Abstract
Relatively little is known about the long-term neurobiological sequelae of chronic stress, which predisposes susceptible patients to neuropsychiatric conditions affecting the prefrontal cortex (PFC). Animal models and human neuroimaging experiments provide complementary insights, yet efforts to integrate the two are often complicated by limitations inherent in drawing comparisons between unrelated studies with disparate designs. Translating from a rodent model of chronic stress where we have shown reversible disruption of PFC function, we show that psychosocial stress induces long-lasting but reversible impairments in behavioral and functional magnetic resonance imaging (fMRI) measures of PFC function in humans. Twenty healthy adults, exposed to 1 month of psychosocial stress, confirmed by a validated rating scale, were scanned while performing a PFC-dependent attention-shifting task. One month later, they returned for a second scanning session after a period of reduced stress, and their performance was compared with a twice-scanned, matched group of low-stress controls. Psychosocial stress selectively impaired attentional control and disrupted functional connectivity within a frontoparietal network that mediates attention shifts. These effects were reversible: after one month of reduced stress, the same subjects showed no significant differences from controls. These results highlight the plasticity of PFC networks in healthy human subjects and suggest one mechanism by which disrupted plasticity may contribute to cognitive impairments characteristic of stress-related neuropsychiatric conditions in susceptible individuals.
Keywords: chronic stress, dorsolateral prefrontal cortex, functional connectivity analysis, perceived stress scale
Chronic stress is a well-known risk factor for several major neuropsychiatric conditions that affect the prefrontal cortex (PFC), including depression, bipolar disorder, schizophrenia, and anxiety disorders (1–7). In healthy subjects, it disrupts creativity, flexible problem solving, working memory, and other PFC-dependent processes (8–10). Efforts to investigate the neurobiological basis of these associations are hampered by our limited understanding of the long-term effects of stress on the PFC. A variety of external stress treatments and monoaminergic pharmacologic manipulations have been shown to alter PFC function acutely in rats, monkeys, and human subjects, acting on a timescale of seconds to minutes to increase monoaminergic tone above optimal levels (8, 9). In contrast, relatively little is known about how naturalistic, chronic psychosocial stressors affect PFC function in the long term.
Functional neuroimaging studies are a powerful tool for assessing PFC function in human subjects, but those studies may be mechanistically less informative in the absence of results from animal models that can be used to constrain hypotheses and data interpretation. Recent studies in rats have begun to address this issue. They show that 21 days of repeated restraint stress reduce dendritic arborization and spine density in the medial prefrontal cortex (mPFC), decreasing axospinous inputs to pyramidal cells by as much as 33% (11–13). These structural changes have significant functional consequences: chronic stress selectively impairs attentional set-shifting, which depends on mPFC integrity, but not reversal learning, a cognitive function of comparable difficulty that is independent of the mPFC (14–16). Importantly, chronic stress effects on the rodent mPFC are reversible 4 weeks after cessation of the stressor, a finding that highlights both the plasticity and the resilience of mPFC pyramidal cells (17).
This study was designed to assess whether comparable changes are detectable in human subjects performing an attentional set-shifting task designed to capture key features of the rodent paradigm, while functional magnetic resonance imaging (fMRI) gauged PFC integrity. Twenty healthy young adults were tested after 4 weeks of psychosocial stress exposure as they prepared for a major academic examination, and their performance was compared with 20 control subjects matched for age, gender, and occupation. Stress exposure was confirmed and quantified using the 10-item Cohen perceived stress scale (PSS), a well-validated questionnaire that gauges chronic stress on a 40-point scale (18, 19) and has been used successfully in related work (20, 21). Finally, the same subjects returned after 4 weeks of reduced stress and were reassessed relative to matched controls with equal task experience, thus yielding an assessment of the reversibility of stress effects on PFC function while controlling for unidentified group differences, selection biases, or other confounding variables.
Results
Chronic Psychosocial Stress Selectively Impaired Attention Shifts.
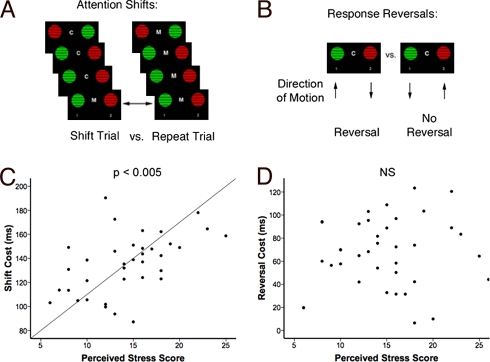
All subjects were trained and tested on a visual discrimination task that yielded dissociable measures of attention shifts and response reversals. The task design and validity are described in detail elsewhere [see supporting information (SI) and ref. 22]. Briefly, subjects viewed two circular square-wave gratings on each trial. The gratings were either red or green and moved either up or down, and the stimuli varied independently along these two dimensions. A centrally located cue (“M” or “C”) instructed subjects to respond to either the motion of the stimuli (select the upward-moving grating) or their color (select the red grating) while ignoring the other dimension. Attention shifts were assessed by contrasting performance on shift trials—defined as those preceded by 2–5 trials of the opposite dimension—with repeat trials, which were preceded by 2–5 trials of the same dimension (Fig. 1A). Response reversals were assessed by contrasting shift trials that required a reversal of the prepotent response learned in the previous block of repeats with those that did not (Fig. 1B). Previous work showed that response reversals are comparably difficult but are mediated by a network independent of the prefrontal areas that mediate attention shifts (22), in analogy to the task paradigm used in the rodent model (15).
Fig. 1.
Chronic psychosocial stress selectively impaired attention shifting. (A) Attention-shift paradigm. Subjects viewed two moving, circular square-wave gratings on each trial and were cued to respond on the basis of either the color (“C”) or the motion (“M”) of the stimuli. Attention shifting was assessed by contrasting shift trials—defined as those preceded by 2–5 trials of the opposite dimension—with repeat trials, which were preceded by 2–5 trials of the same dimension but were otherwise identical. (B) Reversal learning paradigm. On some shift trials (“reversals”), the target response for the color dimension (red) was paired with the nontarget for the motion dimension (down), so the subject was required to override the response learned in the previous block of repeats. On others, the target response was the same in both dimensions. Response reversals were assessed by contrasting shift trials that required a reversal of the prepotent response learned in the previous block of repeats with those that did not. (C) Psychosocial stress impaired attention shifts. Across subjects, PSS scores predicted larger attention shift costs (r = 0.51, P = 0.002). (D) Stress effects on attention shifts were specific: PSS scores were not associated with reversal costs (r = 0.10, P = 0.56).
Consistent with previous work (22), shift trials were slower than repeat trials (t = 33.23, P < 0.001). This attention-shifting cost [mean shift response time (RT) − mean repeat RT] was elevated in chronically stressed subjects, (t = 2.10, P = 0.04), who reported significantly higher PSS scores (t = 4.51, P < 0.001). Across subjects in both groups, individual PSS scores predicted impairments in attention shifts (Fig. 1C: r = 0.51, P = 0.002) but not in response reversals (Fig. 1D: r = 0.10, P = 0.56). This effect did not reflect a general impairment in speed of processing, since repeat trial reaction time was not significantly elevated in stressed subjects (t = 1.59, P = 0.12). Moreover, response reversal performance in the two groups was equivalent (t = 0.64, P = 0.53), indicating that stress selectively disrupted attention shifts and not other processes of comparable difficulty. The association between stress and attention shifting was not confounded by sleep habits. Subjects in the two groups reported sleeping for an equivalent length of time during the night preceding the testing session (t = 0.86, P = 0.40), and across both groups there was no correlation between sleep and attention-shifting costs (r = 0.12, P = 0.46).
Chronic Psychosocial Stress Disrupted Prefrontal Functional Connectivity.
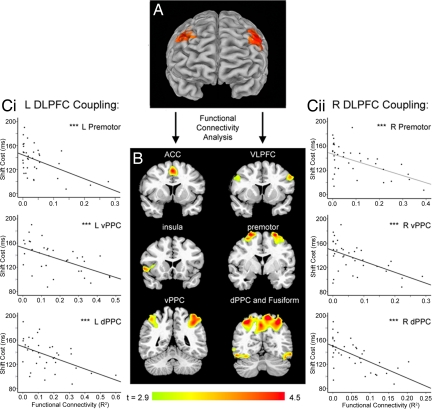
Functional imaging data confirmed that attention shifts engaged a frontoparietal network that included dorsolateral PFC (DLPFC) (P < 0.05, corrected; Fig. 2A), a putative homolog of the rodent dorsal mPFC (23). Studies in rats suggest that attention-shifting impairments may be due in part to chronic stress-related decreases in axospinous inputs to the apical dendrites of prefrontal layer II/III pyramidal cells in this area (15). These dendrites are the target of long-range corticocortical projections and are assumed to play an important computational role in cognitive functions mediated by a distributed network of structures (24). Accordingly, we reasoned that if chronic stress reduces long-range corticocortical axospinous input to the PFC by reducing dendritic arborization, then it may also disrupt fMRI measures of corticocortical connectivity.
Fig. 2.
Flexible attentional control depends on the integrity of a frontoparietal network that includes DLPFC. (A) Attention shifts engaged DLPFC bilaterally (P < 0.05, corrected). (B) The areas depicted in A served as seed volumes for a functional connectivity analysis that quantified coupling between DLPFC and other areas of a frontoparietal network that was active during attention shifts, including anterior cingulate (ACC), ventrolateral prefrontal (VLPFC), insula, premotor, ventral and dorsal areas of the posterior parietal cortex (PPC), and occipitotemporal visual areas including the fusiform cortex (P < 0.05, corrected). (C) Attention-shift performance depended on the integrity of this network. Decreased functional connectivity between left (i) and right (ii) DLPFC and areas of posterior parietal and premotor cortex was correlated with impaired attention shifting, independent of stress effects (P < 0.05, corrected). Scatterplots depict results for peak voxels in each cluster. See SI for details. ***, P < 0.005.
To test this hypothesis, we used functional connectivity analysis to assess whether stress exposure modulated DLPFC coupling with other areas of the attention network (see refs. 25 and 26 and SI for details). This analysis quantified coupling between DLPFC and other areas of a frontoparietal antentional network, including anterior cingulate, ventrolateral prefrontal, insula, premotor, posterior parietal, and occipitotemporal visual areas (Fig. 2B), while controlling for variation in the magnitude of task-related activity. To assess whether attention-shifting performance depends on the integrity of this network, we performed a multivariate linear regression of shift cost on measures of prefrontal connectivity, while controlling for stress (PSS scores) as a covariate. Decreased functional coupling between DLPFC and areas of premotor and posterior parietal cortex was associated with greater impairments in attention shifting (Fig. 2C). This finding shows that effective attention shifting depends in part on the integrity of the frontoparietal network shown in Fig. 2B, suggesting that disrupted connectivity may impair attention shifts.
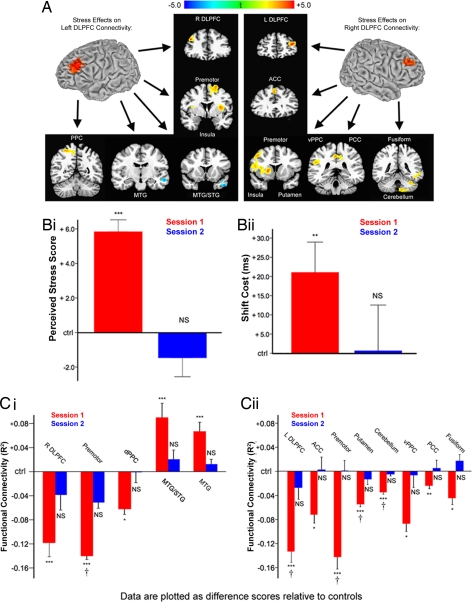
Next, we used multifactorial ANOVA to assess whether psychosocial stress may cause disruptions of this type (Fig. 3A). Chronically stressed subjects showed a relative decoupling of left DLPFC with areas of right DLPFC, left premotor, bilateral ventral PFC, and left posterior parietal cortex, whereas stress increased coupling with temporal lobe areas devoted to visual processing. Analysis of right DLPFC coupling showed decreased connectivity with left DLPFC, right ventral PFC, striatum, right premotor cortex, cingulate cortex, left fusiform cortex, and left cerebellum. Together, these results support the hypothesis that attention shifting depends in part on the integrity of a frontoparietal network that includes DLPFC and that stress-related impairments in DLPFC connectivity may contribute to a decline in flexible attentional control. (See SI for further analytical and statistical details.)
Fig. 3.
Chronic stress reversibly disrupted DLPFC functional connectivity. (A) Psychosocial stress exposure in the month preceding the first scanning session was associated with altered functional connectivity in left and right DLPFC. (Left) Stress decreased coupling between left DLPFC and right DLPFC, premotor, ventral PFC (insula), and posterior parietal cortex (PPC) relative to controls. Stress increased coupling with middle temporal lobe areas. (Right) Psychosocial stress decreased coupling between right DLPFC and left DLPFC, anterior cingulate (ACC), premotor, ventral PFC (insula), putamen, ventral PPC, posterior cingulate (PCC), fusiform cortex, and cerebellum. Maps represent post hoc t-tests of the stress effect. (B) Stress-exposed subjects were retested after 1 month of reduced stress and showed no differences from control subjects on PSS scores (i: t = 0.88, P = 0.39) or attention-shift costs (ii: t = 0.05, P = 0.96). (C) Stress effects on left (i) and right (ii) DLPFC functional connectivity reversed after 1 month of reduced stress for all areas tested except ventrolateral PFC. This reversal was confirmed by a significant stress-by-session interaction for all areas within a search volume that included voxels showing a significant main effect of stress overall. Other areas showed a comparable trend: stressed subjects showed altered connectivity in session 1 (red bars) but not in session 2 (blue bars). Data are plotted relative to mean values in low-stress control subjects. Error bars, SEM; NS, not significant; †, interaction significant at P < 0.05; t-tests, *, P < 0.05; **, P < 0.01; ***, P < 0.005.
Stress Effects on Attention and Prefrontal Connectivity Were Reversible.
In combination with data from rodent models, the data suggest that chronic psychosocial stress effects on attention shifting may be related to alterations in functional properties of the PFC, which may in turn reflect disruptions in dendritic arborization and axospinous input to this region of the type observed in rats. However, those effects may instead reflect other unidentified confounding variables, whereby subjects with impaired prefrontal processing at baseline may also perceive situations and events to be more stressful. Conversely, if psychosocial stress truly does impair PFC function in the long term, it may be clinically useful to know whether these effects are reversible, as they are in rodent models after 1 month of reduced stress (17).
To address these two issues, chronically stressed subjects returned for a second scanning session ≈4 weeks after cessation of the stressor (1 month after their examination date). They were retested on the perceived stress scale to confirm a reduction in psychosocial stress during the interim. Stress-exposed subjects who reported persistently elevated PSS scores in session 2, defined as 1 standard deviation above the normative population mean, were excluded. To control for practice effects, we retested an equal number of control subjects, excluding those whose PSS scores on retest exceeded the normative population mean by 1 standard deviation. (See SI for additional details.)
After 1 month of reduced stress, high-stress subjects from the first session showed no significant differences from the constant, low-stress controls on measures of perceived stress or attention shifting (Fig. 3B). Three-factor (stress, session, subject), mixed-effects ANOVA was used to identify regions showing an effect of stress in session 1, but not session 2, confirmed by a significant session-by-stress interaction. The search volume for this ANOVA comprised all areas showing a significant main effect of stress overall such that the selection of voxels to be analyzed was independent of the interaction being tested. This ensured that the reversibility analysis was not biased by the results obtained in session 1. Stress effects on DLPFC functional coupling reversed in all areas included in the search volume except ventral prefrontal cortex (Brodmann Area 13/47), which remained decoupled relative to controls (Fig. 3C). Areas showing smaller effects in session one—including cingulate, posterior parietal, and higher-order visual areas—did not meet search volume criteria so the significance of a reversal interaction could not be confirmed. However, t-tests of connectivity in these areas revealed the same trend: significantly disrupted connectivity in session 1 but not in session 2 (Fig. 3C). These data suggest that chronic psychosocial stress and not some other confounding variable impairs PFC processing and that these impairments are largely reversible. They also suggest that the effects of stress on PFC function observed here reflect a predominantly state-dependent phenomenon, rather than a trait-dependent one.
Discussion
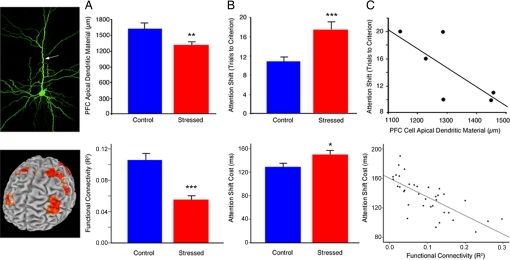
Collectively, our results demonstrate that psychosocial stress induces changes in human PFC function that persist in the absence of any acute stressor and support the utility of the rodent repeated restraint paradigm for modeling aspects of human PFC plasticity in states of chronic stress. Fig. 4 highlights data from our rodent model and the parallel findings observed here. In both studies, chronic stress disrupted attention shifting (Fig. 4A) and PFC circuitry (Fig. 4B), and measures of PFC integrity predicted attention-shifting performance (Fig. 4C).
Fig. 4.
Stress effects on human PFC function (Bottom) are consistent with those observed in a rodent model of chronic stress (Top). Data from the rodent model are reproduced with permission from ref. 15 (Copyright 2006, The Journal of Neuroscience). (A) Chronic stress disrupted DLPFC functional connectivity in human subjects (t = 5.74, P < 0.001) and reduces apical dendritic arborization in rats (t = 2.83, P = 0.007). Human functional connectivity values represent the group means for peak voxels in each of the affected regions depicted in Fig. 3 A and C. (B) Stress-induced corresponding impairments in attention shifting [humans (Bottom), t = 2.10, P = 0.04; rats (Top), t = 3.51, P = 0.002). (C) Measures of PFC integrity predicted attention-shifting impairments in humans (Bottom) (r = −0.64, P < 0.001) and showed a similar trend in rats (Top) (r = −0.74, P = 0.09). Human functional connectivity values represent the means for peak voxels in each of the 6 regions depicted in the scatterplots in Fig. 2C. Error bars, SEM; *, P < 0.05; **, P < 0.01; ***, P < 0.005.
These findings are informative but should be considered in light of several caveats. First, while rodent studies may be useful for constraining data interpretation in neuroimaging experiments, there are limitations in translating between rodents and human subjects. Most importantly, humans' perception of a naturalistic psychosocial stressor differs qualitatively from a rat's response to repeated restraint in an artificial laboratory environment. Likewise, our data confirm that there is considerable variability within human subjects in the degree to which the same trigger—preparing for an academic examination—is perceived to be stressful. Humans evaluate the significance of a psychosocial stressor, and it is this higher-order neocortical processing of the perceived meaning of a stimulus, in conjunction with activity in the medial and central nuclei of the amygdala, that is thought to activate the hypothalamic-pituitary-adrenal axis and the stress response (27–29). Thus, it is probable that variability in this factor may have important mechanistic consequences, even if the end points in both rats and humans—structural and functional remodeling of PFC networks—share certain characteristics. Additional research would be required to address that issue.
A second caveat concerns the degree to which our findings can be generalized to other human subjects. We opted to study medical students preparing for a major examination because the exposure was perceived to be highly stressful for a period of weeks but also came with a preset limit on its duration, followed by a period of reduced stress, thus permitting a within-subjects control and an assessment of reversibility. One prospective study examined the relation between PSS scores and hippocampal volumes in postmenopausal women and found a reduction in hippocampal gray matter volumes (21), suggesting that stress effects on human brain structure may generalize to other contexts. Still, future studies should replicate these results in other populations under other forms of stress.
A third caveat concerns the benefits and limitations of the PSS scale for quantifying stress exposure. The PSS scale is an extensively validated tool designed to quantify subjects' experience of psychosocial stress and trace changes in stress exposure over time (18, 21), but it does not assess stress-related physiological changes. Other studies have followed diurnal measurements of salivary cortisol for this purpose (30–32). They strongly implicate glucocorticoids in the brain's response to stress, although it is likely that they play a permissive role in a cascade that also involves excitatory amino acids, neurotrophins, adhesion molecules, altered glucocorticoid receptor expression patterns, and neuromodulators like serotonin (33). Accordingly, diurnal cortisol is a complex end point for gauging chronic psychosocial stress, which the PSS assesses directly. (See SI for more detailed discussion.)
Even in light of these limitations, our results are provocative and provide several potentially useful insights. First, they show that chronic, psychosocial stress selectively and reversibly disrupts human PFC function. Second, they demonstrate the utility of translating from animal data to constrain hypotheses in human neuroimaging work and support the validity of the rodent repeated restraint stress model for elucidating mechanistic aspects of the response to chronic stress in humans. Chronic stress disrupted coupling within a frontoparietal attentional network in human subjects in a manner that can be easily understood within the framework of rodent studies showing alterations in dendritic arborization and axospinous inputs, which in turn may disrupt both local oscillatory activity within the PFC and long-range corticocortical connections between the PFC and more distant areas. These changes, in turn, may interfere with top—down regulation of activity by the DLPFC and interfere with functional coupling. The DLPFC, acting in concert with a network of higher-order association areas, is believed to regulate attention directly by biasing processing in occipitotemporal visual cortex, favoring one dimension over another (34, 35). Anterior cingulate and posterior parietal cortex act to detect conflicts in information processing (e.g., color vs. motion processing) and signal to the DLPFC the need for increased top—down control (22). In this view, functional coupling within the network would be important for both regulating DLPFC activity and mediating its effects (24), an interpretation supported by the correlations with attention-shifting performance depicted in Fig. 2C.
Third, they add to a complementary body of literature that has elucidated the mechanisms by which stress alters PFC function acutely. Dopamine and norepinephrine enhance PFC function via D1 and α2 adrenergic receptor stimulation, which in turn enhances functional connectivity within PFC networks by inhibiting cAMP-dependent hyperpolarization (8, 36). In contrast, excessive monoamine release associated with acute uncontrollable stressors has just the opposite effect, impairing PFC-dependent working memory and cognitive flexibility in a manner preventable by pretreatment with pharmacologic antagonists of these neuromodulators (8, 10). Other studies indicate that acute alterations in neuromodulatory systems affect attention shifting as well (37). The results presented here complement these studies by identifying long-term but reversible prefrontal functional impairments in chronically stressed human subjects even in the absence of any acute stress treatment. Stress effects on PFC function may therefore be attributable to both acute changes in monoaminergic tone and longer-term structural changes in dendritic arborization and spine density that perturb functional connectivity (12, 15). Of course, additional work in both rodents and human subjects will be required to confirm an association between structural plasticity in the former and functional connectivity in the latter and to establish a causal relationship between these factors and cognitive impairments.
Finally, our results may prove to be clinically informative. The diagnostic criteria for major depression, generalized anxiety disorder, posttraumatic stress disorder, and other stress-related neuropsychiatric conditions include deficits in the cognitive control of attention (38). Our results show that stress induces comparable deficits in healthy human subjects as well, a finding consistent with previous work linking PSS scores with measures of executive function (20) and hippocampal volume (21) in nonclinical populations. Thus, they highlight the therapeutic potential for stress-reduction interventions by delineating 1 neural mechanism by which chronic stress may cause these cognitive deficits.
In some contexts, stress-induced plasticity may be beneficial. In rodents, for example, it may serve a neuroprotective function by reducing excitatory neurotoxicity in the hippocampus (33). It is also notable that prefrontal connectivity did not decrease uniformly: stress increased DLPFC coupling with temporal lobe areas that contribute to visual processing at the expense of association areas mediating flexibility and control. These differences may be due in part to other unidentified changes in frontoparietal attentional circuitry and to selective pruning of some connections but not others. They are consistent with the view that stress effects on prefrontal connectivity may be adaptive in the short term, to the extent that they bias processing in favor of a single, salient stimulus, in a manner that reverses after a period of reduced stress in healthy individuals. When susceptible individuals are exposed to repeated, chronic stress, by contrast, impairments in PFC-mediated flexibility may persist, counteracting short-term benefits in a way that may ultimately contribute to the diverse symptomatology of chronic stress-related neuropsychiatric diseases (2, 39).
Methods
Subjects.
The remaining 40 subjects (20 stressed and 20 controls matched for age, sex, and sleep habits) participated in an initial scanning session that included 3 components: (1) the Cohen perceived stress scale, (2) attentional control task training, and (3) a fMRI scan while being tested on the same task. These are described below, with additional details available as SI. All subjects were asked to return for a second session, ≈1 month after the first, and retested on the same measures. The experimental procedure was approved by the Weill Cornell Medical College Institutional Review Board, and written informed consent was obtained from all subjects before scanning.
Perceived Stress Scale.
Stress was quantified by self-report at the start of each session using the Cohen PSS, a standardized and reliable measure of an individual's perception of chronic psychosocial stress (18, 19).
Attentional Control Task.
The task was as described above (Fig. 1) and in more detail elsewhere (22). On each trial, subjects responded manually by pressing a button with their right (dominant) hand corresponding to the target stimulus as described in SI. Color and motion trials were counterbalanced for trial type, dimension, and side of target presentation. Before each scanning session, subjects were trained on 3 blocks of 36 trials consisting of color discriminations, motion discriminations, and alternating color/motion discriminations, respectively. In the scanner, subjects completed 6 blocks of 72 trials, which were presented in a jittered task design.
Functional MRI Analysis.
Functional MR images were acquired on a GE 3T scanner using a spiral in-and-out sequence (40) while subjects performed this task. MR images were preprocessed and analyzed using the AFNI software package (http://afni.nimh.nih.gov). This analysis was designed to assess whether psychosocial stress modulated DLPFC functional connectivity and included four steps. First, a general linear model and mixed-effects ANOVA were used to identify areas of DLPFC that were more active during shift trials than during repeat trials. Attention shifting was found to engage DLPFC bilaterally. Second, these two areas—left and right DLPFC (Brodmann Area 8/9)—served as seed volumes for functional connectivity analyses (25, 26) that delineated a frontoparietal network coupled to DLPFC, while controlling for global fluctuations in the MR signal and the magnitude of task-dependent activity. Third, to assess whether functional coupling within this network was important for attention shifting independent of stress effects, we performed a multivariate linear regression of shift cost on measures of prefrontal connectivity, while controlling for stress (PSS scores) as a covariate. Fourth, we examined how functional connectivity varied with stress, using a two-factor mixed-effects ANOVA and voxelwise t-tests of connectivity (R2) in stressed vs. control subjects.
Reversibility Analysis.
To control for confounding variables unrelated to stress and to assess the reversibility of stress effects on PFC function, 15 stress-exposed subjects were rescanned after 1 month of reduced stress. To control for practice effects, we retested an equal number of control subjects. Three-factor (stress, high vs. low; session, 1 vs. 2; and subject), mixed-effects ANOVA, and post hoc t-tests (session 1, high vs. low stress; session 2, high vs. low stress) were used to assess reversibility of stress effects on PSS scores, shift costs, and functional connectivity. Stress-by-session interactions were used to confirm the reversibility of connectivity effects within a search volume that included all areas showing a main effect of stress, averaged over the first and second sessions, so that the analysis region was independent of the interaction tested. This search volume excluded several regions showing smaller effects in session 1. To examine whether these areas followed a similar trend, we performed simple t-tests on the peak voxel in each cluster.
Supplementary Material
Acknowledgments.
This work was supported in part by National Institute of Mental Health Grants P50 MH62196-08 (Project IV) and P50 MH79513-01A1 (to B.J.C.). C.L. was supported by National Institutes of Health Medical Scientist Training Program grant GM 07739, a W. M. Keck Foundation Medical Scientist Fellowship, a Paul and Daisy Soros Fellowship, and a Cornell Department of Psychiatry Perry Prize.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807041106/DCSupplemental.
References
- 1.Bishop S, Duncan J, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 2.Caspi A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 3.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 4.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger DR, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 6.Agid O, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 7.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 8.Arnsten AFT. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- 9.Arnsten AFT, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys—evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 10.Beversdorf DQ, Hughes JD, Steinberg BA, Lewis LD, Heilman KM. Noradrenergic modulation of cognitive flexibility in problem solving. Neuroreport. 1999;10:2763–2767. doi: 10.1097/00001756-199909090-00012. [DOI] [PubMed] [Google Scholar]
- 11.Radley JJ, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Radley JJ, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 13.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 14.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Radley JJ, et al. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 19.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 20.Orem DM, Petrac DC, Bedwell JS. Chronic self-perceived stress and set-shifting performance in undergraduate students. Stress- Int J Biol Stress. 2008;11:73–78. doi: 10.1080/10253890701535103. [DOI] [PubMed] [Google Scholar]
- 21.Gianaros PJ, et al. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 24.Dehaene S, Kerszberg M, Changeux JP. A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci USA. 1998;95:14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pezawas L, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 26.Hare TA, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 28.Joels M, Pu ZW, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 30.Ockenfels MC, et al. Effect of chronic stress associated with unemployment on salivary coltisol—overall cortisol-levels, diurnal rhythm, and acute stress reactivity. Psychosom Med. 1995;57:460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom Med. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- 32.Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 33.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 34.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 35.Desimone R, Duncan J. Neural mechanisms of selective visual-attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 36.Wang M, et al. Alpha 2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 39.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 40.Glover GH, Thomason ME. Improved combination of spiral-in/out images for BOLD fMRI. Magn Reson Med. 2004;51:863–868. doi: 10.1002/mrm.20016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.