Abstract
The yolk sac, first site of hematopoiesis during mammalian development, contains not only hematopoietic stem cells but also the earliest precursors of endothelial cells. We have previously shown that a nonadherent yolk sac cell population (WGA+, density <1.077, AA4.1+) can give rise to B cells, T cells, and myeloid cells both in vitro and in vivo. We now report on the ability of a yolk sac-derived cloned endothelial cell line (C166) to provide a suitable microenvironment for expansion of these early precursor cells. Single day 10 embryonic mouse yolk sac hematopoietic stem cells were expanded >100 fold within 8 days by coculture with irradiated C166 cells. Colony-forming ability was retained for at least three passages in vitro, with retention of the ability to differentiate into T-cell, B-cell, and myeloid lineages. Stem cell properties were maintained by a significant fraction of nonadherent cells in the third passage, although these stem cells expressed a somewhat more mature cell surface phenotype than the initial yolk sac stem cells. When reintroduced into adult allogeneic immunocompromised (scid) hosts, they were able to give rise to all of the leukocyte lineages, including T cells, B cells, and myeloid cells. We conclude that yolk sac endothelial cells can support the stable proliferation of multipotential hematopoietic stem cells, thus generating adequate numbers of cells for study of the mechanisms involved in their subsequent development and differentiation, for in vivo hematopoietic restitution, and for potential use as a vehicle for gene transfer.
The yolk sac is the first site of hematopoiesis during mammalian embryogenesis (1, 2). The yolk sac is also the first site of blood vessel development (3). The formation of blood islands in the yolk sac is an integrated process in which these two events, hematopoiesis and vasculogenesis, proceed in concert. We have postulated that these two events are intimately related and causally interdependent. The present study supports this hypothesis by demonstrating the ability of yolk sac-derived endothelial cells (YS-ECs) to support the maintenance and expansion of early yolk sac-derived hematopoietic stem cells (YS-HSCs) in vitro.
Early YS-HSCs comprise a unique cell population: they have not yet developed to the point of expressing histocompatibility antigens, nor do they exhibit the stem cell antigens that characterize stem cells from the fetal liver or bone marrow (4–8). Moreover, their proliferation potential exceeds that of later-appearing hematopoietic stem cells (1, 9). These characteristics would seem to make them ideal candidates for transplantation, both for hematopoietic restitution and as carriers for gene transfer. A hindrance to this aim, however, is the very scant numbers of these cells. Thus, a primary goal of our research has been to establish the conditions that permit these cells to undergo proliferation and expansion without loss of stem cell properties.
There is now ample evidence that hematopoiesis results when bone marrow stromal cells provide the supportive microenvironment for the proliferation of hematopoietic progenitor cells isolated from adult bone marrow as well as from primitive cord blood (10–12). Studies have also shown that yolk sac-adherent endoderm and mesoderm cells can support the proliferation and differentiation of bone marrow-derived hematopoietic stem cells (13, 14). However, direct interaction between YS-ECs and YS-HSCs have only recently begun to be analyzed (cf. refs. 8, 15–18). What now facilitates such an analysis are two recently reported advances: (i) the ability to identify and enrich (>100-fold) a minority cell population within the yolk sac containing most, possibly all, of the YS-HSCs (4); and (ii) the establishment of stable YS-EC lines (16). The present study reports on the ability of one clonally derived YS-EC line to promote both colony formation and persistence and expansion of the stem cells (YS-HSCs) found in the mouse yolk sac as well as their ability, following expansion, to differentiate into T-, B-, and myeloid cells in vitro and in vivo after reintroduction into immunocompromised adult animals.
MATERIALS AND METHODS
Animals.
C57BL/6Au (Thy1.2), C57BL6/KaAu (Thy1.1), and BALB/cAu were maintained and bred in our own colony. BALB/c scid mice were obtained from the University of Wisconsin Gnotobiotic Facility (Madison, WI).
Source of Cells.
The day of appearance of a vaginal plug was designated day 0 of gestation. Yolk sacs were dissected from early day 10 embryos, rinsed to remove maternal blood, and then disrupted mechanically and filtered through 15-μm nylon mesh. S17 bone marrow-derived stromal cells were obtained as a gift from Kenneth Dorshkind (University of California, Riverside). Cloned yolk sac-derived C165, C166, and C167 cells, established initially from yolk sacs isolated from 12-day mouse embryos, were established in our laboratory (16). The Y4 yolk sac line was generated from 8-day embryos by D. Yu, also in our laboratory.
Reagents.
Antibodies included: α-CD45FITC (Ly5, T200), α-Thy1.2FITC, α-CD4FITC, and α-CD8PE from Becton Dickinson; αThy1.2FITC, α-Thy1.1PE, α-CD44FITC (Pgp-1), α-IgMFITC (μ chain), α-HSAPE, α-H-2Kb FITC, α-H-2KdDd, and α-H-2 Ia FITC from PharMingen; AA4.1 antibody from J. P. McKearn (19); α-Sca-1 from I. L. Weissman (Stanford University); JORO 37.5 from R. Palacios (M.D. Anderson Cancer Center); α-B220 (14.8) from P. Kincade (Oklahoma Medical Research Institute); and α-Mac-1 from the American Type Culture Collection. Fluorescein isothiocyanate (FITC)-labeled wheat germ agglutinin (WGAFITC) was purchased from Sigma; tricolor streptavidin conjugates were from Caltag (South San Francisco, CA); and second-step reagents were from various commercial sources.
Isolation of AA4.1+ Yolk Sac Cells.
AA4.1+ yolk sac cells were isolated as described previously (4). In brief, yolk sac cells were separated by using discontinuous gradients of Percoll (1.054, 1.066, and 1.077 g/cm3 in 0.15 M NaCl; Sigma). Fractions were washed in PBS/20% fetal bovine serum (FBS) and resuspended in RPMI 1640 medium containing 20% FBS. Gradient-separated yolk sac cells were incubated in tissue culture dishes at 37°C, 5% CO2 for 1 hr, after which the nonadherent cells were collected. For isolation of AA4.1+ cells by immunocytoadherence (20), polystyrene dishes were precoated with protein G-purified AA4.1 antibody (10 μg/ml) in 10 ml of 0.05 M Tris·HCl/0.15 M NaCl, pH 9.5 at room temperature for 1 hr. After washing and blocking with PBS/1% FBS, cells were layered onto antibody-coated plates in RPMI 1640 medium/5% FBS and incubated at 4°C for 1 hr. Nonadherent cells were collected as AA4.1− cells, after which the plates were washed 8–10 times with PBS/5% FBS. The bound cells were then recovered by forceful pipetting. The purity of AA4.1+ yolk sac cells (>95%) was determined by flow cytometry. In some experiments, cells were then labeled with WGAFITC, and the WGAbright cells were isolated by cell sorting.
Coculture of AA4.1+ Yolk Sac Cells with YS-ECs.
Cloned YS-EC lines (C165, C166, and C167; ref. 13) were trypsinized, irradiated with 25 Gy using a 137Cs source, and plated to confluence in 96-well or 15 × 60 mm culture plates. After overnight incubation in medium consisting of DMEM, 10% FBS, 10−5 M 2-mercaptoethanol (2-ME), and antibiotics, the unseparated yolk sac cells or AA4.1+ enriched yolk sac hematopoietic precursor cells (104 cells) were dispensed on top of the YS-EC monolayers. Cultures were analyzed at 7–8 days for colony numbers. For the analysis of total cell expansion, the cells were recovered from cocultures by vigorous pipetting, after which the nonadherent cells (11.3%) were isolated by plastic adhesion at 37°C for 1 hr. Cells recovered from mixed or nonmixed colonies grown on YS-ECs were evaluated for cell differentiation by Wright–Giemsa (Diffquik; Baxter) staining of cytospin preparations. Development of sorted AA4.1+/WGAbright yolk sac cells was studied by single cell sorting into 96-well plates prepared with irradiated C166 cells, as described above. Nonadherent cells (cf., above) were isolated from mixed colonies formed on C166 feeder layers and aliquoted for in vitro assay of myeloid or lymphoid precursors. The nonadherent cells from mixed colonies were also transferred onto fresh C166 feeder layers at weekly intervals for long-term culture.
Flow Cytometry.
Cells were incubated with antibodies for 30 min, washed, stained with second-step reagents for 30 min where required, washed, then resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS/1% BSA). Flow cytometry was carried out using a FACScan (Becton Dickinson) instrument and lysys-ii or pc-lysys analysis software. Sorting was performed using a FACStarPLUS (Becton Dickinson) instrument (488 nM, 100-mA current, ACDU and Macrosort accessories; Becton Dickinson).
Myeloid Cell Differentiation.
A stock solution of methylcellulose was prepared as described (4). Concanavalin-A-conditioned medium was prepared by culturing 2 × 106 C57BL/6 splenocytes per ml in DMEM/10% FBS with concanavalin-A (Sigma) at 2.5 μg/ml and 50 μM 2-ME for 3 days. L-929-conditioned medium was obtained by culturing L929 cells in DMEM/10%FBS for 7 days. Nonadherent cells (103–105), isolated from colonies formed on YS-EC feeder layers by plastic adherence (see above), were seeded in 0.8% methylcellulose supplemented with 15% concanavalin-A-conditioned medium and 15% L–929-conditioned medium, cultured for 7–10 days, and scored for colony formation. Groups of >40 cells were considered a colony and colonies with diameters >0.5 mm (containing >5000 cells) were considered to be “high proliferating potential” colonies (21). Groups of cells containing <40 cells were considered to be clusters. Colonies were plucked for analysis by cytospin and Wright–Giemsa staining.
B-Cell Differentiation.
Irradiated (25 Gy) S17 cells were plated to near confluence in 35-mm dishes. After 24 hr, nonadherent cells (103-104) were added and cultured in medium consisting of RPMI 1640 medium with 10% FBS, 10−5 M 2-ME, and recombinant human interleukin 7 at 1000 units/ml for 2 weeks. B cells were identified by flow cytometric analysis based on expression of B220 and surface-associated IgM expression.
T-Cell Differentiation.
Embryonic thymic lobes were dissected from day 14 embryos of C57BL6/Ka (Thy1.1) mice. Fetal thymus organ cultures were depleted of stem cells by deoxyguanosine treatment for 5 days as described previously (5, 22). The isolated nonadherent cells (102-104) from secondary cultures were seeded into each fetal thymic lobe placed in each well of a Terasaki plate and cultured as a hanging drop culture for 48 hr. Lobes were washed free of unincorporated cells, then placed on Nuclepore filters (0.8-mm pore size) supported by Gelfoam sponges (Upjohn) embedded in RPMI 1640/10% FBS medium for 14 or more days. Cells dispersed from these lobes were then analyzed by flow cytometry, using αThy1.1 and αThy1.2 to distinguish yolk sac-derived cells from possible thymus-derived cells.
Reconstitution Protocols.
BALB/c scid mice were irradiated with 300 rad of γ (Cs) irradiation. After 3–5 hr, 2 × 104 nonadherent cells from secondary passage were introduced intravenously. After 3 months, cells were obtained from spleens, thymuses, and peripheral blood and analyzed by flow cytometry.
RESULTS
Colony Formation of Stem Cells Grown in Coculture with YS-ECs.
Application of the sequential protocol of adherence to plastic, density gradient centrifugation, and immunocytoadherence to AA4.1 antibody generated a small subset (<0.15%) of yolk sac cells that were nonadherent (density <1.077 and >95% AA4.1+). These cells were plated on YS-EC clones C165, C166, or C167 and on the Y4 primitive YS-EC line, with no added growth factors. No colonies were seen in control cultures grown in the absence of a feeder layer, nor were they found in cocultures with Y4 cells (day 8 YS-ECs). On the other hand, the 12-day cloned YS-ECs showed a significant supportive function of the AA4.1+ hematopoietic stem cells, as evidenced by colony formation (Fig. 1), with one-third of plated cells forming colonies on C166 and C167, and a significant number (one of six) forming colonies on C165. Although there was no significant difference between the total number of colonies formed in cocultures with C166 and C167 cells, there was a major difference in the type of colonies formed: Only C166 led to the generation of mixed colonies (10%; Fig. 2).
Figure 1.
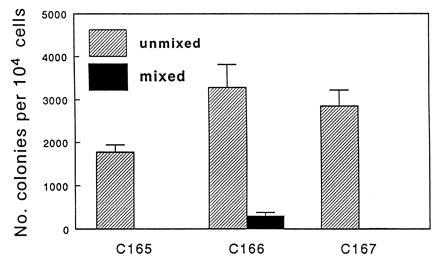
Colony forming activity supported by YS-ECs. Nonadherent, density <1.077 g/cm3, AA4.1+ yolk sac stem cells (104) were isolated from yolk sacs dissected from day 10 mouse embryos and cocultured with YS-ECs. Colonies were counted after 7 days. Bars represent mean ± SE.
Figure 2.
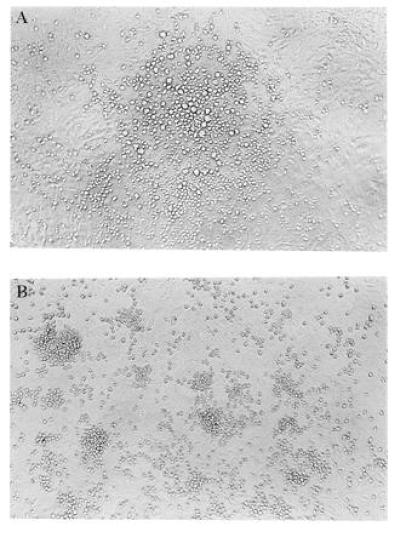
Morphology of colonies on YS-EC. Morphology of colonies formed on C166 feeder layers after 7 days of coculture. (A) Mixed colony (×100); (B) nonmixed colony (×100).
In addition to being effective in supporting mixed colony formation, C166 cells also permitted a 16- to 36-fold increase in the total number of nonadherent cells (11.3% of cells recovered) generated after 1 week of coculture. As shown in Table 1, when 1000 cells were plated initially, >36,000 nonadherent cells could be recovered after 1 week. In contrast, C167 cocultures permitted only a modest (2.3-fold) increase, and there was no net gain when AA4.1+ cells were plated on C165 feeder layers. Recovery of these cells from coculture with C166 cells yielded from 276- to 330-fold amplification.
Table 1.
Number of nonadherent cells recovered following 1 week of coculture of AA4.1+ yolk sac stem cells with YS-ECs
| Endothelial cell clone used for coculture | No. of nonadherent cells recovered | Increase in cell no. |
|---|---|---|
| Control (none) | 0 | 0 |
| C165 | <1000 | <1× |
| C166 | 36,194 ± 5762 | 36.2× |
| C167 | 2359 ± 487 | 2.3× |
AA4.1+ yolk sac stem cells (103 cells) were cocultured with cloned YS-ECs for 7 days. Nonadherent cells were removed from individual cultures and enumerated. The results represent data from three independent experiments ± SD.
Single-cell deposition was carried out by sorting WGAbright cells (top 25%) from suspensions containing preselected AA4.1+, nonadherent, density <1.077 yolk sac cells onto irradiated C166 cells plated to confluence in 96-well plates. Colonies were found in 38.8% of wells, including both mixed and unmixed colonies (Table 2). Nonadherent cells isolated from individual mixed colonies were capable of forming secondary colonies on C166 cells, and the colony forming activity was maintained for at least three passages at weekly intervals.
Table 2.
Colony formation by single YS-HSCs (day 10) following coculture with irradiated C166 YS-ECs
| Colony size (no. of cells per well) | Number of
colony-positive wells
|
Total no. of colonies | |
|---|---|---|---|
| Mixed | Unmixed | ||
| <5,000 | 0 | 21 | 21 |
| 5,000–10,000 | 5 | 29 | 34 |
| >10,000 | 2 | 19 | 21 |
| Total no. (%) | 7 (9%) | 69 (91%) | 76 |
| Positive wells, % | 3.5 | 34.9 | 38.4 |
Single cell sorting of WGA+ cells (brightest, 25%) was carried out on AA4.1+, low-density, nonadherent cells isolated from freshly dissected yolk sacs (10-day embryos). A total of 198 wells were first seeded with C166 YS-ECs, which were permitted to grow to confluence and were then irradiated (25 Gy). Cultures were assayed following 8 days of coculture.
Morphological and Phenotypic Characteristics of Colony-Forming Cells.
Fig. 1 illustrates that AA4.1+ yolk sac hematopoietic cells formed mixed and nonmixed colonies on C166 cells. Further characterization of cell types found in these colonies was determined by examination of cytospin preparations (Fig. 3). Mixed colonies from cells grown on C166 feeder layers included macrophages, granulocytes, megakaryocytes, blast cells, and monocytes in various stages of maturation. In contrast, colonies grown on C165 or C167 feeder layers gave rise almost exclusively to macrophages or macrophage/granulocytes (data not shown).
Figure 3.
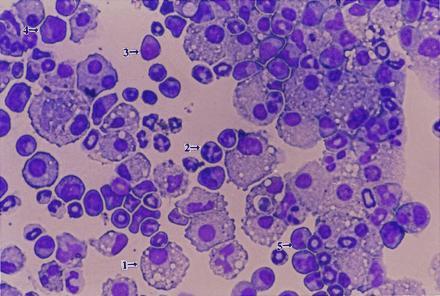
Analysis of cells found in mixed colonies formed on C166 cells. Cells were removed from mixed colonies grown on C166 feeder layers and evaluated for cell differentiation by Wright–Giemsa staining of cytospin preparations. Representative examples of various cell types are indicated by arrows: 1, macrophage; 2, granulocyte; 3, blast cell; 4, megakaryocyte; and 5, monocyte. (×160.)
Nonadherent cells from mixed colonies were assessed for expression of both stem-cell and lineage-specific markers (Fig. 4). By using plastic adherence, 11.2% nonadherent cells were isolated from mixed colonies formed on C166 cells, of which >95% were strongly positive for HSA+, an antigen whose expression decreases during hematopoietic differentiation. Of the cells assessed, >50% were positive for AA4.1 and Sca-1. In contrast, the vast majority of cells (>98.8%) failed to express Thy 1.2, and no cells were found to express either B220 or JORO 35.7. The cell surface phenotype analysis supports the conclusion that a large fraction of the progeny of AA4.1+ yolk sac cells grown on C166 feeder layers are primitive multipotential stem cells.
Figure 4.
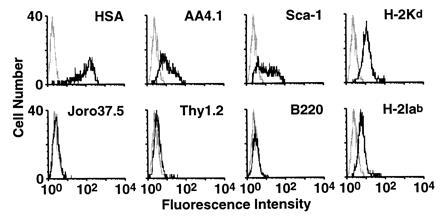
Phenotypic analysis of nonadherent cells expanded by C166 cells. Surface antigens expressed on nonadherent cells obtained from mixed colonies on C166 cells were analyzed by flow cytometry. Gating on the lymphoid population was carried out on the basis of low forward and side scatter signals.
Identification of B-Cell Precursors in Mixed Colonies Formed on C166 Feeder Layers.
The potential of nonadherent cells from mixed colonies to differentiate into B-cell lineages was determined by transferring nonadherent cells (103–104 cells) from secondary cultures onto S17 stromal cells with added interleukin 7. When tested after 15 days, significant B-cell differentiation was observed, as demonstrated by the expression of B220 and surface-associated IgM (Fig. 5).
Figure 5.
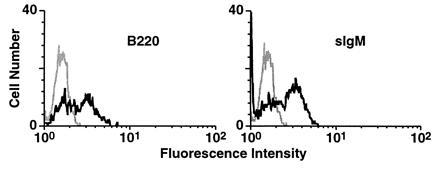
Generation of B-cell precursors in mixed colonies formed on C166 cells. Nonadherent cells (103–104) isolated from secondary cocultures of YS-HSCs with irradiated C166 cells were cultured on S17 bone marrow stromal cells with interleukin 7 for 14 days. Expression of B220 and surface-associated IgM on cultured cells was determined by flow cytometric analysis.
Identification of T-Cell Precursors in Mixed Colonies Formed on C166 Feeder Layers.
Fetal thymus organ cultures were used to test the potential of nonadherent cells obtained from mixed colonies to differentiate into T-cell lineages. A Thy1 allelic difference between cells of the thymus rudiment (Thy1.1) and those derived from yolk sacs (Thy1.2) was used to provide positive identification of yolk sac-derived T cells. The deoxyguanosine pretreatment of the thymic rudiments was effective inasmuch as no Thy1.1+ cells could be detected. Three-color analysis revealed that 97% of the lymphocytes isolated from thymus rudiment cultures after 15 days were Thy1.2+. Donor-derived T cells included double-positive (CD4+CD8+), double-negative (CD4−CD8−), and single-positive (CD4+CD8−; CD4−CD8+) cells (Fig. 6).
Figure 6.
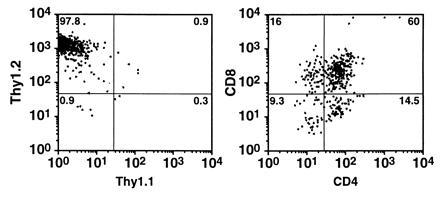
Generation of T-cell precursors in mixed colonies formed on C166 feeder layers. (A) Donor-derived (yolk sac) cells were distinguished on the basis of Thy1.1 (host) and Thy1.2 (donor) antigen expression. (B) Correlated dot plot shows CD4 and CD8 expression of donor-derived T cells.
Identification of Myeloid Precursors in Mixed Colonies.
To evaluate whether the isolated nonadherent cells from mixed colonies were capable of differentiating along myeloid lineages, cells from secondary culture were cultivated in methylcellulose. After 7–12 days of culture, myeloid colonies could be identified (Table 3). Cytospin preparations revealed various types of myeloid cells in these colonies (Fig. 7). The frequency of colonies ranged from 1/100 to 1/150. Myeloid colonies were virtually absent in cells isolated from coculture of AA4.1+ yolk sac cells grown in coculture with C165 or C167 cells, or from nonmixed colonies formed on C166 feeder layers (data not shown).
Table 3.
Myeloid cell colony formation in methylcellulose by nonadherent cells obtained from secondary cultures grown on irradiated YS-ECs
| Endothelial cell clone | No. of colonies per 105 nonadherent cells |
|---|---|
| C165 | 2 ± 1 |
| C166 | 840 ± 170 |
| C167 | 11 ± 5 |
Data are in mean ± SD. Myeloid precursors were identified by morphology and Wright–Giemsa staining of cytospin preparations of cells isolated from individual colonies.
Figure 7.
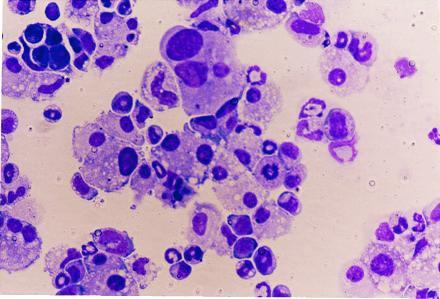
Morphology of colony-forming cells in methylcellulose culture. Nonadherent cells from colonies grown on YS-ECs were evaluated for their ability to form colonies after 7–10 days in methylcellulose culture. Cells were prepared by cytospin preparation.
Reconstitution of Adult Mice with Passaged YS-HSCs.
Nonadherent cells from secondary cultures originating from expansion of AA4.1+, WGA+, nonadherent, density <1.077, 10-day C57BL/6 (H-2b) yolk sac-derived cells grown on C166 yolk sac feeder cells were injected into mildly irradiated BALB/c (H-2d) scid mice (2 × 104 nonadherent cells per host animal). This number of cells was chosen on the basis of in vitro limiting dilution assays, from which we estimated that ≈1/75 nonadherent cells recovered from these cultures would be multipotential stem cells. Thymuses, spleens, and peripheral blood were assayed 3 months after cell transfer. Irradiated, unreconstituted scid mice served as controls. The results are shown in Table 4. In the spleen of reconstituted animals, 75% were found to be donor-derived (H-2b+; H-2d−), and, similarly, 80% of peripheral blood leukocytes expressed the donor haplotype marker. Whereas no mature (CD4+CD8−; CD4−CD8+) cells were present in control thymuses, half of the thymocytes in reconstituted animals were of these phenotypes. Similarly, 70% of the lymphocytes in the thymuses of experimental animals expressed the α/β T-cell receptor, whereas such cells were not present in control thymuses.
Table 4.
In vivo reconstitution of BALB/C scid mice by in vitro-passaged YS-HSCs
| Cells | CD4− CD8+ | CD4+ CD8+ | CD4− CD8− | CD4+ CD8− | TCRαβ+ | B220+ sIgM+ | B220+ sIgM− | Mac-1+ H-2Kb+ | H-2Kb+ H-2Kd− |
|---|---|---|---|---|---|---|---|---|---|
| Thymus | |||||||||
| scid | 3.3 | 68.9 | 26.4 | 1.0 | 0.5 | ||||
| + YS | 25.6 | 30.8 | 17.3 | 26.8 | 69.7 | ||||
| Spleen | |||||||||
| scid | 0.7 | 0.0 | 98.3* | 0.8 | 0.9 | 0.03 | 0.6 | 0.5 | 0.1 |
| + YS | 4.4 | 0.2 | 69.2* | 27.2 | 33.8 | 16.2 | 1.6 | 15.6 | 74.7 |
| PBL | |||||||||
| scid | 0.6 | 0.3 | 97.2 | 0.7 | 7.3 | 0.4 | 1.1 | 0.8 | 2.3 |
| + YS | 13.7 | 0.8 | 53.2 | 32.7 | 55.6 | 35.2 | 6.3 | 20.1 | 80.3 |
Nonadherent cells (2 × 104) from two-passage expansion of AA4.1+, WGA+, nonadherent, density <1.077 C57BL/6 yolk sacs were injected into irradiated BALB/c scid mice. Irradiated, unreconstituted scid mice served as controls. Cells from thymus, spleen, and peripheral blood were analyzed 12 weeks after transfer. Data are presented as % of total cells recovered. PBL, peripheral blood lymphocyte; TCR, T-cell receptor; sIgM, surface-associated IgM.
Number includes all lymphocytes, identified and gated on the basis of forward and side scatter signals.
A similar pattern was seen in the spleen: all of the control lymphocytes were double-negative (CD4−CD8−), whereas 32% of the splenic lymphocytes in reconstituted animals were single-positives and expressed the α/β T-cell receptor phenotype. Control animals had no surface-associated IgM-expressing B cells, whereas 16% of the cells in the spleens of reconstituted animals showed surface expression of IgM. Donor-derived (Mac-1+) myeloid cells (16%) were identified by H-2 haplotype staining. The peripheral blood results paralleled those obtained for the spleen (Table 4).
DISCUSSION
We have demonstrated that primitive definitive hematopoietic stem cells can be expanded in vitro through several passages to yield cells that retain the ability to differentiate into B lymphocytes, T lymphocytes, and various myeloid cells. Not only do these cells have the capacity to differentiate in vitro, but they are capable of reconstituting adult, allogeneic, immunocompromised host animals. This expansion with retention of stem cell properties has been accomplished by using a coculture system consisting of a cloned endothelial cell line, C166, derived from the yolk sac of mid-gestation (12-day) embryos.
Various types of cloned stromal cells have been isolated and used by other investigators to study their role in expansion and differentiation of bone marrow-derived hematopoietic stem cells (10, 11, 23). To date, no combination of growth factors has equaled the ability of stromal cell lines to provide these conditions. Two major mechanisms responsible for the supportive function of stromal cells are thought to be involved in the control of hematopoiesis in vitro: (i) production of diffusible hematopoietic regulatory factors (24–26); and (ii) physical cell–cell interaction between hematopoietic cells and stromal cells. A considerable degree of selectivity and specificity appears to be involved in these cell–cell interactions, with some stromal cell lines supporting myelopoiesis, others B-cell differentiation, and, possibly, still others, T-cell development. Our work with YS-ECs does not address this question of selectivity, focusing rather on earlier events—i.e., those interactions that permit maintenance and expansion of multipotential stem cells. Whether YS-ECs can support differentiation of hematopoietic precursor cells obtained from later sites of hematopoiesis (e.g., cord blood, fetal liver, or bone marrow) remains to be determined.
As many as 50% of the cells retained after subculture maintain a stem cell profile as manifested by the expression of the AA4.1+ phenotype and as demonstrated by their ability to give rise to both T and B lymphocytes as well as mixed colonies comprised of diverse leukocytes. It should be noted, however, that the original yolk sac stem cell, although AA4.1+, is Sca-1-negative and major histocompatiblity complex (MHC) class I- and II-negative (4)—i.e., it has not yet acquired the primitive stem cell antigens that identify stem cells of the fetal liver (ref. 24; cf. ref. 8 for extended discussion). However, the preponderant phenotypes of the stem cells found after coculture and passage in vitro are Sca-1+ and MHC class I+. This suggests that C166 permits the progression from the more primitive to the somewhat more developmentally advanced stem cell phenotype. It is tempting to speculate that this feature of C166 cells reflects the origin of these cells from day 12 embryos and that YS-ECs obtained from earlier stage embryos might maintain the more primitive stem cells as Sca-1− cells. Y4 cells, obtained from day 8 embryos, did not support yolk sac stem cell maintenance or proliferation. However, this cell line may have failed to support hematopoiesis for purely technical reasons, inasmuch as feeder layers of these cells do not remain well-attached to the plastic substrate following irradiation. We have now established additional yolk sac endothelial cell lines from 7- to 11-day-old embryos, which will enable us to test the validity of this explanation.
Our experiments differ in significant ways from earlier studies (13, 16, 17) in the use of yolk sac endothelial cells as inducers of hematopoietic stem cell proliferation. Yoder et al. (13) used oncogene-mediated transformed endothelial cell lines and examined the in vitro differentiation of cells from bone marrow. Fennie et al. (17) also used oncogene-mediated immortalized endothelial cell lines and restricted their analysis to myeloid and erythroid lineages. Godin et al. (18) described clonal proliferation and subsequent in vitro differentiation of individual stem cells isolated from a highly confined intraembryonic site as well as from the embryonic yolk sac but did not examine the role of endothelial cells for maintaining stem cell properties. Most importantly, none of these studies addressed the critical questions of in vivo function of these stem cells.
The fact that single AA4.1+ yolk sac cells can be induced to proliferate and to give rise to the various leukocyte lineages indicates that C166 cells provide a suitable microenvironment for both proliferation and differentiation of primitive yolk sac hematopoietic precursor cells. However, only one of three consecutively isolated clonal cell lines tested (C165, C166, and C167) provided this microenvironment, suggesting that the yolk sac vascular endothelium represents a heterogeneous group of cells. Current studies in our laboratory (S.-J.W. and R.A., unpublished work) indicate that among these three clones, only C166 cells produce detectable levels of mRNA for stem cell factor. Whether exogenous addition of stem cell factor adequately supplements C165 or C167 cell monolayers in coculture with AA4.1+ cells is under investigation.
Our study has not included an assessment of erythropoietic competence of the expanded yolk sac stem cell population. Previous work in our laboratory (H. Huang and R.A., unpublished observations) showed that the initial AA4.1+, WGA+, density <1.077, nonadherent cells obtained from 11-day embryos could form erythroid colonies in vitro, provided interleukin 7 and erythropoietin were added to the culture medium. Although it is possible that erythroid potential of these cells was maintained on passage and on transfer into irradiated scid host animals, the fact that the yolk sac cells expressed sca-1 after passage in vitro raises the likelihood that divergence of the erythroid and leukocyte lineages may have occurred during culture. Experiments have now been initiated to document erythropoietic properties of the cultured stem cells and to determine whether donor-type hemoglobin is produced when these stem cells are transferred into irradiated host animals.
A challenging problem is the need to maintain stem cells under conditions that inhibit the differentiation of MHC antigens. The yolk sac, in contrast to later hematopoietic organs, does not express either class I or class II antigens, making it an ideal source of cells for transfer into histoincompatible recipients. However, the stem cells we have obtained in secondary cultures do express, albeit weakly, MHC class I antigens (Fig. 4). This is further evidence that these stem cells are further along the developmental pathway than the cells originally placed in C166 cells.
Perhaps our most pivotal finding is that, even after extensive expansion in vitro, there are a significant number of cells capable of reconstituting adult immunocompromised allogeneic host animals. The fact that C57BL/6 (H-2b) yolk sac-derived cells could be recovered several months after transfer into BALB/c (H-2d) scid mice is encouraging, especially because these host mice have a normal complement of NK cells that might have been expected to eliminate the donor cells when, after differentiation, they begin to express MHC alloantigens (27, 28).
There has been a prolonged controversy concerning the primary site of hematopoietic stem cells during embryonic development. What has been clearly shown is that at an earlier stage of development (day 6–7), embryonic stem cells have the ability to give rise to “blood islands”—i.e., be induced to form hematopoietic as well as vascular endothelial cells (29, 30). This is not surprising, given the ability of such cells to form complete embryos. Between day 7 and 9, however, there appear to be two sites, one intraembryonic [the aorta/gonad/mesonephric, or AGM, region, which includes the primordial germ cells (cf. refs. 8, 16, and 30)], and the other extraembryonic (the yolk sac). True vascular traffic of stem cells cannot be taking place at this time, because a circulating vascular system arises only at late day 9 or day 10 of development (1, 2, 31). However, the present report involves study of AA4.1+ cells from day 10, which could conceivably represent cells that have migrated to the yolk sac from an intraembryonic site. Studies with yolk sac-derived AA4.1+ cells from day 8–9, now in progress, may help to resolve this question.
The long-term maintenance of proliferating yolk sac-derived stem cells has two practical implications: (i) the generation of cells that can serve as a vehicle for gene transfer; and (ii) the generation of a pool of cells that can be used for hematopoietic restitution (32–35). A recent report describes experiments that show that yolk sac-derived stem cells transfected in vitro with cDNA for human growth hormone subsequently secrete this hormone in amounts detectable in the serum of genetically compatible or immunocompromised recipient animals (35). It remains to be established, however, whether complete immunological functions will be restored following in vitro expansion and subsequent transfer of YS-HSCs into transiently immunocompromised histoincompatible host animals.
Acknowledgments
We thank Larry Morrissey for cell sorting, Louis Kubai for help with dissections, Jim Roth for flow cytometry, Barbara Gilligan for overall laboratory management, and Wanda Auerbach for significant editorial assistance. We appreciate the antibodies and cell lines contributed by Paul Kincade, Irving Weissman, Kenneth Dorshkind, Linda Cioffi, and J. P. McKearn, as well as growth medium supplements generously provided by Progenitor, Inc (Columbus, OH). The endothelial cell studies were supported by National Institutes of Health Grants EY 4203 and HL 52148. L.-S.L. was supported by a gift from Progenitor, Inc. to the University of Wisconsin Foundation.
Footnotes
Abbreviations: YS-EC, yolk sac-derived endothelial cell; YS-HSC, yolk sac-derived hematopoietic stem cell; MHC, major histocompatibility complex; FBS, fetal bovine serum.
References
- 1.Moore M A, Metcalf D. Br J Haematol. 1970;18:279–295. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 2.Russell E S. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- 3.Wang R, Clark R, Bautch V L. Development (Cambridge, UK) 1992;114:303–316. doi: 10.1242/dev.114.2.303. [DOI] [PubMed] [Google Scholar]
- 4.Huang H, Auerbach R. Proc Natl Acad Sci USA. 1991;90:10110–10114. doi: 10.1073/pnas.90.21.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C P, Auerbach R. Development (Cambridge, UK) 1991;113:1315–1323. doi: 10.1242/dev.113.4.1315. [DOI] [PubMed] [Google Scholar]
- 6.Huang H, Auerbach R. Exp Hematol (Charlottesville, Va) 1994;22:19–25. [PubMed] [Google Scholar]
- 7.Palacios R, Imhof B A. Proc Natl Acad Sci USA. 1993;90:6581–6585. doi: 10.1073/pnas.90.14.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auerbach R, Huang H, Lu L S. Stem Cells. 1996;14:269–280. doi: 10.1002/stem.140269. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa M. Blood. 1993;81:2844–2853. [PubMed] [Google Scholar]
- 10.Sutherland H J, Lansdorp P M, Henkelman D H, Eaves A C, Eaves C J. Proc Natl Acad Sci USA. 1990;87:3584–3588. doi: 10.1073/pnas.87.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verfaillie C, Blakolmer K, McGlave P. J Exp Med. 1990;172:509–520. doi: 10.1084/jem.172.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye Z Q, Burkholder J K, Qiu P, Schultz J C, Shahidi N T, Yang N S. Proc Natl Acad Sci USA. 1994;91:12140–12144. doi: 10.1073/pnas.91.25.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoder M C, Papaioannou V E, Breitfeld P P, Williams D A. Blood. 1994;83:2436–2443. [PubMed] [Google Scholar]
- 14.Yoder M C, King B, Hiatt K, Williams D A. Blood. 1995;86:1322–1330. [PubMed] [Google Scholar]
- 15.Lu L-S, Wang S-J, Auerbach R. Colloq INSERM. 1995;235:33–36. [Google Scholar]
- 16.Wang S J, Greer P, Auerbach R. In Vitro Cell Dev Biol Anim. 1996;32:292–299. doi: 10.1007/BF02723062. [DOI] [PubMed] [Google Scholar]
- 17.Fennie C, Cheng J, Dowbenko D, Young P, Lasky L A. Blood. 1995;86:4454–4467. [PubMed] [Google Scholar]
- 18.Godin I, Dieterlen-Lievre F, Cumano A. Proc Natl Acad Sci USA. 1995;92:773–777. doi: 10.1073/pnas.92.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKearn J P, Baum C, Davie J M. J Immunol. 1984;132:332–339. [PubMed] [Google Scholar]
- 20.Wysocki L J, Sato V L. Proc Natl Acad Sci USA. 1978;75:2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNiece I K, Bertoncello I, Kriegler A B, Quesenberry P J. Int J Cell Cloning. 1990;8:146–160. doi: 10.1002/stem.5530080302. [DOI] [PubMed] [Google Scholar]
- 22.Jenkinson E J, Franchi L L, Kingston R, Owen J J. Eur J Immunol. 1982;12:583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 23.Aizawa S, Yaguchi M, Nakano M, Toyama K, Inokuchi S, Imai T, Yasuda M, Nabeshima R, Handa H. Exp Hematol (Charlottesville, Va) 1994;22:482–484. [PubMed] [Google Scholar]
- 24.Muench M O, Schneider J G, Moor M A. Exp Hematol (Charlottesville, Va) 1992;20:339–349. [PubMed] [Google Scholar]
- 25.Guba S C, Sartor C I, Gottschalk L R, Jing Y H, Mulligan T. Blood. 1992;80:1190–1198. [PubMed] [Google Scholar]
- 26.Lyman S D, James L, Bos T V, Vries P, Brasel K, Gliniak B, Hollingsworth L T, Picha K S, McKenna H J, Splett R R, Fletcher F A, Maraskovsky E, Farrah T, Foxworthe D, Williams D E, Beckmann M P. Cell. 1993;75:1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- 27.Flamme I, Risau W. Development (Cambridge, UK) 1992;116:435–439. doi: 10.1242/dev.116.2.435. [DOI] [PubMed] [Google Scholar]
- 28.Murphy W J, Kumar V, Bennett M. J Exp Med. 1987;165:1212–1230. doi: 10.1084/jem.165.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Risau W, Sariola H, Zerwes H G, Sasse J, Ekblom P, Kemler R, Doetschman T. Development (Cambridge, UK) 1988;102:471–478. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 30.Dzierzak E, Medvinsky A. Trends Genet. 1995;11:359–366. doi: 10.1016/s0168-9525(00)89107-6. [DOI] [PubMed] [Google Scholar]
- 31.Haar J L, Ackerman G A. Anat Rec. 1971;170:199–223. doi: 10.1002/ar.1091700206. [DOI] [PubMed] [Google Scholar]
- 32.Aguila H L, Weissman I L. Blood. 1996;87:1225–1231. [PubMed] [Google Scholar]
- 33.Nolta J A, Smogorzewska E M, Kohn D B. Blood. 1995;86:101–110. [PubMed] [Google Scholar]
- 34.Dunbar C E, Emmons R V. Stem Cells. 1994;12:563–576. doi: 10.1002/stem.5530120604. [DOI] [PubMed] [Google Scholar]
- 35.Corn B J, Reed M A, Dishong S L, Li Y, Wagner T E. Clin Biotechnol. 1991;3:15–19. [Google Scholar]


