphate phosphohydrolase I proteins. J. Gen. Viol. 78:3115-3123. [DOI] [PubMed] [Google Scholar]
- 53.Tanne, E., E. Boudon-Padieu, D. Clair, M. Davidovich, S. Melamed, and M. Klein. 2001. Detection of phytoplasma by polymerase chain reaction of insect feeding medium and its use in determining vectoring ability. Phytopathology 91:741-746. [DOI] [PubMed] [Google Scholar]
- 54.Wagner, M., C. Fingerhut, H. J. Ross, and A. Schon. 2001. The first phytoplasma RNase P RNA provides new insight into the sequence requirement of this ribozyme. Nucleic Acids Res. 19:2661-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White, D. T., L. L. Blackall, P. T. Scott, and K. B. Walsh. 1998. Phylogenetic positions of phytoplasmas associated with dieback, yellow crinkle and mosaic diseases of papaya, and their proposed inclusion in “Candidatus Phytoplasma australiense” and a new taxon, “Candidatus Phytoplasma australasia.” Int. J. Syst. Bacteriol. 48:941-951. [DOI] [PubMed] [Google Scholar]
- 56.Woodfield, K. Y., N. T. Price, and A. P. Halestrap. 1997. cDNA cloning of rat mitochondrial cyclophilin. Biochem. Biophys. Acta 1351:27-30. [DOI] [PubMed] [Google Scholar]
- 57.Yu, Y. l., K. W. Yeh, and C. P. Lin. 1998. An antigenic protein gene of a phytoplasma associated with sweet potato witches' broom. Microbiology 144:1257-1262. [DOI] [PubMed] [Google Scholar]