Abstract
The role of various p73 isoforms in tumorigenesis has been controversial. However, as we have recently shown, the generation of TAp73-deficient (TAp73−/−) mice reveals that TAp73 isoforms exert tumor-suppressive functions, indicating an emerging role for Trp-73 in the maintenance of genomic stability. Unlike mice lacking all p73 isoforms, TAp73−/− mice show a high incidence of spontaneous tumors. Moreover, TAp73−/− mice are infertile and produce oocytes exhibiting spindle abnormalities. These data suggest a link between TAp73 activities and the common molecular machinery underlying meiosis and mitosis. Previous studies have indicated that the spindle assembly checkpoint (SAC) complex, whose activation leads to mitotic arrest, also regulates meiosis. In this study, we demonstrate in murine and human cells that TAp73 is able to interact directly with several partners of the SAC complex (Bub1, Bub3, and BubR1). We also show that TAp73 is involved in SAC protein localization and activities. Moreover, we show that decreased TAp73 expression correlates with increases of SAC protein expression in patients with lung cancer. Our results establish TAp73 as a regulator of SAC responses and indicate that TAp73 loss can lead to mitotic arrest defects. Our data suggest that SAC impairment in the absence of functional TAp73 could explain the genomic instability and increased aneuploidy observed in TAp73-deficient cells.
Keywords: Bub1, meiosis, mitotic arrest, p73, spindle checkpoint
Accurate chromosome segregation during meiosis and mitosis is critical to the preservation of euploidy in eukaryotic cells (1). Errors in the molecular mechanisms regulating segregation result in aneuploidy, a hallmark of spontaneous abortions, birth defects, and many cancers (2, 3). The spindle assembly checkpoint (SAC) is a regulatory mechanism that senses the improper attachment of sister chromatids to the mitotic or meiotic spindle and delays anaphase until all chromosomes are correctly oriented for segregation (4, 5). More than 20 proteins participate in, or are functionally associated with, the SAC, including MAD2, BUB1, BUB3, BUBR1, cyclin B, Rae1, and Aurora B (6).
The high levels of chromosome mis-segregation in cancer cells suggest that they may have undergone SAC inactivation. Several SAC-related genes have been linked to tumors in humans and mice. Human gastric cancers frequently show mutations of MAD2 (7), and bi-allelic mutations of BUB1B, which encodes BUBR1 in humans, lead to the rare familial disorder known as mosaic variegated aneuploidy. This disease features developmental abnormalities (e.g., severe intrauterine growth retardation, microcephaly, eye anomalies, mild dysmorphism, variable developmental delay, and a broad spectrum of additional congenital abnormalities), widespread aneuploidy, and increased risk of tumorigenesis (8). In mice, a deficiency of BubR1 promotes infertility and premature aging (9), and mice with heterozygous mutations of Mad2, BubR1, Bub3, or Rae1 are prone to spontaneous or carcinogen-induced tumorigenesis (10 –12). Dominant-negative mutations in Bub1 or BubR1 have been identified in thymic lymphomas from mice homozygous for a truncating mutation of the Brca2 gene (13). Recently, two studies reinforced the link between SAC components and genomic instability by revealing that decrease of SAC component activity induces genomic instability and aneuploidy (14, 15). However, mutations in the known SAC genes are relatively rare in human carcinomas, suggesting that the genomic instability in these tumors either results from mutations in currently unknown SAC genes or is driven by an epigenetic mechanism. It has been proposed that a mutation in any SAC protein may trigger chromosome mis-segregation when combined with another defect (16), such as insufficient p53 function (17). However, several cancer lines known to have both SAC component mutations and p53 mutations are diploid or chromosomally stable (18). In addition, chromosomal instability appears early in tumorigenesis, whereas p53 mutations usually occur much later (19). These conflicting findings could result from the functional redundancy of p53 family member isoforms.
The p53 family is composed of three members: p53 itself, p63, and p73. Whereas p63 seems more related to stem cell maintenance and development (20), p73 is tightly related to p53 in terms of functional abilities. However, the interrelation between p53 and p73 is highly complex as a result of the numerous protein isoforms of p73. Indeed, the Trp-73 gene has two promoters that drive the expression of two major p73 isoform subfamilies with opposite effects: isoforms of the TAp73 sub-family show pro-apoptotic activities similar to those of p53, whereas members of the ΔNp73 subfamily (lacking the TA domain) have an anti-apoptotic function (21). Thus, these isoforms can compete with, synergize with, or be unrelated to those of p53 (22, 23).
Although the precise roles of p73 isoforms in tumorigenesis remain unclear (24 –27), strong advances were made in the past few years by the use of transgenic mouse models (23, 28). Recently, from the analysis of TAp73-deficient mice, we showed that TAp73 is a p73 isoform with tumor suppressor activity (28, 29). Indeed, TAp73-null mice are prone to spontaneous tumor development. These mice are also subject to other defects such as a male and female infertility and a hippocampal dysgenesis. We also showed that loss of TAp73 is correlated with an increase in aneuploidy, suggesting that TAp73 is playing a role in the maintenance of genomic stability. This is supported by previous studies showing a connection among p53 family members, SAC regulation, and genomic instability (30 –33), a crucial player in tumorigenesis (34). In view of these data, we reasoned that the infertility and tumor-prone phenotype of TAp73−/− mice might be caused by a common cellular defect. In the present study, we investigated whether defects observed in TAp73-deficient mice could be related to defaults in SAC activity. Moreover, we analyzed whether TAp73 is able to directly interact with components of the spindle assembly checkpoint, and consequently regulate its activity.
Results
Spindle Assembly Checkpoint Components Are Mis-Localized in TAp73−/− Mice.
The aneuploidy of p73−/− cells (33) and the spindle defects observed in TAp73−/− cells (28) prompted us to examine the expression and localization of Bub1 and BubR1 in TAp73+/+ and TAp73−/− oocytes. The localization of Bub1 (Fig. 1 A and B) and BubR1 (Fig. 1 C–E) to the meiotic spindle and its associated chromosomes was diminished in TAp73−/− oocytes resuming meiosis I in vitro as well as in ovulated oocytes arrested in prophase of meiosis II (Fig. 1 A and C). Shortly after germinal vesicle breakdown, Bub1 localized to chromosomes of WT oocytes and tubulin assembly was evident (Fig. 1 B). Both these events were delayed in TAp73-deficient oocytes. Whereas Bub1 failed to associate with chromosomes and remained cytoplasmic in a subset of ovulated oocytes—particularly those with scattered chromosomes (Fig. 1 A)—BubR1 formed distinct foci around kinetochores, which appeared diffused in TAp73-deficient cells. Quantification of BubR1 protein levels in whole oocytes or in the metaphase plate area alone revealed that whole TAp73−/− oocytes showed an overall increase in BubR1 protein (Fig. 1 D), but the amount of BubR1 protein specifically located within the metaphase plate was decreased in the mutant oocytes (Fig. 1 E). Immunostaining to detect BubR1 in human cells treated with control or TAp73 siRNA [Fig. 1 F and supporting information (SI) Fig. S1] resulted in similarly diffused and disorganized distribution of BubR1 in the absence of TAp73. These observations indicate that, in the absence of TAp73, kinetochore scaffold formed by Bub1 is either absent or altered, causing insufficient tethering of BubR1 to the kinetochore complex.
Fig. 1.
TAp73 deficiency causes mis-localization of SAC components. (A and B) Mis-localization of Bub1 in ovulated oocytes (A) and 3 h after initiation of in vitro maturation (B). Blue staining (arrows), chromosomes; green staining (arrowheads), Bub1 staining; red staining, tubulin. (Magnification: 100 × 10.) (C) Decreased expression of BubR1. Ovulated oocytes from TAp73+/+ and TAp73−/− mice were immunostained for tubulin (green), DNA (blue), and BubR1 (red). (Magnification: 40 × 10; Insets, 100 × 10.) Arrows indicate specific localization of BubR1. (D) Increased total BubR1 protein. Total BubR1 and tubulin protein levels in TAp73+/+ and TAp73−/− MII oocytes were determined by using immunostaining and deconvolution imaging software. Results shown are mean ± SE for the indicated number (n) of oocytes per group (**P < 0.001, Student t test). (E) Decreased BubR1 localization at the metaphase plate area. The DNA concentration/BubR1 protein ratio in TAp73+/+ and TAp73−/− ovulated oocytes was determined by using immunostaining and deconvolution imaging software. Results shown are the mean DNA/BubR1 ratio ± SE for the indicated number (n) of oocytes per group (**P < 0.001, Student t test). (F) Mis-localization of BubR1. HeLa cells treated for 48 h with control siRNA (siCt) or TAp73 siRNA (siTAp73) were exposed for 8 h to 0.6 μM nocodazole and stained with anti-BubR1 and DAPI. Arrows indicate BubR1 staining; arrowheads indicate co-localization (siCt) and non-co-localization (siTAp73) of BubR1 with chromosomes. (Magnification: 63 × 10.)
As in the TAp73 selective knockout, high levels of BubR1 were detected in the nuclei of TAp73-depleted human cells (Fig. 1 F Left, Lower), but BubR1 co-localization to chromosome kinetochores appeared disorganized (Fig. 1 F Right, Lower). Elevated levels of BubR1 protein were also found in TAp73−/− mouse embryo fibroblasts (MEFs) that had been synchronized in culture and treated with the microtubule-disrupting agent nocodazole for 24 h (Fig. S2A), as well as in H1299 human lung carcinoma cells engineered to over-express various TAp73 isoforms (Fig. S2B). Overexpression of the ΔNp73β isoform did not have any impact on Bub1 or BubR1 protein levels (Fig. S2B). Taken together, these results show that an imbalance of p73 isoforms induces a modification in the expression and localization of SAC components such as Bub1 and BubR1.
To determine whether TAp73 plays a direct role in controlling SAC protein localization, we immunostained TAp73+/+ and TAp73−/− oocytes to detect TAp73. In TAp73+/+ ovulated oocytes, TAp73 was present in the cytoplasm and associated specifically with the meiotic spindle at metaphase II (Fig. S3). Immunostaining to detect ΔNp73 with a specific antibody (35) showed that this isoform did not co-localize with either the kinetochore or the spindle (data not shown). These data suggest that TAp73 is required for correct SAC protein expression and localization.
TAp73 Physically Interacts with the SAC Components BubR1 and Bub1.
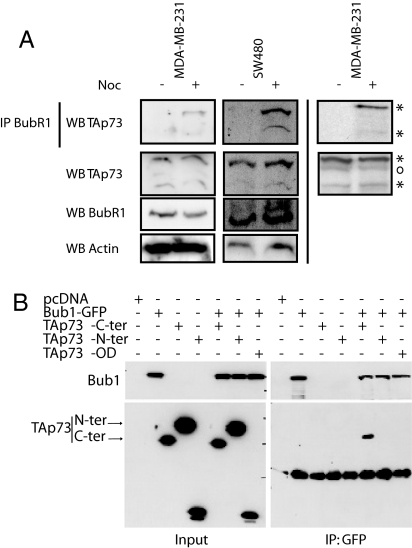
We next investigated whether TAp73 might physically interact with the SAC proteins BubR1 and Bub1. In parallel, we analyzed whether ΔNp73 could disrupt TAp73-SAC protein interaction by competing with TAp73 for SAC protein binding. We carried out co-immunoprecipitation experiments using HeLa cells engineered to over-express isoforms of TAp73 or ΔNp73 and determined whether any of these proteins bound directly to the SAC components BubR1 or Mad2. Only TAp73 (not ΔNp73) was found to bind BubR1 endogenously in two different human cancer cell lines—MDA-MB-231 (breast) and SW480 (colon)—that had been synchronized and treated with nocodazole before immunoprecipitation with anti-BubR1 and blotting with the TAp73-specific antibody (35) H-79 (Fig. 2 A Left, Center) or IMG-246 Imgenex antibody (data not shown). Similarly, when MDA-MB-231 cells were immunoblotted using an anti-p73 antibody that recognizes both TAp73 and ΔNp73, only the binding of endogenous TAp73 to endogenous BubR1 was detected; no interaction between ΔNp73 and BubR1 was observed (Fig. 2 A Right). Using truncated protein mutants, we determined that it was the C-terminal part of TAp73 (which is also present in ΔNp73) that interacts with Bub1 (Fig. 2 B) and Bub3 (Fig. S4A). However, during overexpression, both TAp73 and ΔNp73 interacted in vitro with BubR1 (but not with Mad2; Fig. S4B). We conclude that, in vivo, only TAp73 is able to physically interact with SAC components.
Fig. 2.
Interaction of TAp73 with SAC components. (A) Only TAp73 (not ΔNp73) interacts with BubR1 in vivo. Human cancer cell lines MDA-MB-231 and SW480 were treated (+) or not treated (-) with nocodazole for 16 h and extracts were immunoprecipitated with BubR1 and blotted with either TAp73-specific antibody (Left, Center), or antibody recognizing both TAp73 and ΔNp73 (Right). (B) In vitro interaction of TAp73 with Bub1 determined as in A. OD, oligomerization domain.
TAp73 Is Involved in BubR1 Function.
We hypothesized that the loss of TAp73 in TAp73−/− mice might lead to a weakened SAC response. We therefore analyzed BubR1 activity after the induction of mitotic arrest in the presence or absence of TAp73. BubR1 was originally characterized as a protein kinase that controls the activation of the anaphase-promoting complex by binding and inhibiting p55cdc20 (p55), the major anaphase-promoting complex regulatory protein (36). Recently, activated BubR1 was shown to phosphorylate specific targets, including itself, and to induce mitotic cell death (31, 37). When we examined the effect of TAp73 deficiency on the ability of BubR1 to bind to p55, we found that BubR1-p55 binding was impaired in TAp73−/− MEFs (Fig. 3 A) and HeLa cells treated with TAp73 siRNA (Fig. 3 B). Moreover, the binding of BubR1 with p55 was increased when TAp73 isoforms (mainly the TAp73β isoform) were overexpressed in H1299 cells, with or without nocodazole (Fig. 3 C Left). Conversely, when TAp73 protein was reduced in HeLa cells via siRNA treatment, a decrease in SAC activity was observed, as evidenced by reduced phosphorylation of BubR1 and phospho-histone H3 (Fig. 3 D Upper). Moreover, the increase in p55 level after nocodazole treatment (Fig. 3 D Right) was lost in the absence of TAp73 (Fig. 3 D Left), reflecting the absence of p55 binding with BubR1. Finally, when TAp73 was overexpressed, BubR1-mediated phosphorylation of histone H1 was increased (Fig. 3 E, lanes 1–3). Thus, TAp73 is able to potentiate BubR1 activity, and TAp73 deficiency compromises BubR1 functions. Moreover, although overexpressed ΔNp73 increased BubR1/p55 binding to some extent, no modification of BubR1 kinase activity was observed after ΔNp73 (or p53) overexpression (Fig. 3 E, lanes 4–5). These data indicate that TAp73, and not ΔNp73, plays a role in the SAC response in vivo.
Fig. 3.
TAp73 binding modifies BubR1 activity. TAp73 depletion decreases BubR1 activity. TAp73+/+ and TAp73−/− MEFs (A) or HeLa cells treated for 48 h with TAp73 siRNA (B) were treated with nocodazole, and extracts were immunoprecipitated with anti-p55/cdc20 and blotted with anti-BubR1 or anti-Mad2. (C) Increase in BubR1 activity induced by TAp73 overexpression. HeLa cells overexpressing TAp73 or ΔNp73 isoforms were treated and immunoprecipitated as in A and B. (D) Decreased TAp73 correlates with reduced SAC activity. Levels of phospho-BubR1 (P) and phospho-histone H3 (p-histh3) (representing SAC activity) were determined by Western blotting of HeLa cells treated with either control (siC) or TAp73 siRNA and subjected to nocodazole for the indicated number of hours. (E) TAp73 potentiates BubR1 kinase activity. HeLa cells overexpressing control, TAp73α, TAp73β, ΔNp73β, or p53 DNA were assayed for BubR1 kinase activity using histone-H1 as the substrate.
Correlation of TAp73, ΔNp73, and hBUBR1 Expression in Human Lung Cancer.
hBUBR1 expression is up-regulated in numerous lung cancer cell lines (38), and mitotic checkpoint defects are a feature of human lung cancers exhibiting chromosomal instability (39). When we assessed the expression of TAp73, ΔNp73, and hBUBR1 in matched normal and tumoral lung tissue samples from 18 patients with lung cancer, we found that TAp73 down-regulation, ΔNp73 up-regulation, a decreased TAp73/ΔNp73 ratio, and hBUBR1 up-regulation were all correlated in these samples (Fig. 4 A; Pearson score, P = 0.001). The large increases in hBUBR1 mRNA levels seen in this experiment were consistent with the increased BubR1 protein observed in TAp73−/− oocytes and MEFs. However, these increases in BubR1 protein were not associated with increased BubR1 activity. Instead, the increase in total hBUBR1 protein may be a hallmark of the loss of hBUBR1 activity, which may be associated in some cases with protein mis-localization. These results suggest that a decrease in the TAp73/ΔNp73 ratio indicates a weakened SAC response more accurately than the determination of TAp73 or ΔNp73 expression alone.
Fig. 4.
Correlation of TAp73, ΔNp73, and BubR1 expression in human lung cancer. Correlation of expression levels of TAp73, ΔNp73, and hBubR1 in human lung cancers. (Top) Orange bars indicate that the expression of the indicated mRNA was decreased in the tumor sample compared with the paired normal tissue. Green bars indicate that the expression of the indicated mRNA was increased in the tumor sample compared with the paired normal tissue. Pearson R, P = 0.001.
Discussion
Here we show that a p53 family member, TAp73, has a role in regulating SAC functions during meiosis and mitosis. As previously described, TAp73-deficient mice are sterile and TAp73−/− oocytes show defects in spindle organization that lead to impaired early embryogenesis. Moreover, TAp73−/− mice develop spontaneous tumors, particularly lung adenocarcinomas, and are more sensitive to chemical carcinogens. The phenotype of TAp73-deficent mice and cells suggested that TAp73 could be involved in the formation or maintenance of the proper mitotic and meiotic spindles required for chromosome alignment and genomic stability. In the absence of TAp73, MEFs and human cells have a reduced ability to initiate and maintain proper mitotic arrest as a result of insufficient BubR1 and Bub1-mediated SAC functions, leading to genomic instability. The influence of TAp73 on the SAC response may underlie the tumor suppressor activity of TAp73 and account for the infertility and the spontaneous malignancies observed in TAp73−/− mice. Fig. S5 illustrates how abrogation of TAp73-mediated regulation of the SAC might lead to infertility and tumorigenesis.
The precise molecular mechanisms through which SAC defects might result in tumorigenesis is subject to intense investigation. Alterations in genes encoding mitotic regulators (40) are frequently associated in human tumors with a chromosome instability (CIN) “signature” (34). Conversely, 29 of the 70 genes identified in a cancer CIN signature function as mitotic regulators (41). Gene-targeted mice lacking mitotic regulators readily develop tumors (42), and mice heterozygous for a deficiency of a mitotic regulator show enhanced sensitivity to induced tumorigenesis (12). Thus, subtle changes in expression levels of specific mitotic regulators may have important consequences for genomic stability and tumorigenicity (43). Our findings support this hypothesis by demonstrating that TAp73 is one of these regulators whose loss leads to a weakened SAC response.
Our results are also consistent with studies showing that premature exit from mitosis occurs when essential mitotic checkpoint regulators are deleted, non-functional, or only partially functional (44). Indeed, cells from mice with a hypomorphic BubR1 mutation display increased aneuploidy and infertility (9). BubR1 has been implicated in the control of p53 functions (31), and p73 is necessary for the mitotic death elicited by Bub1 deregulation (45). The interaction between Bub1 and p73 was not investigated in the latter study but our work provides direct evidence of a molecular link between p73 and Bub1. The impact of aberrant chromosomal segregation and aneuploidy on tumorigenesis is slowly becoming better understood (40, 46 –48), and our data have shed more light on this issue by linking TAp73 to SAC components.
Our findings provide a potential molecular mechanism for the association, at least in some tumors, of tumorigenicity and chemo-resistance with TAp73 loss of function and a reduced TAp73/ΔNp73 ratio. A relative lack of TAp73 not only predisposes a cell to aneuploidy and a CIN phenotype, but also compromises the ability of a tumor cell to die, either spontaneously or in response to chemotherapy. Although a relative TAp73 loss is not necessarily the only mechanism responsible for tumorigenesis in this context, it would be interesting to evaluate the karyotypes and chemo-sensitivity of tumor cells in which the normal TAp73/ΔNp73 balance is lost.
Materials and Methods
Immunohistochemical Analysis of Oocyte Sections.
Female mice (3–7 weeks of age) were superovulated, and germinal vesicle or ovulated oocytes were collected as previously described (49). Oocyte fixation and immunocytochemistry were performed as previously described (50). Samples were viewed on a deconvolution fluorescence microscope. Oocytes were serially scanned and 10 optical sections each were analyzed using DeltaVision software (Applied Precision) as previously described (51).
Statistical Analysis.
Data were analyzed by Student t test or Pearson test. For Student t test, data are presented as mean ± SD, and P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments.
The authors gratefully acknowledge D. Barcaroli and V. De Laurenzi for technical support and M. Saunders and R.A. Knight for scientific editing. This work was supported by grants from AIRC, EU (Epistem), FIRB, Alleanza Contro Cancro, MIUR, and MinSan (to G.M.), and Canadian Institute of Health Research (to T.M.). R.T. was supported by l'Association pour la Recherche contre le Cancer. R.T and K.T were supported by fellowships from CIHR. A.J. was supported by the Canada Research Chair Program and by CIHR grant MOP84328.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812096106/DCSupplemental.
References
- 1.Yuen KW, Montpetit B, Hieter P. The kinetochore and cancer: what's the connection? Curr Opin Cell Biol. 2005;17:576–582. doi: 10.1016/j.ceb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 3.Thomas NS, et al. Maternal sex chromosome non-disjunction: evidence for X chromosome-specific risk factors. Hum Mol Genet. 2001;10:243–250. doi: 10.1093/hmg/10.3.243. [DOI] [PubMed] [Google Scholar]
- 4.Gardner RD, Burke DJ. The spindle checkpoint: two transitions, two pathways. Trends Cell Biol. 2000;10:154–158. doi: 10.1016/s0962-8924(00)01727-x. [DOI] [PubMed] [Google Scholar]
- 5.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SS, Scott MI, Holland AJ. The spindle checkpoint: a quality control mechanism which ensures accurate chromosome segregation. Chromosome Res. 2004;12:599–616. doi: 10.1023/B:CHRO.0000036610.78380.51. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, et al. Frequent mutations of human Mad2, but not Bub1, in gastric cancers cause defective mitotic spindle checkpoint. Mutat Res. 2005;578:187–201. doi: 10.1016/j.mrfmmm.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Hanks S, et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat Genet. 2004;36:1159–1161. doi: 10.1038/ng1449. [DOI] [PubMed] [Google Scholar]
- 9.Baker DJ, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 10.Babu JR, et al. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J Cell Biol. 2003;160:341–353. doi: 10.1083/jcb.200211048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai W, et al. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 2004;64:440–445. doi: 10.1158/0008-5472.can-03-3119. [DOI] [PubMed] [Google Scholar]
- 12.Michel LS, et al. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- 13.Lee H. Impaired phosphorylation and mis-localization of Bub1 and BubR1 are responsible for the defective mitotic checkpoint function in Brca2-mutant thymic lymphomas. Exp Mol Med. 2003;35:448–453. doi: 10.1038/emm.2003.58. [DOI] [PubMed] [Google Scholar]
- 14.Bohers E, et al. Gradual reduction of BUBR1 protein levels results in premature sister-chromatid separation then in aneuploidy. Hum Genet. 2008 doi: 10.1007/s00439-008-0572-y. in press. [DOI] [PubMed] [Google Scholar]
- 15.Privette LM, Weier JF, Nguyen HN, Yu X, Petty EM. Loss of CHFR in human mammary epithelial cells causes genomic instability by disrupting the mitotic spindle assembly checkpoint. Neoplasia. 2008;10:643–652. doi: 10.1593/neo.08176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haruta M, et al. Combined BubR1 protein down-regulation and RASSF1A hypermethylation in Wilms tumors with diverse cytogenetic changes. Mol Carcinog. 2008;47:660–666. doi: 10.1002/mc.20412. [DOI] [PubMed] [Google Scholar]
- 17.Burds AA, Lutum AS, Sorger PK. Generating chromosome instability through the simultaneous deletion of Mad2 and p53. Proc Natl Acad Sci USA. 2005;102:11296–11301. doi: 10.1073/pnas.0505053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eshleman JR, et al. Chromosome number and structure both are markedly stable in RER colorectal cancers and are not destabilized by mutation of p53. Oncogene. 1998;17:719–725. doi: 10.1038/sj.onc.1201986. [DOI] [PubMed] [Google Scholar]
- 19.Baker SJ, et al. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990;50:7717–7722. [PubMed] [Google Scholar]
- 20.Finlan LE, Hupp TR. p63: the phantom of the tumor suppressor. Cell Cycle. 2007;6:1062–1071. doi: 10.4161/cc.6.9.4162. [DOI] [PubMed] [Google Scholar]
- 21.Ozaki T, Nakagawara A. p73, a sophisticated p53 family member in the cancer world. Cancer Sci. 2005;96:729–737. doi: 10.1111/j.1349-7006.2005.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourdon JC, et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang A, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 24.Moll UM, Slade N. p63 and p73: roles in development and tumor formation. Mol Cancer Res. 2004;2:371–386. [PubMed] [Google Scholar]
- 25.Melino G, Lu X, Gasco M, Crook T, Knight RA. Functional regulation of p73 and p63: development and cancer. Trends Biochem Sci. 2003;28:663–670. doi: 10.1016/j.tibs.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Melino G. p73, the “assistant” guardian of the genome? Ann N Y Acad Sci. 2003;1010:9–15. doi: 10.1196/annals.1299.002. [DOI] [PubMed] [Google Scholar]
- 27.Oswald C, Stiewe T. In good times and bad: p73 in cancer. Cell Cycle. 2008;7:1726–1731. doi: 10.4161/cc.7.12.6148. [DOI] [PubMed] [Google Scholar]
- 28.Tomasini R, et al. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev. 2008;22:2677–2691. doi: 10.1101/gad.1695308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbluth JM, Pietenpol JA. The jury is in: p73 is a tumor suppressor after all. Genes Dev. 2008;22:2591–2595. doi: 10.1101/gad.1727408. [DOI] [PubMed] [Google Scholar]
- 30.Tomasini R, Mak TW, Melino G. The impact of p53 and p73 on aneuploidy and cancer. Trends Cell Biol. 2008;18:244–252. doi: 10.1016/j.tcb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Ha GH, et al. p53 activation in response to mitotic spindle damage requires signaling via BubR1-mediated phosphorylation. Cancer Res. 2007;67:7155–7164. doi: 10.1158/0008-5472.CAN-06-3392. [DOI] [PubMed] [Google Scholar]
- 32.Oikawa T, et al. Transcriptional control of BubR1 by p53 and suppression of centrosome amplification by BubR1. Mol Cell Biol. 2005;25:4046–4061. doi: 10.1128/MCB.25.10.4046-4061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talos F, Nemajerova A, Flores ER, Petrenko O, Moll UM. p73 suppresses polyploidy and aneuploidy in the absence of functional p53. Mol Cell Biol. 2007;27:647–659. doi: 10.1016/j.molcel.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 34.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 35.Sayan AE, et al. New antibodies recognizing p73: comparison with commercial antibodies. Biochem Biophys Res Commun. 2005;330:186–193. doi: 10.1016/j.bbrc.2005.02.145. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Lees E. Identification of an overlapping binding domain on Cdc20 for Mad2 and anaphase-promoting complex: model for spindle checkpoint regulation. Mol Cel Biol. 2001;21:5190–5199. doi: 10.1128/MCB.21.15.5190-5199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong OK, Fang G. Cdk1 phosphorylation of BubR1 controls spindle checkpoint arrest and Plk1-mediated formation of the 3F3/2 epitope. J Cell Biol. 2007;179:611–617. doi: 10.1083/jcb.200708044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seike M, et al. The promoter region of the human BUBR1 gene and its expression analysis in lung cancer. Lung Cancer. 2002;38:229–234. doi: 10.1016/s0169-5002(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 39.Haruki N, et al. Molecular analysis of the mitotic checkpoint genes BUB1, BUBR1 and BUB3 in human lung cancers. Cancer Lett. 2001;162:201–205. doi: 10.1016/s0304-3835(00)00675-3. [DOI] [PubMed] [Google Scholar]
- 40.Perez de Castro I, de Carcer G, Malumbres M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis. 2007;28:899–912. doi: 10.1093/carcin/bgm019. [DOI] [PubMed] [Google Scholar]
- 41.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38:1043–1048. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 42.Baker DJ, Chen J, van Deursen JM. The mitotic checkpoint in cancer and aging: what have mice taught us? Curr Opin Cell Biol. 2005;17:583–589. doi: 10.1016/j.ceb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Malmanche N, Maia A, Sunkel CE. The spindle assembly checkpoint: preventing chromosome mis-segregation during mitosis and meiosis. FEBS Lett. 2006;580:2888–2895. doi: 10.1016/j.febslet.2006.03.081. [DOI] [PubMed] [Google Scholar]
- 44.Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Niikura Y, Dixit A, Scott R, Perkins G, Kitagawa K. BUB1 mediation of caspase-independent mitotic death determines cell fate. J Cell Biol. 2007;178:283–296. doi: 10.1083/jcb.200702134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Weaver BA, Cleveland DW. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer Res. 2007;67:10103–10105. doi: 10.1158/0008-5472.CAN-07-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2007;11:25–36. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Perez GI, et al. A central role for ceramide in the age-related acceleration of apoptosis in the female germline. FASEB J. 2005;19:860–862. doi: 10.1096/fj.04-2903fje. [DOI] [PubMed] [Google Scholar]
- 50.Yao LJ, et al. Aurora-A is a critical regulator of microtubule assembly and nuclear activity in mouse oocytes, fertilized eggs, and early embryos. Biol Reprod. 2004;70:1392–1399. doi: 10.1095/biolreprod.103.025155. [DOI] [PubMed] [Google Scholar]
- 51.Acton BM, Jurisicova A, Jurisica I, Casper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod. 2004;10:23–32. doi: 10.1093/molehr/gah004. [DOI] [PubMed] [Google Scholar]