Abstract
Purpose: To investigate whether combining pulsed high-intensity focused ultrasound (HIFU) with the chemotherapeutic drug bortezomib could improve antitumor activity against murine squamous cell carcinoma (SCC) tumors.
Materials and Methods: All experiments were conducted with animal care and use committee approval. Murine SCC cells were implanted subcutaneously in C3H mice. When tumors reached 100 mm3, mice were randomized to one of three groups for twice weekly intraperitoneal injections of 1.5 mg of bortezomib per kilogram of body weight, a proteasome inhibitor (n = 10); 1.0 mg/kg bortezomib (n = 11); or a control vehicle (n = 12). Within each group, half of the mice received pulsed HIFU exposure to their tumors immediately prior to each injection. The time for tumors to reach 650 mm3 was compared among groups. Additional tumors were stained with terminal deoxynucledotidyl transferase-mediated dUTP nick end labeling and CD31 to assess apoptotic index and blood vessel density, respectively.
Results: Tumors in the control group, pulsed HIFU and control group, and 1.0 mg/kg of bortezomib alone group reached the size end point in 5.2 days ± 0.8 (standard deviation), 5.3 days ± 0.8, and 5.6 days ± 1.1, respectively. However, pulsed HIFU and 1.0 mg/kg bortezomib increased the time to end point to 9.8 days ± 2.9 (P < .02), not significantly different from the 8.8 days ± 2.1 in tumors treated with 1.5 mg/kg bortezomib alone (P > .05). Combination therapy was also associated with a significantly higher apoptotic index (P < .05).
Conclusion: Treatment of tumors with pulsed HIFU lowered the threshold level for efficacy of bortezomib, resulting in significant tumor cytotoxicity and growth inhibition at lower dose levels.
© RSNA, 2008
Keywords: HIFU = high-intensity focused ultrasound, NF-κB = nuclear factor-kappa B, SCC = squamous cell carcinoma, TUNEL = terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
High-intensity focused ultrasound (HIFU) is emerging clinically as a noninvasive modality, whereby continuous deposition of acoustic energy results in thermal ablation of targeted tissues such as prostate, liver, and breast cancers and uterine fibroids (1). Alternatively, pulsed HIFU uses low duty cycle pulses that minimize heat generation and alter tissue properties by using nonthermal mechanisms to overcome barriers to the delivery of therapeutic molecules. This unique HIFU operation mode has potential applications in gene therapy (2,3), thrombolysis (4,5), and solid tumor therapy, where irregular vessels, elevated interstitial pressure, and dense fibrillar collagen matrices pose formidable drug delivery barriers (6,7). In a murine squamous cell carcinoma (SCC) model (SCC7), exposure of the tumor to pulsed HIFU significantly enhanced growth inhibition of tumors directly injected with tumor necrosis factor-α plasmid deoxyribonucleic acid (8). Pulsed HIFU has also been combined with low temperature–sensitive liposomes to enhance local drug deposition and impair tumor growth (9).
Bortezomib (Millenium Pharmaceuticals, Cambridge, Mass), a potent dipeptidyl boronic acid analogue and 20S proteasome inhibitor, has anticancer efficacy mediated by using downstream effects on a number of targets altered in cancer. Antiproliferative and cytotoxic effects are linked to inhibition of activation of prosurvival transcription factors such as nuclear factor-kappa B (NF-κB) and hypoxia-inducible factor (10,11), and increased activation of cell cycle inhibitory and proapoptotic factors including p21, p27, p53, and jun kinase (12,13). Antiangiogenic effects are associated with inhibition of NF-κB mediated expression of angiogenesis factors such as interleukin-8, growth-related oncogene-α, and vascular endothelial growth factor and induction of apoptosis in endothelium (10,14). In addition to its Food and Drug Administration approval and activity against multiple myeloma (15), bortezomib has induced partial responses alone and in combination with other agents or radiation therapy in some patients with solid malignancies such as head and neck SCC and prostate, lung, and colon cancers (16,17). However, in both preclinical murine studies (10) and human phase I clinical trials of head and neck SCC (17), activity of systemically administered bortezomib has largely been limited to partial responses at maximally tolerated doses and schedules.
This observation of a relatively narrow therapeutic window for bortezomib in the treatment of head and neck SCC, along with the demonstration of pulsed HIFU–enhanced delivery of systemically administered genes (2), proteins (5), and liposomes (9), led to the hypothesis that pulsed HIFU combined with chemotherapeutic drugs could increase intratumoral drug concentration and either enhance antitumor efficacy at existing doses or reduce the side effects from chemotherapy by lowering the threshold level for efficacy and permitting a lower systemic dose. Thus, the purpose of our study was to prospectively investigate whether combining pulsed HIFU with the chemotherapeutic drug bortezomib could improve cytotoxic activity against, and growth inhibition of, murine SCC tumors.
MATERIALS AND METHODS
Millenium Pharmaceuticals donated bortezomib through a materials cooperative research and development agreement with one author (C.V.W.). All authors had full control of the data and information submitted for publication.
Bortezomib Preparation and Storage
The synthesis and purification of bortezomib was described previously by Adams et al (12). For in vitro and in vivo studies, bortezomib was reconstituted in 0.9% of normal saline at a concentration of 2.6 × 10−3 mol/L. Appropriate dilutions in 0.9% of normal saline were performed for each study. Solutions were sterilized by using filtration with a 0.45-μm syringe filter before use and shielded from light and stored at 4°C between uses. Fresh bortezomib solutions were prepared after 5 days in these storage conditions (18).
Assessment of in Vitro Cytotoxicity
SCC7 cells were exposed in vitro to bortezomib similar to Sunwoo et al (10) (J.A.P., with 3 years experience). Equal numbers of cells were seeded in 96-well culture plates in triplicate, incubated for 24 hours, and then either exposed to bortezomib at 10−8 or 10−7 mmol/L, or untreated to serve as controls. At the 4-, 12-, 24-, and 72-hour points, the viable cell count was determined by using a cell counting kit (CCK-8; Alexis Biochemical, San Diego, Calif). At each time, the percentage of cells in the treated wells was calculated as compared with the control wells.
In Vivo Experimental Setup
Animal tumor model.—All animal work was carried out under a National Institutes of Health (NIH) Clinical Center Animal Care and Use Committee–approved protocol, in accordance with NIH guidelines. The SCC7 cell line was derived from a spontaneously arising SCC in a C3H mouse (19) and has well-characterized growth properties (20). The cell line was maintained at 37°C and 5% CO2 in an RPMI 1640 medium, supplemented with 10% fetal bovine serum, l-glutamine, and penicillin/streptomycin . The C3H mice used were between 6 and 8 weeks of age, female, and weighed 20 g or less. For in vivo experiments, a suspension of 1 × 106 SCC7 cells in 0.1 mL of phosphate-buffered saline was injected subcutaneously in the flanks of C3H mice. The flanks were shaved and prepared with isopropyl alcohol prior to inoculations. On average, tumors required 10 days after inoculation to reach 100 mm3 in size.
Pulsed HIFU exposures.—Pulsed HIFU exposures were conducted with a custom-built image-guided HIFU system, modified from an existing device (Sonoblate 500; Focus Surgery, Indianapolis, Ind). The probe was comprised of a therapeutic 1-MHz transducer with a focal length of 4 cm and a colinear single-element 10-MHz imaging transducer. The spherical concave therapeutic transducer had a diameter of 5 cm; the aperture of the imaging transducer was 0.8 cm. The focal zone of the therapeutic transducer was an elongated ellipsoid with an axial length of 7.2 mm (−3 dB) and radial diameter of 1.38 mm (−3 dB).
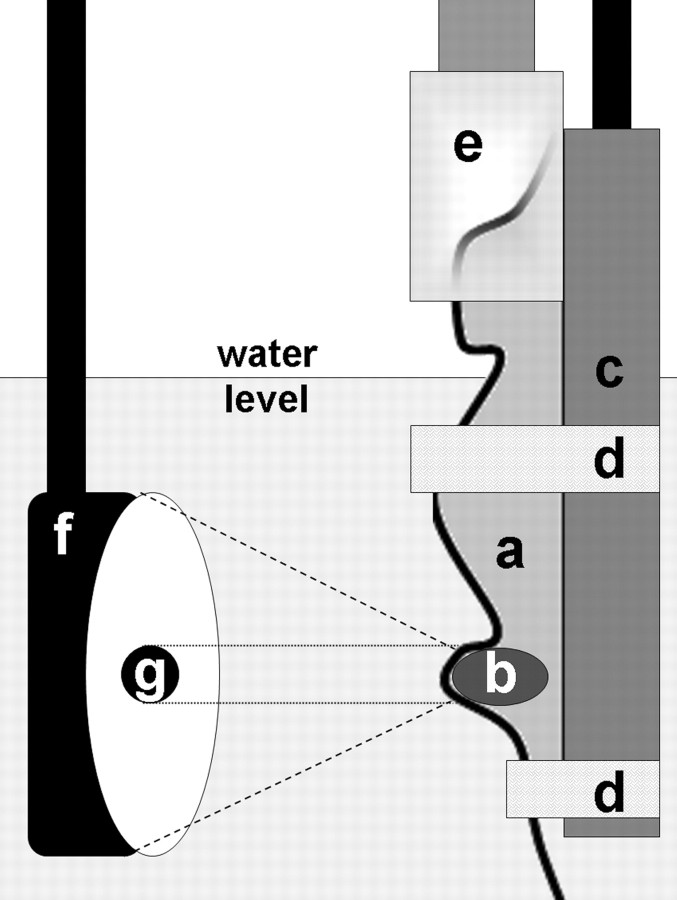
Pulsed HIFU exposures were carried out as previously described (2,21). Briefly, all animals were anesthetized with inhalation of 2% of isoflorane throughout the treatment process. Tumors were covered with ultrasonographic (US) coupling gel to exclude trapped gas bubbles at the skin surface. Individual mice were secured in a custom-built holder that attached to a three-dimensional stage, and inserted upright into a tank of degassed water maintained at body temperature (37°C), with the head of the mice above water. The submerged tumors were imaged in both the x (lateral) and y (vertical) planes with the colinear 10-MHz imaging transducer and positioned symmetrically in the center of the focal zone of the therapeutic transducer. By using the HIFU system graphic user interface, a two-dimensional grid of raster points was drawn over the image of the tumors, with a raster spacing of 2 mm in the x and y dimensions. The system then treated the tumors automatically, moving successively through each raster point. The exposure at each point had the following parameters: spatial average and temporal average intensity, 130 W/cm2; 100 pulses; pulse repetition frequency, 1 Hz; duty cycle, 5% (50 msec on and 950 msec off). With this treatment regimen, a typical exposure for an entire tumor lasted 15–20 minutes. A schematic representation of the setup for carrying out the exposures is provided (Fig 1).
Figure 1:
Schematic of in vivo experimental setup for providing pulsed HIFU exposures. a = mouse, b = subcutaneous flank tumor, c = holder, d = restraining straps, e = anesthesia nose cone, f = concave therapeutic HIFU transducer, g = colinear single-element 10-MHz US imaging transducer.
Gross Tumor Growth
Murine SCC7 cells were inoculated subcutaneously in the right flank of 33 immunocompetent syngeneic C3H mice (Charles River Laboratories, Wilmington, Mass) (J.X., J.A.P., and B.T., with 17, 3, and 3 years experience, respectively). When the tumors reached a size of 100 mm3, mice were individually randomized in one of three bortezomib dose groups to begin treatment: 1.5 mg bortezomib per kilogram of body weight (n = 10); 1.0 mg/kg bortezomib (n = 11); or control (0.0 mg/kg), where mice received normal saline (n = 12). The higher dose of bortezomib (1.5 mg/kg) was shown to be the maximally tolerated dose in mice and partially inhibit tumor growth while the lower dose (1.0 mg/kg) showed lower activity in an SCC model studied previously (10). Bortezomib was administered by using sterile technique intraperitoneal injection two times per week on a Monday/Thursday schedule (B.T. and J.A.P., each with 3 years experience), as given in human clinical trials for head and neck SCC (17). Within each of the three dose groups, one-half of the mice were randomly assigned on the first day of treatment to have their tumors exposed to pulsed HIFU immediately prior to each intraperitoneal injection for the length of the study. The tumors of the remaining mice in each dose group never received pulsed HIFU exposures. Tumor volumes were measured every other day with digital calipers in three orthogonal dimensions, by longest axis length, shortest axis width, and height, by a blinded investigator (J.A.P.). Tumor volumes were calculated as length times width times height. Mice were euthanized when tumors reached a volume of 650 mm3, selected as an end point on the basis of a pilot study and to adhere to animal euthanasia criteria for tumor size. The number of days to reach this end point was noted for each tumor.
Apoptosis and Blood Vessel Density
A second experiment involving a separate group of mice was conducted to procure tumors for immunohistochemical analysis of apoptosis and blood vessel density. Both flanks of 18 C3H mice were inoculated with SCC7 cells (J.X., J.A.P., and B.T.), as mice were sacrificed well before tumors could reach euthanasia size criteria. When both flank tumors reached 100 mm3 in size, mice were randomized to receive either 1.0 mg/kg of bortezomib (n = 9) or a control (n = 9) on the same twice weekly schedule as was used in the tumor growth study, with only one of the flank tumors in each mouse receiving pretreatment with pulsed HIFU prior to each bortezomib injection (V.F. and B.T.). The 1.0-mg/kg bortezomib dose was used to explore the significant growth differences observed in the first in vivo experiment between tumors treated with and without HIFU at this dose level. Mice were euthanized 1, 3, and 6 days after the initial treatment (n = 3 mice per time per dose group), and the extracted tumors were stained with a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay and CD31 (C.T.A. and Z.C., with 2 and 24 years experience, respectively) for determination of apoptotic index and blood vessel density, respectively (J.A.P., C.T.A., and A.C., each with 3 years experience).
Extracted tumors were frozen in a compound (Tissue-Tek OCT; Sakura Finetek, Torrance, Calif) at −80°C immediately following euthanasia. Frozen tumor tissues were sectioned at a thickness of 10 μm at the largest tumor area and placed on silanated glass slides. Apoptosis of tumor cells was assessed by using a TUNEL kit (ApoTag S7100; Chemicon, Temecula, Calif), following the manufacturer protocol with a 4% paraformaldehyde 1× phosphate-buffered saline fixation. By using serial sections of the same tumors stained for apoptosis, blood vessel density was evaluated via mouse CD31 immunostaining by using the standard avidin-biotinylated peroxidase method (Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, Calif). For each tumor section, images of representative high-power fields of at least 100 tumor cells selected in a blinded fashion were photographed with a camera (DP70; Olympus, Center Valley, Pa) and a microscope (BX51; Olympus). The apoptotic index for each tumor was calculated in blinded fashion from three high-power fields of the TUNEL-stained section as the average of the percentage of TUNEL-positive neoplastic cells, defined as brown nuclear or cytoplasmic staining (22). The blood vessel density for each tumor was calculated in a blinded fashion from three high-power fields of the CD31-stained section as the average vessel count per high-power field (10) by using the vessel definition of Seshadri et al (23).
Statistical Analysis
Times to reach end points from the tumor growth study and apoptotic indices and blood vessel densities from the immunohistochemistry studies were compared (all possible pairs) with a Tukey-Kramer significant difference test by using software (JMP; SAS Institute, Cary, NC). For the in vitro cytotoxicity experiment, statistical differences between the treatment and control groups were analyzed at each time with a two-tailed Student t test by using software (JMP; SAS Institute). A P value of less than .05 was considered to indicate a significant difference.
RESULTS
In Vitro Cytotoxicity
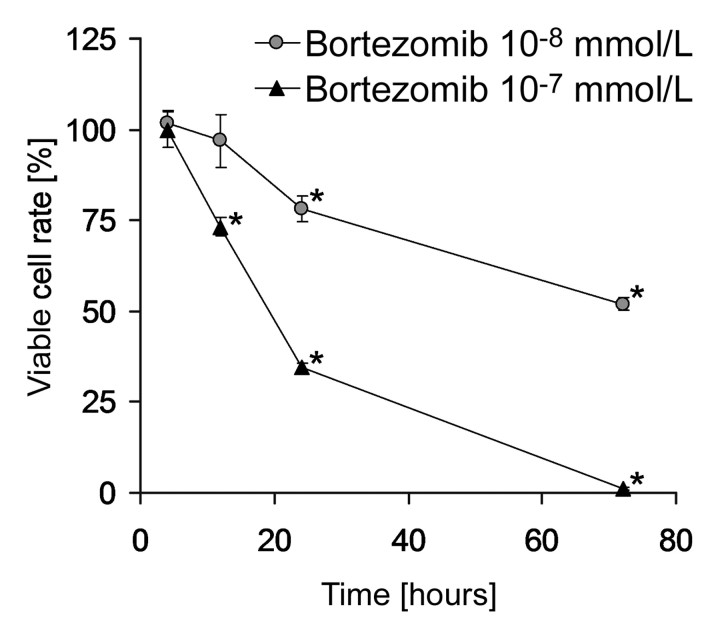
A significant reduction in viable SCC7 cells was detectable by 12 hours at the 10−7 mmol/L bortezomib concentration (P < .001) with a three-fold reduction by 24 hours (Fig 2). At the 10−8 mmol/L bortezomib concentration, reduced viability was apparent by 24 hours (P < .01).
Figure 2:
SCC7 cell line exposed in vitro to bortezomib. Percentages of viable cells as compared with untreated control cells are shown after 4, 12, 24, and 72 hours of bortezomib exposure. Data are mean values and error bars indicate standard error of mean. * = significant cytotoxicity compared with untreated control (P < .01).
Tumor Growth
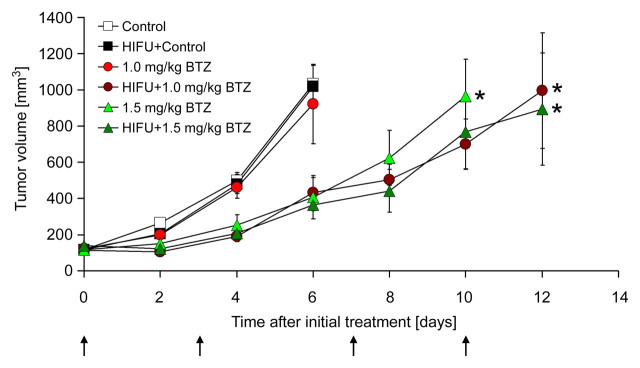
No significant differences in the time to reach the end point size of 650 mm3 were found among tumors treated with control alone, the combination of pulsed HIFU and control, or 1.0 mg/kg of bortezomib alone (5.2 days ± 0.8, 5.3 days ± 0.8, or 5.6 days ± 1.1, respectively). Compared with each of these groups, however, the combination of pulsed HIFU and 1.0 mg/kg of bortezomib significantly slowed tumor growth (9.8 days ± 2.9; P < .005, P < .005, and P < .02, respectively) (Fig 3). Moreover, the growth inhibition resulting from the combination of pulsed HIFU and 1.0 mg/kg of bortezomib was not significantly different than that from 1.5 mg/kg of bortezomib alone (9.8 days ± 2.9 vs 8.8 days ± 2.1, P > .05). At the 1.5-mg/kg dose level, the addition of pulsed HIFU did not further slow tumor growth compared with the drug alone (11.8 days ± 2.5 vs 8.8 days ± 2.1, respectively; P > .05).
Figure 3:
Graph shows effect of twice-weekly treatment with pulsed HIFU and bortezomib on growth of SCC7 xenografts in C3H mice. Data are mean values and error bars indicate standard error of mean. Arrows denote treatment days. BTZ = bortezomib. * = significant difference (P < .05); no significant difference exists between curves with asterisk.
Apoptosis and Blood Vessel Density
The combination of pulsed HIFU and 1.0 mg/kg of bortezomib significantly enhanced apoptosis at 1 day after the initiation of treatment when compared with controls (11.5% ± 3.0 vs 3.1% ± 1.9, P < .05), and again at 3 days after the initiation of treatment when compared with bortezomib alone, the combination of pulsed HIFU and control, or control alone (12.7% ± 3.5, 5.2% ± 1.7, 5.9% ± 0.3, and 5.0% ± 0.9, respectively; P < .05 for all three comparisons) (Fig 4). By 6 days after the initiation of treatment, no differences in apoptosis were detected between treatment groups.
Figure 4:
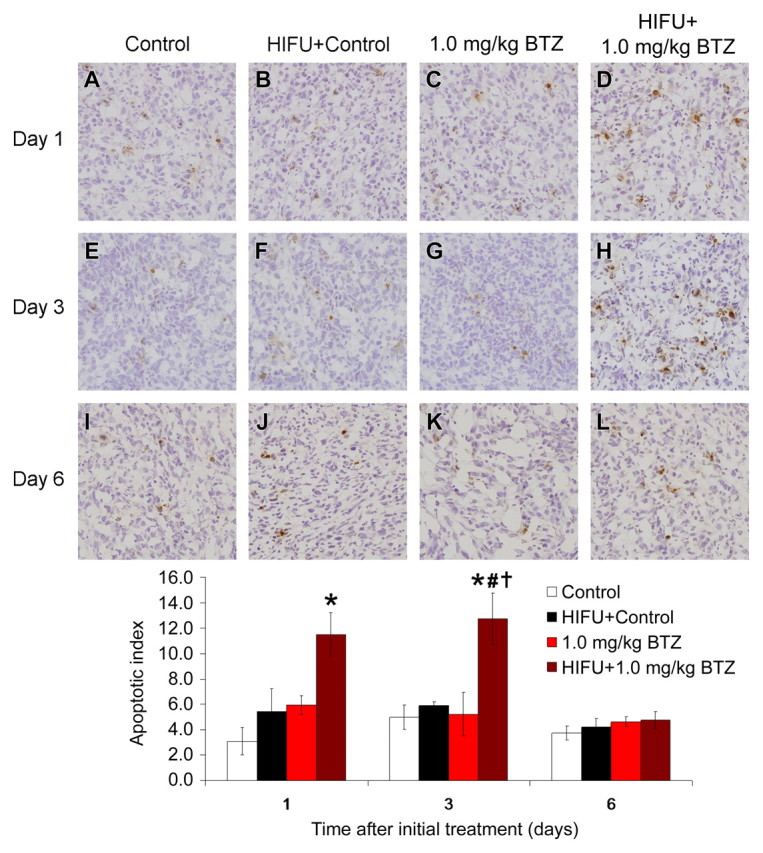
Photomicrographs show effect of pulsed HIFU and 1.0 mg/kg bortezomib (BTZ) treatment regimens on apoptosis in SCC7 xenografts in C3H mice at 1, 3, and 6 days after start of treatment (TUNEL stain; original magnification, ×400). Graph quantitatively shows effects of treatment on apoptosis at each time. Data are mean values and error bars indicate standard error of mean. * = P < .05 vs control, # = P < .05 vs HIFU, † = control, P < .05 vs bortezomib.
The combination of pulsed HIFU and 1.0 mg/kg of bortezomib decreased blood vessel density compared with controls at 1, 3, and 6 days after the initiation of treatment (P < .05, P < .01, and P < .01, respectively), and compared with the combination of pulsed HIFU and control at 3 and 6 days after the initiation of treatment (both P < .01) (Fig 5). Compared with bortezomib alone, however, the combination of pulsed HIFU and bortezomib did not produce a further significant reduction in blood vessel density at any time.
Figure 5:
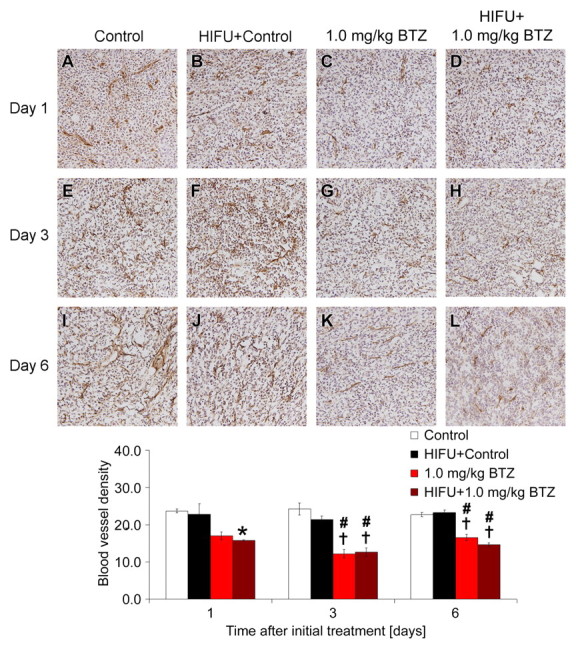
Photomicrographs show effect of pulsed HIFU and 1.0 mg/kg bortezomib (BTZ) treatment regimens on blood vessel density in SCC7 xenografts in C3H mice at 1, 3, and 6 days after start of treatment (CD31 stain; original magnification, ×200). Graph quantitatively shows effects of treatment on blood vessel density at each time. Data are mean values and error bars indicate standard error of mean. * = P < .05 vs control, # = P < .01 vs control, † P < .01 vs HIFU and control.
DISCUSSION
Our study demonstrates that preexposure of murine SCC xenografts to pulsed HIFU results in tumor growth inhibition and induction of apoptosis by using systemic bortezomib at a lower dose than is required when bortezomib is given alone. As reported in a similar murine tumor model (10), lowering the dose of bortezomib from 1.5 to 1.0 mg/kg can significantly improve the side effect profile. We also found that exposure of naïve SCC7 tumors to pulsed HIFU alone neither reduced growth rates nor increased apoptosis on TUNEL assay, which is consistent with previous adenocarcinoma (9,21) and SCC studies (2,8). Similar to results with the PAM-LY2 SCC tumor model (10), tumor growth inhibition was not achieved with bortezomib alone until a dose of 1.5 mg/kg was used. However, the combination of pulsed HIFU and 1.0 mg/kg of bortezomib caused a substantial growth delay, nearly doubling the time to end point compared with control (Fig 3), and produced substantially greater apoptosis of carcinoma cells compared with 1.0 mg/kg of bortezomib alone (Fig 4). The results of the apoptosis assay corroborate the findings of the initial tumor growth study, suggesting that the slowing of tumor growth by using the combination therapy resulted from the early enhancement of apoptosis.
The beneficial interactions observed in this study may potentially be explained by thermal or nonthermal mechanisms of pulsed HIFU. The pulsed HIFU exposures used in this study produce minimal and noncytotoxic temperature elevations of 4°–5°C locally in targeted tissues (21), which have been used to trigger low temperature–sensitive liposomes for enhanced delivery of doxorubicin, and ultimately greater antitumor effect when compared with heat-stable liposomes (Doxil) (9). The transient local hyperthermia could increase blood flow to the targeted tumor because of vasodilation (24,25), possibly improving drug delivery during the early passes through the tumor, since small molecule drugs such as bortezomib are rapidly cleared from the circulatory system (26). Pulsed HIFU also induces structural changes in the extracellular matrix and at cellular junctions that are visible with transmission electron microscopy (8) and may play a role in improving drug extravasation.
The diverse molecular effects of bortezomib and pulsed HIFU must also be considered. Bortezomib, in addition to various effects on p53, p21, p27, jun kinase, and other cell cycle regulatory proteins (11,27), inhibits proteasome-mediated degradation of the inhibitor of κBα, IκBα, decreasing NF-κB activation, and inducing apoptosis of head and neck SCC cells (10). HIFU upregulates members of the heat shock protein (HSP) family, such as HSP70, as demonstrated in the SCC7 tumor model (28), and clinically relevant scenarios that employ HSP promoter-associated genes to spatially and temporally control gene therapy (29). Recent studies have revealed HSP70, like bortezomib, to be an inhibitor of NF-κB activation through stabilization of IκBα (30–32). Thus, an interesting hypothesis for future study is that pulsed HIFU, by upregulating HSP70, causes a degree of IκBα stabilization that, while not enough to induce apoptosis alone, sensitizes the cancer cells to bortezomib and lowers the threshold level for bortezomib-induced apoptosis.
This study was limited in that a single animal tumor model was examined, although previous studies have demonstrated the efficacy of pulsed HIFU in combination with a variety of biomolecules in different settings, including other tumor models (2,5,8,9). Additionally, only indirect histologic evidence of enhanced drug delivery was reported, as the small drug doses administered yield tissue concentrations at or below detection limits owing to the high potency and short half-life of bortezomib. Finally, human tumors are likely to be substantially larger than the murine tumors in this study and would require long exposure times with a single pulsed HIFU transducer. The development of phased-array HIFU devices, however, could lead to major improvements in time efficiency of exposures (33).
In summary, our findings indicate that pulsed HIFU allows antitumor and cytotoxic effects of bortezomib at a dose below that which was required for anticancer activity when given alone.
Practical application: Pulsed HIFU may prove a valuable clinical tool if it allows the use of lower doses of bortezomib, given its narrow therapeutic window. As bortezomib is already U.S. Food and Drug Administration–approved for use in multiple myeloma, results of this preclinical pulsed HIFU study, with further clarification of the exact mechanism, could potentially direct this paradigm toward enhancement of clinical activity of systemically administered bortezomib in head and neck SCC and other relevant clinical models of antineoplastic drugs used with image-guided focused-energy devices. Indeed, the joining of magnetic resonance imaging and HIFU on commercial systems should be the harbinger of more work in this fertile area of image-guided drug delivery for enhanced therapeutics.
ADVANCE IN KNOWLEDGE
Exposure of squamous cell carcinoma tumors in mice to pulsed-high intensity focused US prior to chemotherapy with bortezomib expands the therapeutic window of the drug, resulting in significant anticancer effects at lower systemic doses.
Acknowledgments
We gratefully thank Mary Angstadt, BS, for support with all animal work, Dr. Matthew R. Dreher, PhD, for reviewing the manuscript, and Hilary Hancock, BS, for contributions to the figures.
Pulsed high-intensity focused ultrasound allows antitumor and cytotoxic effects of bortezomib at a dose below that which was required for anticancer activity when given alone.
See Materials and Methods for pertinent disclosures.
See also Science to Practice in this issue.
Author contributions: Guarantors of integrity of entire study, B.J.W., C.V.W., V.F.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors; literature research, J.A.P., B.T., B.J.W., V.F.; experimental studies, J.A.P., C.T.A., B.T., A.C., J.X., Z.C., V.F.; statistical analysis, J.A.P., C.T.A., V.F.; and manuscript editing, all authors
Supported in part by Howard Hughes Medical Institute NIH Research Scholars Program (J.A.P.), NIDCD Intramural Research Project DC-00016 (C.V.W., Z.C., C.T.A.), NIH Clinical Center, and a Materials Cooperative Research and Development Agreement with Millennium Pharmaceuticals (C.V.W.).
References
- 1. Kennedy JE High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 2005; 5(4): 321–327. [DOI] [PubMed] [Google Scholar]
- 2. Dittmar KM, Xie J, Hunter F, et al. Pulsed high-intensity focused ultrasound enhances systemic administration of naked DNA in squamous cell carcinoma model: initial experience. Radiology 2005; 235(2): 541–546. [DOI] [PubMed] [Google Scholar]
- 3. Frenkel V, Li KC. Potential role of pulsed-high intensity focused ultrasound in gene therapy. Future Oncol 2006; 2(1): 111–119. [DOI] [PubMed] [Google Scholar]
- 4. Frenkel V, Oberoi J, Stone MJ, et al. Pulsed high-intensity focused ultrasound enhances thrombolysis in an in vitro model. Radiology 2006; 239(1): 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stone MJ, Frenkel V, Dromi S, et al. Pulsed-high intensity focused ultrasound enhanced tPA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thromb Res 2007; 121(2): 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med 2003; 9(6): 713–725. [DOI] [PubMed] [Google Scholar]
- 7. Jain RK Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307(5706): 58–62. [DOI] [PubMed] [Google Scholar]
- 8. Traughber B, Frenkel V, Xie J, et al. Pulsed-high intensity focused ultrasound (HIFU) exposures combined with intratumoral injection of TNF-a plasmid enhance growth inhibition in a murine squamous cell carcinoma model: Proceedings of the 6th International Symposium on Therapeutic Ultrasound, Oxford UK, August 30–September 2, 2006
- 9. Dromi S, Frenkel V, Luk A, et al. Pulsed-high intensity focused ultrasound and low temperature sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res 2007; 13(9): 2722–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sunwoo JB, Chen Z, Dong G, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res 2001; 7(5): 1419–1428. [PubMed] [Google Scholar]
- 11. Mani A, Gelmann EP. The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol 2005; 23(21): 4776–4789. [DOI] [PubMed] [Google Scholar]
- 12. Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res 1999; 59(11): 2615–2622. [PubMed] [Google Scholar]
- 13. Hideshima T, Mitsiades C, Akiyama M, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood 2003; 101(4): 1530–1534. [DOI] [PubMed] [Google Scholar]
- 14. Williams S, Pettaway C, Song R, Papandreou C, Logothetis C, McConkey DJ. Differential effects of the proteasome inhibitor bortezomib on apoptosis and angiogenesis in human prostate tumor xenografts. Mol Cancer Ther 2003; 2(9): 835–843. [PubMed] [Google Scholar]
- 15. Cavo M Proteasome inhibitor bortezomib for the treatment of multiple myeloma. Leukemia 2006; 20(8): 1341–1352. [DOI] [PubMed] [Google Scholar]
- 16. Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu Rev Med 2006; 57: 33–47. [DOI] [PubMed] [Google Scholar]
- 17. Van Waes C, Chang AA, Lebowitz PF, et al. Inhibition of nuclear factor-kappaB and target genes during combined therapy with proteasome inhibitor bortezomib and reirradiation in patients with recurrent head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2005; 63(5): 1400–1412. [DOI] [PubMed] [Google Scholar]
- 18. Andre P, Cisternino S, Chiadmi F, et al. Stability of bortezomib 1-mg/mL solution in plastic syringe and glass vial. Ann Pharmacother 2005; 39(9): 1462–1466. [DOI] [PubMed] [Google Scholar]
- 19. Hirst DG, Brown JM, Hazlehurst JL. Enhancement of CCNU cytotoxicity by misonidazole: possible therapeutic gain. Br J Cancer 1982; 46(1): 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Malley BW Jr, Cope KA, Johnson CS, Schwartz MR. A new immunocompetent murine model for oral cancer. Arch Otolaryngol Head Neck Surg 1997; 123(1): 20–24. [DOI] [PubMed] [Google Scholar]
- 21. Frenkel V, Etherington A, Greene M, et al. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Acad Radiol 2006; 13(4): 469–479. [DOI] [PubMed] [Google Scholar]
- 22. Schiffer IB, Gebhard S, Heimerdinger CK, et al. Switching off HER-2/neu in a tetracycline-controlled mouse tumor model leads to apoptosis and tumor-size-dependent remission. Cancer Res 2003; 63(21): 7221–7231. [PubMed] [Google Scholar]
- 23. Seshadri M, Mazurchuk R, Spernyak JA, Bhattacharya A, Rustum YM, Bellnier DA. Activity of the vascular-disrupting agent 5,6-dimethylxanthenone-4-acetic acid against human head and neck carcinoma xenografts. Neoplasia 2006; 8(7): 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dewhirst MW, Jones E, Samulski T, Vujaskovic Z, Li CY, Prosnitz L. Hyperthermia. In: Kufe D, Pollack R, Weichselbaum R, et al, eds. Cancer medicine 6. Hamilton, Ontario: Decker, 2003; 623 –636. [Google Scholar]
- 25. Vaupel P, Kallinowski F. Physiological effects of hyperthermia. Recent Results Cancer Res 1987; 104: 71–109. [DOI] [PubMed] [Google Scholar]
- 26. Stanford BL, Zondor SD. Bortezomib treatment for multiple myeloma. Ann Pharmacother 2003; 37(12): 1825–1830. [DOI] [PubMed] [Google Scholar]
- 27. Orlowski RZ The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death Differ 1999; 6(4): 303–313. [DOI] [PubMed] [Google Scholar]
- 28. Ringler MD, Pandit SD, Khaibullina A, Kable A, Bur M, Li KCP. Pulsed focused ultrasound-induced differential gene expression in murine SCCVII tumors. Proceedings of the 95th Annual Meeting of the American Association for Cancer Research, 2004; 215 [Google Scholar]
- 29. Moonen CT Spatio-temporal control of gene expression and cancer treatment using magnetic resonance imaging-guided focused ultrasound. Clin Cancer Res 2007; 13(12): 3482–3489. [DOI] [PubMed] [Google Scholar]
- 30. Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol 2000; 164(10): 5416–5423. [DOI] [PubMed] [Google Scholar]
- 31. Sun D, Chen D, Du B, Pan J. Heat shock response inhibits NF-kappaB activation and cytokine production in murine Kupffer cells. J Surg Res 2005; 129(1): 114–121. [DOI] [PubMed] [Google Scholar]
- 32. Shi Y, Tu Z, Tang D, et al. The inhibition of LPS-induced production of inflammatory cytokines by HSP70 involves inactivation of the NF-kappaB pathway but not the MAPK pathways. Shock 2006; 26(3): 277–284. [DOI] [PubMed] [Google Scholar]
- 33. Clement GT Perspectives in clinical uses of high-intensity focused ultrasound. Ultrasonics 2004; 42(10): 1087–1093. [DOI] [PubMed] [Google Scholar]