Abstract
Recombinant expression of the Aryl Hydrocarbon Receptor (AhR) yields small amounts of ligand-binding competent AhR. Therefore, Spodoptera frugiperda (Sf9) cells and baculovirus have been evaluated for high level and functional expression of AhR. Rat and human AhR were expressed as soluble protein in significant amounts. Expression of ligand-binding competent AhR was sensitive to the protein concentration of Sf9 extract, and co-expression of the chaperone p23 failed to affect the yield of functional ligand-binding AhR. The expression system yielded high levels of functional protein, with the ligand-binding capacity (Bmax) typically 20-fold higher than that obtained with rat liver cytosol. Quantitative estimates of the ligand-binding affinity of human and rat AhR were obtained; the Kd for recombinant rat AhR was indistinguishable from that of native rat AhR, thereby validating the expression system as a faithful model for native AhR. The human AhR bound TCDD with significantly lower affinity than the rat AhR. These findings demonstrate high-level expression of ligand-binding competent AhR, and sufficient AhR for quantitative analysis of ligand-binding.
Keywords: Dioxin, PCB, ligand-binding, aryl hydrocarbon receptor, species difference
Introduction
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a ubiquitous toxin, and a prototypical representative of a series of chemicals which effect toxicity through a common mechanism, binding to the Aryl Hydrocarbon Receptor (AhR) [1, 2]. Mouse genetic studies demonstrated that dioxin toxicity is mediated through the AhR locus [2], and the subsequent cloning of the AhR [3, 4] characterised the AhR as a transcription factor. The creation of AhR knockout mice enabled a confirmation that AhR was required for dioxin toxicity [5] [6] [7] [8].
For a variety of persistent ligands, the affinity of the AhR for the ligand is directly related to toxicity endpoints [1, 2]. Hence determining the affinity of ligands for AhR gives key information for evaluating toxicity. The AhR ligand-binding assay is exquisitely sensitive to minor changes in methodology e.g. [9, 10], and this leads to large differences (greater than 100-fold) in measured affinity of AhR for TCDD between different laboratories, e.g. [1, 10-12]. Therefore, measured affinity constants for AhR ligands are not necesarily directly comparable between laboratories.
There are marked species differences in the toxicity of TCDD [2], and it is important to determine if these differences are due to binding to the AhR, and if they are common to other ligands of the AhR. The relative affinity of the human and mouse AhR for TCDD is known [13-15], but the affinity of the human AhR for other ligands has not been determined [12, 16]; this knowledge deficit arises partly because of difficulties in obtaining fresh human tissue, but also because analysis of AhR in human tissues is affected by the lability and low levels of the human AhR [16, 17]. Consequently, it is problematic to compare the affinity of human AhR for ligands with AhR affinity data for toxicologically relevant model species, if this latter data is generated in a different laboratory.
Hence a recombinant expression system would enable reliable comparison between AhR preparations in the same laboratory under controlled conditions. Whilst reticulocyte lysates are capable of producing AhR with ligand-binding functionality [3], the amount of AhR produced in this system is small and quantitative assays require considerable amounts of lysate [18], effectively precluding this method for comparison of multiple ligands in multiple species. Mammalian cells are also capable of producing small amounts of functional AhR, but Ramadoss et al. [19] found limitations in saturation binding analysis in vitro with this system, and were unable to determine a Kd for ligand-binding to AhR in vitro. Baculovirus infection of insect (Spodoptera frugiperda) cells is a system capable of producing high levels of recombinant protein, that has been exploited for a variety of transcription factors. Whilst human AhR has been expressed in this system [20], there was no quantitative measurement of the amount or affinity of the ligand-binding AhR in this system. This is a prerequisite for studies on ligand-binding, and for validating that the AhR is correctly folded. Thus there is a pressing need for a recombinant expression system that can yield high levels of functional AhR, and that can be used in a saturation binding analysis to give quantitative measures of ligand affinity with AhRs from different species.
We have investigated the use of baculovirus for recombinant expression of the AhR. We characterise the biochemical parameters required for functional expression of the AhR in this system, quantify the amount and ligand-binding functionality of expressed AhR, and show that it faithfully reflects the native receptor. The high-level expression of functional AhR has numerous applications, and as an example, we use the recombinant rat and human AhR to demonstrate and quantify binding affinity for a number of ligands.
Materials and Methods
Materials
TCDD and congeners (PCDD, 1,2,3,7,8-pentachlorodibenzo-p-dioxin; TCDF, 2,3,7,8-tetrachlorodibenzofuran; PCDF, 2,3,4,7,8-pentachlorodibenzofuran; PCB126, polychlorinated biphenyl 126) were obtained from Cambridge Isotope laboratories, Mass, USA, at high purity (99%). 2,3,7,8-tetrachloroazoxybenzene (TCAOB) was a kind gift from Dr. A. G. Smith (MRC Toxicology Unit, Leicester, UK). [3H]-TCDD was obtained from Eagle-Picher (Kansas, USA), and was at 27.7 Ci mmol-1. All other chemicals were also of the highest quality available. Rats were obtained from Charles River Laboratories, UK.
Sub-cloning of rat and human AhR cDNAs
A rat (Charles River Wistar- CRL:WI) was killed, and liver was homogenised in TRIzol Reagent (Invitrogen). Total RNA was extracted from liver according to the manufacturer's manual. mRNA was purified by Oligotex mRNA Spin-column (Qiagen) according to the manufacturer's manual. RT-PCR (Prostar Ultra HF RT-PCR kit (Stratagene)) was used to clone rat AhR and its ligand-binding domain sequences according to the manufacturer's instructions. The PCR primers were designed based on the known Sprague-Dawley rat AhR cDNA sequence (Table 1), and the first nine amino acids (leader sequence) were deleted. Rat AhR cDNA was divided into two pieces (primers rA5 and rALB3; rABam3 and rLBDreverse), amplified separately and ligated together at the BamHI site. Rat AhR ligand-binding domain was amplified by primers rALB5 (forward), which contains a novel Sal I site, and rALB3 (reverse) containing the Hind III site and six-histidine tag. All PCR products were subcloned into either pGEMT-Easy (Promega) or pPCR Script (Stratagene) plasmid, and analysed by double-strand DNA sequencing. The AhR cDNA clones were then inserted into plasmid pFastBac1 between the SalI and HindIII sites.
Table 1. Sequence of oligonucleotides used in PCR.
| Gene name primer | Sequence |
|---|---|
| Rat AhR
AAA56897 |
|
| rALB5 (forward) | gtcgacatgGCAATGAATTTCCAAGGGAGGTTAAAGTATC |
| rALB3 (reverse) | aagcttctagtgatggtgatggtgatgCAAGGGATCCATTATGGGAGAGAAAGG |
| rA5 (forward) | gtcgacatgGGCAGCCGCAAGCGGCGC |
| rABam3 (forward) | CTCTCCCATAATGGATCCCTTGC |
| rLBDreverse | AAGCTTCTAGTGATGGTGATGGTGATGCAGGAATCCGCTGGGTGTGATATCAGG |
|
| |
| Human AhR
AAA16210 |
|
| hA5 (forward) | gtcgacatgGCCAGTCGCAAGCGGCGGAAG |
| hA3 (reverse) | aagcttctagtgatggtgatggtgatgCAGGAATCCACTGGATGTCAAATCAGG |
| hALB5 (forward) | gtcgacatgGCAATGAATTTCCAAGGGAAGTTAAAGTATC |
| hALB3 (reverse) | aagcttctagtgatggtgatggtgatgTAAGGGATCCATTATGGCAGGAAAAGG |
| V381A | CCAGATTATATCATTGCAACTCAGAGACCGTTAACAGATGAGGAAGGAAC |
PCR Primers for cloning rat and human AhR and its ligand-binding domain. Sequences based on SD rat AhR are shown in capital letters. Introduced restriction sites are shown by underlining, the start codon is shown in bold, and the hexa-histidine sequence is also in bold font. For the mutagenesis oligo (V381A), the mutated codon is in bold, and the selection restriction enzyme site is underlined. Sequences are shown 5′ to 3′.
Human AHR cDNA, and its ligand-binding domain, were amplified by PCR from plasmid pSporthAhr2 (Susan Moran, Mcardle Lab. for Cancer Research, University of Wisconsin-Madsion Medical School); the primers are listed in Table 1. The human AHR-His and hAHR-LBD-His fragments were then subcloned into Sal I and Hind III sites of plasmid pFastBac1.
The plasmid pFastBac1.Gus (Invitrogen) was used as a positive control to prepare recombinant β-glucuronidase (GUS) baculovirus, using the same method as described above. Baculovirus expressing human p23 was described previously [21], and was a kind gift from David O. Toft (Mayo Graduate School, Rochester, MN 55905, USA).
Site-directed mutagenesis
Quick-change mutagenesis kit (Stratagene) was used to generate the V381A mutant of the hAhR LBD construct, according to the manufacturer's instructions. The amino acid at 381 is variant between rat and human (V/A), and mutation of this amino acid is known to confer high affinity binding [13, 14]. The mutant primer is shown in Table 1. The plasmid pFast-Bac1/hAhR-LBD was used as the template. The mutation was confirmed by double-stranded DNA sequencing.
Expression of recombinant AhRs
The Bac-to-Bac (Invitrogen) system was used to express recombinant AhRs in insect cells. Briefly, the plasmids pFastBac1/hAhR-His, pFastBac1/hAhR-LBD-His, pFastBac1/V381A-His, pFastBac1/rAhR-His and pFastBac1/rAhR-LBD-His were transformed into bacterial strain DH10Bac. Bacmids were prepared by Qiagen miniprep, and inserts were confirmed by PCR analysis. The bacmids then transfected into Sf9 cells, baculovirus was harvested after 5-7 days incubation and then further amplified in infected Sf9 cells to obtain high titre virus. Virus was directly titred in a plate assay, and high titre virus stocks were used to infect Sf9 cells and express recombinant AhRs. Virus was added to Sf9 cell culture, and infected Sf9 cells were harvested by centrifugation at 500g for 10 minutes at 48 hours after infection, or as indicated, and all subsequent steps were on ice or at 4°C. Cell pellets were re-suspended in MDEG buffer (25mM MOPS, 1mM DTT, 1mM EDTA, 10% glycerol, pH7.5) containing 20mM molybdate. Cells were broken by sonication, and cell debris was removed by centrifugation at 12,000g for 10 minutes. The supernatant was further centrifuged at 200,000g for 30 minutes. The final cytosolic supernatants were divided into small aliquots and stored at -80°C. For diafiltration, cytosol was diafiltered with a 10k Molecular Weight cut-off membrane with MDEG buffer at 4°C. For addition of ATP/ Mg2+, these were added in MDEG buffer with 2mM ATP, 5mM MgCl2.
Ligand binding assay
The method for [3H]-TCDD binding to AhR was previously established by [1]. Typically, rat liver cytosol was diluted to 5mg/ml in MDEG buffer (25mM MOPS, 1mM DTT, 1mM EDTA, 10% glycerol, pH7.5) containing 20mM molybdate. Then the sample was incubated with [3H]-TCDD or [3H]-TCDD plus a 200-fold excess of competitor 2,3,7,8-tetrachloroazoxybenzene (TCAOB) at 4°C overnight. After incubation, 30μl of dex-tran-coated charcoal suspension (67mg/ml, prepared in MDEG buffer) was added into a 200μl sample of the mixture. The suspension was incubated on ice for 10min, and then was centrifuged at 25,000g for 10min. 150μl of the supernatant was removed and radioactivity was measured in a scintillation counter. Specific binding was defined as the difference of radioactivity between without (total binding) and with competitor TCAOB (non-specific binding). For recombinant rat AhR protein, either 0.25mg or 0.5mg/ml cytosol protein was used for binding assay, and supplemented with BSA to a final protein concentration of 5mg/ml; 1mg/ml recombinant human AhR with 4mg/ml BSA was used for assay unless otherwise stated. For competition assay, a serial dilution of competitor was incubated with 0.5nM [3H]-TCDD (except where otherwise stated), and the specific binding was determined as described above. Specific binding or Ki was determined by using non-linear regression, using Graphpad 4/5, fitting a saturation binding isotherm, or one-site competitive binding equation, to the experimental data. Ki was derived from the IC50 using the Cheng-Prusoff equation, as implemented in Graphpad.
Hydroxylapatite assay
200μl sample was treated with charcoal as described above; the supernatant was transferred into a fresh tube, then 200μl 50% hydroxyapatite (HAP) was added. After incubation on ice for 30 minutes, the HAP resin was spun down at 25000g for 1 minute at 4°C. The pellet was washed twice with 1ml MDEG buffer containing 0.1% Tween 20, and then the protein was eluted into 0.5ml ethanol. The supernatant was assayed for radioactivity by liquid scintillation counting. Analysis of saturation-binding data was as described for the charcoal assay above.
Western blotting
Protein was run on polyacrylamide gel electrophoresis (PAGE) (either SDS-PAGE, or pre-cast gels (Invitrogen) using lithium dodecyl sulphate), and transferred to a blotting membrane. Signal was detected using Enhanced Chemiluminescence (ECL; GE Healthcare) and exposure to a film. The antibody against p23 was obtained from Stressgen Bioreagents Corporation (Assay Designs, Inc., 5777 Hines Drive, Ann Arbor, Michigan 48108 USA). The LBD of the mouse AhRb-1 allele cDNA [14] was amplified with oligos 282 and 416 (amino acids 282-416; Table 1), and subcloned into the BamHI site of pRSETc (Invitrogen). The AhR LBD was induced in BL21(DE3) bacteria, and the insoluble protein was solubilised in 6M guanidine HCl, 50 mM Tris, pH 8.0, and purified by Ni2+-affinity chromatography. The purified protein was used to immunise rabbits, and antiserum harvested when the bleeds were capable of detecting <1ng of antigen.
Statistical analysis
Data is presented as mean and standard deviation, except where otherwise noted. Statistical analysis used t-test where appropriate. For calculation of confidence intervals from saturation, and competition ligand-binding, Graphpad 5 was used for calculation of 95% confidence limits subsequent to non-linear curve-fitting.
Results
Generation of baculovirus constructs and expression of protein
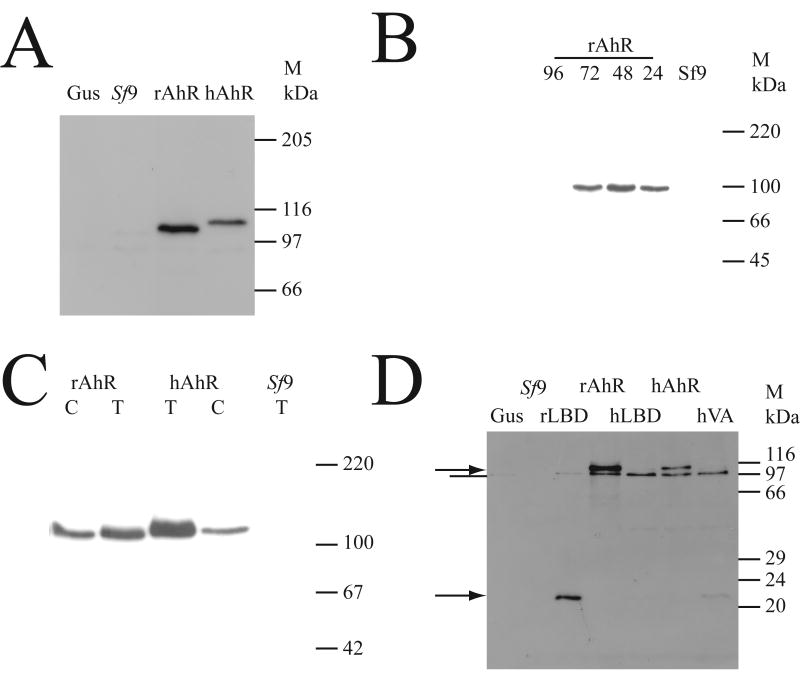
The AhR cDNA was cloned from the liver of a CRL:WI rat, and the sequence of the constructs (Accession number AJ821851) was confirmed by double-stranded sequencing to be identical for that of the Sprague-Dawley rat (AAA56897). The human and rat cDNAs were cloned into recombinant baculovirus, used to infect Sf9 insect cells, and the presence of AhR determined using western blotting (Fig. 1A). Cytosol from uninfected cells showed no noticeable cross-reactivity with the antibody, and cells infected with a virus expressing β-glucuronidase also failed to show any cross-reactivity, demonstrating that viral infection did not cause any cross-reacting proteins. Virus expressing rat and human AhR both gave rise to strong immunoreactive bands in cytosol, with the human AhR migrating at a slightly higher apparent molecular mass than the rat AhR, consistent with previous reports [22]. This shows that the AhR protein was specifically expressed and detected in Sf9 cells. The time course of expression of cytosolic AhR protein was examined (Fig. 1B); AhR protein was maximally induced at 48 hours after infection, and this time point was used for further studies. Comparison of the amount of AhR protein in total cell lysates versus cytosol (Fig. 1C) showed that both the human and rat AhR were more abundant in total cell protein, compared to cytosol. This effect was more obvious for the human AhR, than for the rat AhR. The full-length and Ligand-Binding Domain (LBD) constructs were then expressed at an equal Multiplicity of Infection with virus (MOI), to determine if there was adequate expression of the recombinant proteins in cytosol (Fig. 1D). While the expression of the full-length human and rat AhR proteins was adequate, the expression of the LBD constructs was less than that seen for the full-length constructs. The rat LBD was present in cytosol at acceptable levels, but the human AhR LBD and the LBD-Val381Ala mutant were present at too low a level for comparison with the rat LBD or full-length constructs (Fig. 1D). Thus the Sf9 expression system yielded high levels of expression of both rat and human full-length AhR proteins in cytosolic extracts.
Fig. 1. Recombinant expression of AhR protein in Sf9 cells.
(A) Sf9 cells were uninfected (Sf9), or infected with baculovirus (at an MOI of 10) encoding β-glucuronidase (Gus), rat AhR (rAhR) or human AhR (hAhR); cells were lysed 48 hours after infection, and cytosol prepared as described in the methods section. 10 μg of cytosolic protein was western blotted, and detected with a polyclonal antibody against the AhR; signal was visualised by ECL. The position of the molecular weight markers (M) is shown in kDa. (B) Sf9 cells were uninfected (Sf9), or infected with rat AhR virus (rAhR), and cytosol prepared at the indicated time after infection. 10 μg of cytosolic protein was western blotted for AhR as described in (A). (C) Cytosol (C) or total protein (T) was isolated from Sf9 cells infected (MOI=10) with rat AhR, or human AhR, at 48 hours after infection; total protein from uninfected Sf9 cells is a control lane. 10 μg of protein was western blotted for AhR as described in (A). (D) Sf9 cells were infected at MOI=10 and cytosol prepared 48 hours later. Cells were uninfected (Sf9), or infected with virus encoding β-glucuronidase (Gus), rat AhR (rAhR), rat AhR Ligand-Binding Domain (LBD) (rLBD) or human AhR (hAhR), human AhR LBD (hLBD), or the V381A mutant of the human AhR LBD (hVA); samples were western blotted with an antibody against the AhR, and detected by ECL. The position of the full-length AhR and the AhR LBD proteins is shown by an arrow; a band of cross-reactive protein is indicated by a line. The position of the molecular weight markers (M) is shown in kDa.
Ligand-binding assay
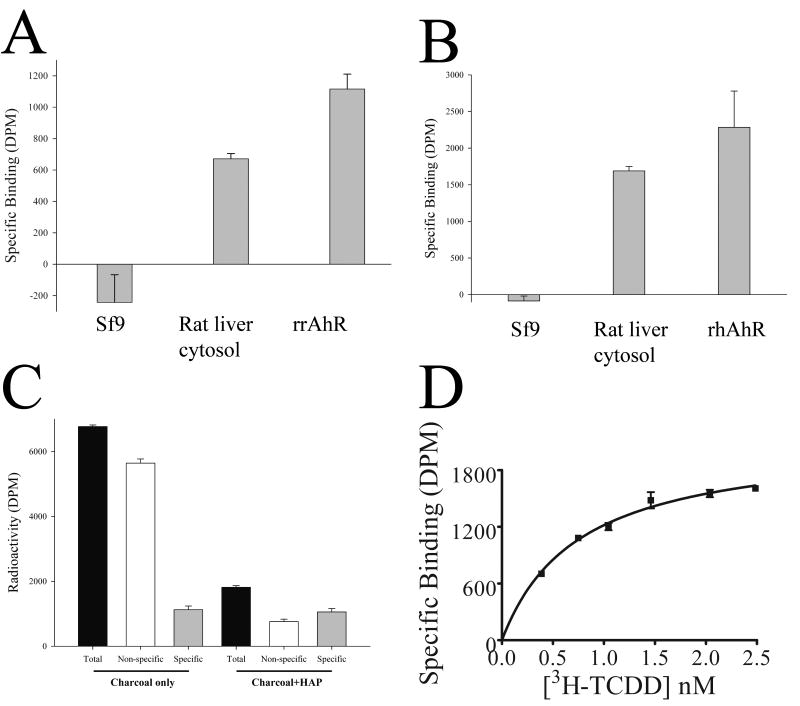
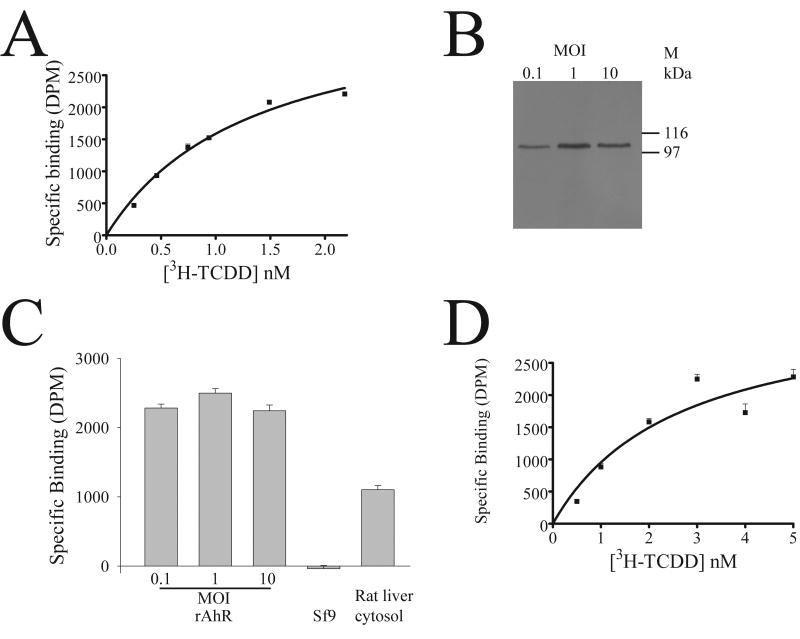
Given that the expression system yielded cytosolic full-length human and rat AhR protein, it was necessary to test whether the protein was capable of specifically binding a ligand; the prototypical ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was used, with rat liver cytosol as a positive control reagent. Fig. 2A, B shows that uninfected Sf9 cytosol (a negative control) showed no specific binding to tritiated TCDD, but that rat liver cytosol bound TCDD. Cytosol from Sf9 cells infected with either rat AhR (A) or human AhR (B) showed specific binding to TCDD, demonstrating that the recombinant AhR protein is capable of binding ligand. The binding assay was robust to the use of 2,3,7,8-tetrachlorodibenzofuran (TCDF) or tetrachloroazoxybenzene (TCAOB) as a competitor to determine specific binding (unpublished data), and the amount of specific binding was not significantly different when using a protocol with charcoal, or charcoal followed by an additional hydroxyapatite step (Fig. 2C). The assay showed specific binding to TCDD with rat liver cytosol (Fig. 2D), with saturation binding analysis for rat cytosol yielding an apparent Kd for TCDD of 1.0 ± 0.45 nMolar (mean ± Standard deviation, n=6), and a maximal binding capacity (Bmax) of 52 fmol/mg, within the range of previous estimates [1, 10, 12].
Fig. 2. Specific binding of TCDD by recombinant baculovirus extracts.
(A) cytosol was prepared from rat liver, Sf9 cells (Sf9) or Sf9 cells infected with rat AhR (rrAhR), as described in Fig. 1. Cytosol was assayed for ligand-binding as described in materials and methods, with rat liver and Sf9 cytosol at 5 mg/ml, and 0.5 mg/ ml rat AhR cytosol made up to 5 mg/ml with BSA; [3H]-TCDD is present in the binding assay at a concentration of 1nM. Specific binding is shown in disintegrations per minute (DPM). Results are shown as mean ± SD (n=3); results are representative of multiple experiments. (B) the binding assay was carried out as for A, except that the cytosol from Sf9 cells infected with virus encoding human AhR (rhAhR), had a protein concentration of 1 mg/ml, made up to 5 mg/ml with BSA. (C) rat liver cytosol was subjected to a binding assay, as described in materials and methods. After charcoal adsorption (Charcoal only), the total radioactivity in solution (Total) was compared to that with a 200-fold excess of TCAOB (Non-specific), and the Specific binding (Specific) is the total minus non-specific binding. Alternatively, the post-charcoal supernatant was subjected to hydroxyapatite treatment (Charcoal + HAP), and the Total, Non-specific and Specific are shown. Results are shown as mean ± SD (n=3). (D) rat liver cytosol (5 mg/ml) was subjected to saturation binding analysis, and specific binding analysed by non-linear regression, as described in the materials and methods. For this experiment, the Kd is 0.75 nM (95% Confidence limits 0.54-0.96nM), and the Bmax is 52 fmol/mg cytosolic protein.
Optimisation of recombinant expression for ligand-binding competent AhR
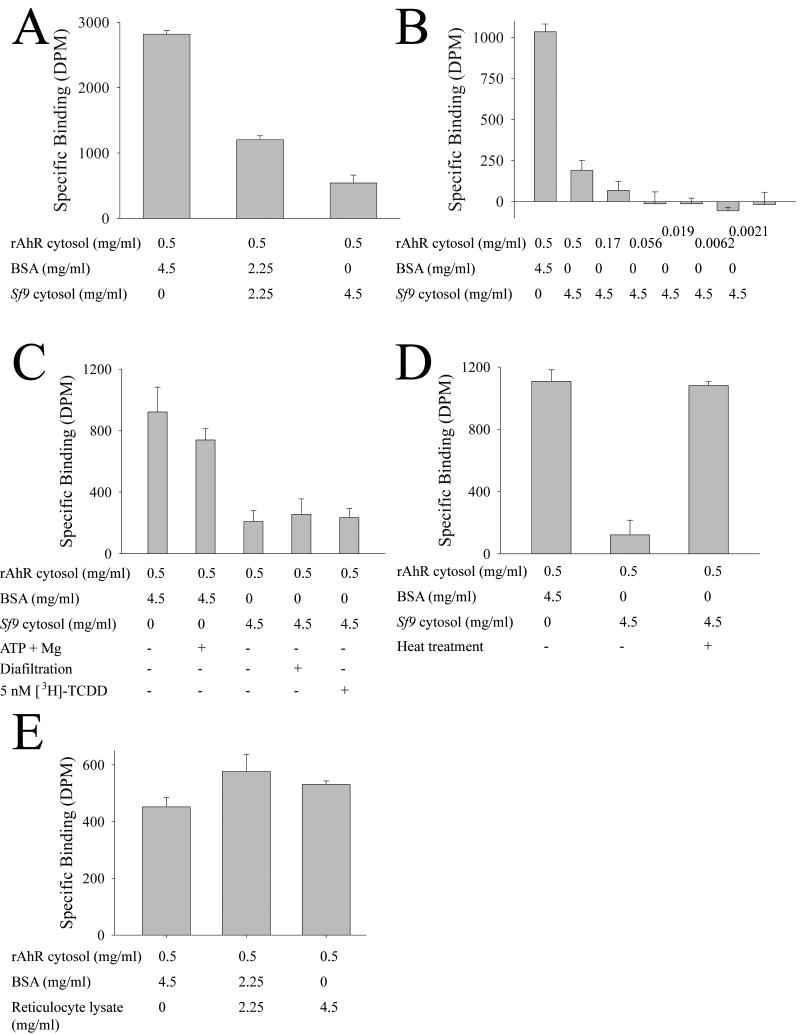
Protein concentration is a key variable for TCDD binding assays, since TCDD solubility is critically dependent upon protein concentration (DRB, unpublished data; [9, 10]), and hence the binding assays were made up to 5 mg/ml final protein concentration with BSA. Although the Sf9 cytosols expressing AhR showed specific binding, the specific binding was dependent upon the concentration of Sf9 cytosol (DRB, unpublished data). Fig. 3A shows that addition of uninfected Sf9 cytosol inhibited the binding of TCDD to recombinant rat AhR in a dose-dependent manner, and that the non-specific protein, BSA, did not inhibit binding of TCDD to the AhR. Since the binding assay represents the difference between total TCDD in solution, and the TCDD present after addition of a ∼200-fold molar excess of unlabelled competitor (i.e. the high capacity and “non-specific” binding), it was possible that this result could have been caused by the addition of the Sf9 cytosol increasing the amount of high-affinity binding sites, such that the unlabelled competitor simply bound to the high-affinity binding sites, but was no longer able to displace [3H]-TCDD since the high affinity binding sites are in excess. However, Fig. 3B shows that dilution of the rat AhR cytosol results in a decrease in the specific binding, and excludes the possibility that the Sf9-mediated decrease in binding is due to an increase in the amount of specific binding sites. The addition of ATP and Mg2+ to the BSA solution did not affect the specific binding of the rat AhR, although these agents are known to be essential for hsp90-mediated protein interactions with AhR [23]. Extensive diafiltration of the Sf9 cytosol failed to reduce the ability of Sf9 cytosol to inhibit the binding of rat AhR to TCDD, excluding the possibility of a low molecular weight AhR ligand being present in the Sf9 cytosol (Fig. 3C). Finally, performing the ligand-binding assay in the presence of a high concentration of TCDD also had no effect on the inhibition of rat AhR by Sf9 cytosol, confirming that the effect is not caused by a surfeit of AhR binding sites. The known dependence of AhR folding on protein interactions (e.g. [23, 24]) suggested that proteins in the Sf9 cytosol may be causing the AhR to adopt an improperly folded conformation; to test this, the Sf9 cytosol was heat treated, whereupon it then failed to inhibit the binding of rat AhR to TCDD (Fig. 3D). Reticulocyte lysates are known to be able to support the folding of AhR into a ligand-binding capable form (e.g. [25] [14]), and so reticulocyte lysate protein was used to determine if the inhibitory effect of Sf9 protein was specific to Sf9 cells, or a general phenomenon of cell lysates. Fig. 3E shows that the specific binding of rat AhR to TCDD was unaffected by reticulocyte lysate protein over a range of 0-5 mg/ml, thereby showing that the effect of Sf9 cytosol in inhibiting the binding of TCDD to recombinant rat AhR is specific to the Sf9 cytosol, as opposed to reticulocyte lysate or BSA solution.
Fig. 3. Factors affecting specific binding of rat AhR in Sf9 cytosol.
(A) TCDD binding assays were performed as described in Figure 2, with the exception that cytosol from uninfected Sf9 cells (Sf9 cytosol) was titrated in at the indicated concentration. Results are shown as mean ± SD (n=3), and are representative of results obtained on at least two occasions. (B) as for A, but the concentration of Sf9 cytosol containing rat AhR was varied. (C) as for A, with the exception that Sf9 cytosol from uninfected cells was diafiltered as indicated, or the TCDD binding assay was performed in the presence of 5nM TCDD (instead of 1nM) where indicated. (D) As for A, with the exception that the Sf9 cytosol from uninfected cells was subject to heat treatment (65°C for 10 minutes) prior to use in the assay, as indicated. (E) as for A, but rabbit reticulocyte lysate and BSA were used to make the rat AhR up to 5 mg/ ml protein concentration.
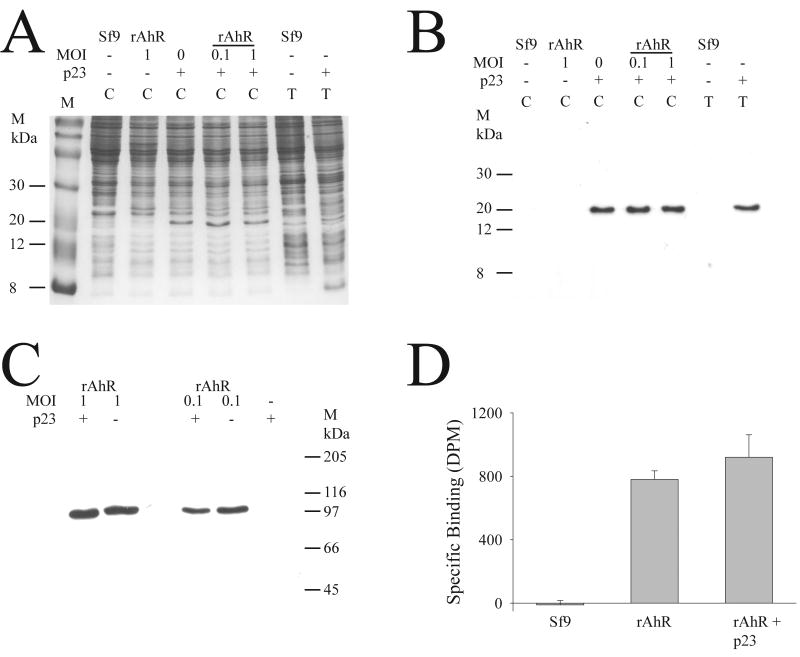
It has previously been shown that p23 is an essential chaperone for correct folding of the glucocorticoid receptor [21], and it is known that p23 associates with the AhR. In order to determine if p23 could enhance the folding of AhR in Sf9 cells, rat AhR was co-expressed with human p23. Fig. 4A shows that baculovirus expression of p23 yields sufficient soluble p23 for visual detection in a coomassie-stained gel, and immunodetection confirmed that the induced band was indeed p23, and neither Sf9 cells, nor AhR-encoding baculovirus caused any cross-reactivity (Fig. 4B). AhR was co-expressed with p23 protein, and there was no obvious effect on the amount of AhR protein that was present in cytosolic extracts in the presence and absence of co-expression of p23 (Fig. 4C); this was true when the rat AhR was expressed at low levels (MOI of 0.1) and at higher levels of expression (MOI=1). The functionality of the expressed rat AhR protein was examined by ligand-binding assay, and there was no significant difference in the amount of specific binding of TCDD between cytosols containing rat AhR with and without p23 protein (Fig. 4D). Thus the expression of p23 had no effect on the yield of cytosolic protein, nor the functionality of expressed AhR under these conditions in Sf9 cells.
Fig. 4. Coexpression of p23 and rat AhR in Sf9 cells.
(A) Sf9 cells were infected with p23 (at an MOI of 1; p23), and/ or with rat AhR (rAhR), at the indicated MOI. Uninfected Sf9 cells (Sf9) were used as a control. Total (T) or cytosolic (C) fractions were isolated, and 10 μg of protein was electrophoresed, followed by staining of the gel with coommassie blue. The position of molecular weight markers (M) is indicated. (B) the samples in A were subjected to western blotting and immunodetected with an antibody against p23, using methodology as described in Fig. 1. (C) cytosol was prepared from cells infected with p23 (MOI=1), and rat AhR (rAhR) at the indicated MOI; cells infected with p23 alone served as a control. The samples were western blotted with an antibody to AhR, as described in Fig. 1. (D) Samples of cytosol prepared from an infection with rat AhR at an MOI of 0.1 (rAhR), with or without p23 at an MOI of 1 (rAhR+p23), and uninfected Sf9 cells (Sf9) were used for a TCDD binding assay, using standard conditions as described in materials and methods. Results are shown as mean ± SD (n=3).
Determination of affinity of rat and human AhR for TCDD
In view of the consistent cytosolic expression of rat and human AhR, and qualitative determination of ligand-binding ability, saturation binding analysis was undertaken to quantitate the amount and affinity of binding. When cytosol containing recombinant rat AhR was produced at an MOI of 0.1, the resulting AhR showed saturable binding to TCDD (Fig. 5A), with a Kd of 1.34 ± 0.32 nM (n=6 independent determinations). This value was not significantly different from the values we obtained for the AhR in rat liver cytosol (1.0 ± 0.45nM, n=6, Fig. 2D), demonstrating that the baculovirus expression system yields faithful expression of functional rat AhR. The Bmax was 1.5±0.37 pmol/ mg cytosolic protein, ∼20-fold higher than in rat liver cytosol (Fig. 2D). Expression of rat AhR with higher MOI yielded slightly higher amounts of cytosolic AhR protein (Fig. 5B), but no increase in the amount of specific binding to TCDD (Fig. 5C). In order to confirm that the recombinant expression system could functionally express AhR from different organisms, the affinity of TCDD for human AhR under optimised conditions was determined, yielding a Kd of 2.77± 0.94 nM (n=6), with a Bmax of 0.46±0.12 pmol/mg cytosolic protein (Fig. 5D). Although we did not determine the affinity of native human liver AhR, this data is consistent with previous reports in showing that the human AhR has a significantly reduced affinity for TCDD compared to rat [13] [12, 15] [16].
Fig. 5. Effect of expression level of AhR on AhR functionality.
(A) Sf9 cytosol was prepared after infection with rat AhR-encoding baculovirus at an MOI of 0.1, and assayed for saturation binding; a typical experiment is shown, with each data point representing triplicate determinations, and showing mean ± SD (n=3). The Kd was 1.35nM, and the Bmax was 1.83 pmol/mg. (B) western blotting of AhR. Sf9 cells were infected with virus encoding rat AhR at an MOI of 0.1-10 (as indicated). Cytosol samples were prepared at 48 hours after infection, and 10 μg of each sample western blotted for AhR, as described in Fig. 1. (C) cytosol samples prepared at different MOIs, as described in (B), were assayed for TCDD binding, with the addition of uninfected Sf9 cytosol (Sf9), and rat liver cytosol as a positive control. Results are shown as mean ± SD (n=3). (D) A representative saturation binding isotherm for recombinant human AhR is shown, with each data point representing triplicate determinations, and showing mean ± SD (n=3). The Kd was 2.6 nM, and the Bmax was 0.42 pmol/mg.
Determination of ligand affinity by competitive binding assay
In order to determine if the expressed AhR could be used to determine the affinity of other ligands, the rat AhR was used in a competition binding assay for a number of congeners of TCDD (Table 2). The competition assays showed good fit to a one-site competitive binding assay equation, and were robust to replication (data not shown). The rat AhR yielded a Ki for TCDD which was not significantly different from the Kd determined from rat liver, or recombinant rat AhR (Fig. 5A), demonstrating that the competitive binding assay with recombinant AhR is robust. Comparison of the ability of various congeners to bind to rat AhR showed that TCDF and PCDD showed a similar affinity as TCDD, but that PCDF was a ∼three-fold less potent ligand than PCDD, and PCB126 was ∼50-fold less potent than TCDD. For the human AhR, TCDD and PCDF were ∼two-fold less potent than TCDF and PCDD, and PCB126 was ∼40-fold less potent as a ligand than TCDD (Table 2). The Ki values for the human AhR consistently showed the same rank order of potency as in the rat; however, as with the Kd values, the human AhR showed a lower affinity for ligands when compared with the rat. Thus these data demonstrate that the recombinant AhR can be used for the determination of ligands in a competitive ligand-binding assay.
Table 2. Competitive binding assay for AhR ligands.
| Compound | Rat AhR Ki (nM) | Human AhR Ki (nM) |
|---|---|---|
| TCDD | 0.55 [0.15-2.1] | 15.4 [9.1-26] |
| 2,3,7,8-tetrachlorodibenzofuran | 0.36 [0.097-1.4] | 6.27 [4.4-8.9] |
| 1,2,3,7,8-pentachlorodibenzo-p-dioxin | 0.46 [0.3-0.71] | 7.62 [4.7-12] |
| 2,3,4,7,8-pentachlorodibenzofuran | 1.5 [0.99-1.71] | 14.1 [8.1-24] |
| PCB126 | 24.7 [15-42] | 736 [410-1300] |
Competitive binding assays were carried out using rat or human AhR, as described in materials and methods. Each experiment was carried out in triplicate, and results are the mean, with 95% confidence limits shown in square brackets.
Discussion
Recombinant expression of ligand-binding competent AhR would have significant advantages for determining the ligand-binding affinity of AhR from a variety of organisms, but expression of AhR is problematic. Bacterial expression of AhR yields insoluble protein [18], and even in insect cells, the majority of the AhR protein produced was in an insoluble 10 000 g pellet (MQF, TJ, unpublished data, [20]). However, the full-length AhR proteins produced cytosolic AhR in insect cells, and these levels were readily detectable (Fig. 1C, D). Thus high levels of cytosolic expression of full-length AhR proteins was achieved, and it was necessary to determine whether these proteins were able to bind ligand.
Surprisingly, the specific binding of TCDD to infected Sf9 cytosols was dependent upon the concentration of Sf9 cytosol in the binding reaction (data not shown, Fig. 3A). The ligand-binding assay for AhR is a complex assay, which depends upon maintaining TCDD in solution by low-affinity binding of TCDD to protein or other macromolecules, and so can be prone to artefactually induced interpretation arising from distinct constituents between Sf9 cytosol and other reagents tested. We firstly demonstrated that this was not due to an artefact, such as the presence of small molecular weight ligands in Sf9 cytosol (Fig. 3C), or the failure of the cold competitor to saturate specific binding (Fig. 3B, C). The fact that heat treatment of Sf9 cytosol abolishes the inhibition of AhR binding (Fig. 3D) suggests that the inhibition is caused by proteins, consistent with previous findings that protein chaperones are essential for AhR folding (e.g. [23, 26-28]). We specifically tested whether p23 could enhance AhR folding; p23 is known to enhance folding of the baculovirus-expressed glucocorticoid receptor in Sf9 cells, and is required for baculovirus-expressed human AhR and ARNT to bind to DNA response elements [29]. However, p23 alone had no effect on AhR solubility or ligand-binding (Fig. 4); this is in contrast to the requirement for p23 with glucocorticoid receptor in Sf9 cells [21]. Thus it remains unclear exactly what proteins are involved in the proper folding of AhR, and this is a key issue for obtaining high yields of AhR for structural studies.
Recombinant cytosolic AhR was tested for ligand-binding functionality, and reproducibly produced saturable ligand-binding to TCDD (Fig. 5A). The Kd for the recombinant expressed AhR was not significantly different from that determined with rat liver cytosols using the same experimental protocol (Fig. 2, Fig. 5), and these are in turn directly comparable with previous determinations [12] [10] [30]. We did not have access to fresh human liver to undertake an analysis of native human AhR ligand affinity, but the affinity of the recombinant human AhR was comparable to literature values [31] [16] [32]. This demonstrates that expression of the AhR using baculovirus yields protein with similar ligand-binding functionality to the native form, and validates the use of recombinant AhR. The relatively high levels of expression of functional recombinant AhR described in this report is an important advance, as this is the first system that generates sufficient functional receptor for e.g. comprehensive ligand-binding studies or protein purification/ structural analyses.
Several invertebrate species have been characterised to have non-ligand-binding AhR genes [33-35], and so it is likely that Spodoptera cells also lack a ligand-binding AhR. In agreement with this, we were unable to detect any reproducible or significant specific binding of [3H]-TCDD in Sf9 cells. However, Sf9 cells are capable of folding the rat and human AhR into a ligand-binding conformation (Fig. 5) that is functionally indistinguishable from the native receptor in terms of Kd. It is apparent that there are no species-specific co-factors that are required for functional reconstitution of ligand-binding functionality of the human or rat AhR. Given the low endogenous background of binding, and the ability to fold heterologous AhR, it is likely that this system will have broad applicability in functional expression of AhR from diverse species.
The human and rat AhR showed robust and saturable ligand-binding affinity for TCDD (Fig. 5), consistent with previous data [12-14], and hence can be used for further ligand-binding analysis. We undertook a comparison of the ability of rat AhR to bind TCDD, and the value in competitive binding assay was not significantly different from that obtained in an equilibrium binding analysis (Table 2). The rat AhR showed specific binding to a range of dioxins and furans, in agreement with prior estimates [1, 2]. The human AHR bound specific ligands in the same rank order of affinity as the rat, but with lower affinity than the rat AhR (Table 2), consistent with the results in Figure 5. The lower affinity of the human AHR for these ligands may lead to artefactually high values for Ki, as the solubility of e.g. TCDD in protein solution declines dramatically at concentrations much in excess of 2 nM (unpublished data). These experiments have been performed under the same conditions, which enhances the reliability of the comparison, and are novel information in defining the affinity of human AHR for environmentally-important ligands. The congeners described account for a substantial proportion (∼90%) of the human dietary exposure to dioxin-like congeners, or TEQ (MR, AF, SW, unpublished data, [36]). Since receptor-ligand binding is required for any subsequent activation of a receptor, and coupling to biological effect, it follows that the affinity of the AhR for ligand is a key determinant for the subsequent biological actions. Thus the use of the baculovirus expression system for functional expression of AhR should have important applications in risk assessment.
In summary, these results show that functional rat and human AhR can be expressed using the baculovirus expression vector system, and that robust and saturable ligand-binding is obtained which is directly comparable to the native receptor. The high yield of functional AhR is a significant advantage for this system, and consequently quantitative analysis of ligand-binding can readily be performed. Our results characterise the binding of a variety of ligands and show that this expression system can be readily applied for determining the affinity of ligands for AhR from two model species.
Acknowledgments
This work was funded by a contract (T01034) from the UK Food Standards Agency.
The authors wish to thank Declan Brady for expert technical assistance, and John Fenlon for help in preparing the AhR antibody. We wish to acknowledge the Food Standards Agency and its expert reviewers (Professors G. Gibson, A.G. Renwick, and Dr A.G. Smith) for helpful comments and guidance, and Lesa Aylward for helpful comments on the manuscript. We thank Susan Moran and Christopher Bradfield for the gift of the human AhR cDNA, and David Toft for baculovirus containing p23.
Abbreviations
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- AhR
Aryl Hydrocarbon Receptor
- GST
glutathione S-transferase
- GGAG
GST-green fluorescent protein-AhR- green fluorescent protein
- LBD
ligand-binding domain
- TEF
TCDD toxic equivalency factors
- GUS
β-glucuronidase
- MDEG
25mM MOPS, 1mM DTT, 1mM EDTA, 10% glycerol, pH 7.5
- Sf9
Spodoptera frugiperda 9 cells
- ECL
enhanced chemiluminescence
- PAGE
polyacrylamide gel electrophoresis
- MOI
muliplicity of infection
- PCDD
1,2,3,7,8-pentachlorodibenzo-p-dioxin
- TCDF
2,3,7,8-tetrachlorodibenzofuran
- PCDF
2,3,4,7,8-pentachlorodibenzofuran
- PCB126
polychlorinated biphenyl 126
- TCAOB
tetrachloroazoxybenzene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poland A, Glover E, Kende AS. Stereospecific, High Affinity Binding Of 2,3,7,8-Tetrachlorodibenzo-Para-Dioxin By Hepatic Cytosol - Evidence That Binding Species Is Receptor For Induction Of Aryl- Hydrocarbon Hydroxylase. J Biol Chem. 1976;251:4936–46. [PubMed] [Google Scholar]
- 2.Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-Para-Dioxin And Related Halogenated Aromatic-Hydrocarbons - Examination Of The Mechanism Of Toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–54. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 3.Burbach KM, Poland A, Bradfield CA. Cloning Of The Ah-Receptor Cdna Reveals A Distinctive Ligand- Activated Transcription Factor. Proc Natl Acad Sci U S A. 1992;89:8185–9. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ema M, Sogawa K, Watanabe N, Chujoh Y, Matsushita N, Gotoh O, et al. Cdna Cloning And Structure Of Mouse Putative Ah Receptor. Biochem Biophys Res Commun. 1992;184:246–53. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- 5.Fernandezsalguero P, Pineau T, Hilbert DM, McPhail T, Lee SST, Kimura S, et al. Immune-System Impairment And Hepatic-Fibrosis In Mice Lacking The Dioxin-Binding Ah Receptor. Science. 1995;268:722–6. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 6.FernandezSalguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140:173–9. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt JV, Su GHT, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, et al. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2000;97:779–82. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradfield CA, Kende AS, Poland A. Kinetic And Equilibrium Studies Of Ah Receptor-Ligand Binding - Use Of (I-125)2-Lodo-7,8-Dibromodibenzo-Para-Dioxin. Mol Pharmacol. 1988;34:229–37. [PubMed] [Google Scholar]
- 10.Farrell K, Safe S. Absence Of Positive Cooperativity In The Binding Of 2,3,7,8-Tetrachlorodibenzo-Para-Dioxin To Its Cytosolic Receptor Protein. Biochem J. 1987;244:539–46. doi: 10.1042/bj2440539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradfield CA, Poland A. A Competitive-Binding Assay For 2,3,7,8-Tetrachlorodibenzo- Para-Dioxin And Related Ligands Of The Ah Receptor. Mol Pharmacol. 1988;34:682–8. [PubMed] [Google Scholar]
- 12.Connor KT, Aylward LL. Human response to dioxin: Aryl hydrocarbon receptor (AhR) molecular structure, function, and dose-response data for enzyme induction indicate an impaired human AhR. J Toxicol Env Health-Pt b-Crit Rev. 2006;9:147–71. doi: 10.1080/15287390500196487. [DOI] [PubMed] [Google Scholar]
- 13.Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa S, et al. Dioxin Binding Activities Of Polymorphic Forms Of Mouse And Human Arylhydrocarbon Receptors. J Biol Chem. 1994;269:27337–43. [PubMed] [Google Scholar]
- 14.Poland A, Palen D, Glover E. Analysis Of The 4 Alleles Of The Murine Aryl-Hydrocarbon Receptor. Mol Pharmacol. 1994;46:915–21. [PubMed] [Google Scholar]
- 15.Harper PA, Golas CL, Okey AB. Characterization Of The Ah Receptor And Aryl-Hydrocarbon Hydroxylase Induction By 2,3,7,8-Tetrachlorodibenzo-P-Dioxin And Benz(A)Anthracene In The Human A431 Squamous-Cell Carcinoma Line. Cancer Res. 1988;48:2388–95. [PubMed] [Google Scholar]
- 16.Manchester DK, Gordon SK, Golas CL, Roberts EA, Okey AB. Ah-Receptor In Human-Placenta - Stabilization By Molybdate And Characterization Of Binding Of 2,3,7,8-Tetrachlorodibenzo-Para-Dioxin, 3-Methylcholanthrene, And Benzo(A)Pyrene. Cancer Res. 1987;47:4861–8. [PubMed] [Google Scholar]
- 17.Poland A, Glover E. Ca-2+-Dependent Proteolysis Of The Ah Receptor. Arch Biochem Biophys. 1988;261:103–11. doi: 10.1016/0003-9861(88)90109-9. [DOI] [PubMed] [Google Scholar]
- 18.Coumailleau P, Poellinger L, Gustafsson JA, Whitelaw ML. Definition Of A Minimal Domain Of The Dioxin Receptor That Is Associated With Hsp90 And Maintains Wild-Type Ligand-Binding Affinity And Specificity. J Biol Chem. 1995;270:25291–300. doi: 10.1074/jbc.270.42.25291. [DOI] [PubMed] [Google Scholar]
- 19.Ramadoss P, Perdew GH. Use of 2-azido-3-[I-125]iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human versus mouse aryl hydrocarbon receptor in cultured cells. Mol Pharmacol. 2004;66:129–36. doi: 10.1124/mol.66.1.129. [DOI] [PubMed] [Google Scholar]
- 20.Chan WK, Chu RY, Jain S, Reddy JK, Bradfield CA. Baculovirus Expression Of The Ah Receptor And Ah Receptor Nuclear Translocator - Evidence For Additional Dioxin-Responsive Element-Binding Species And Factors Required For Signaling. J Biol Chem. 1994;269:26464–71. [PubMed] [Google Scholar]
- 21.Morishima Y, Kanelakis KC, Murphy PJM, Lowe ER, Jenkins GJ, Osawa Y, et al. The Hsp90 cochaperone p23 is the limiting component of the multiprotein Hsp90/Hsp70-based chaperone system in vivo where it acts to stabilize the client protein center dot Hsp90 complex. J Biol Chem. 2003;278:48754–63. doi: 10.1074/jbc.M309814200. [DOI] [PubMed] [Google Scholar]
- 22.Holmes JL, Pollenz RS. Determination of aryl hydrocarbon receptor nuclear translocator protein concentration and subcellular localization in hepatic and nonhepatic cell culture lines: Development of quantitative Western blotting protocols for calculation of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein in total cell lysates. Mol Pharmacol. 1997;52:202–11. doi: 10.1124/mol.52.2.202. [DOI] [PubMed] [Google Scholar]
- 23.Bell DR, Poland A. Binding of aryl hydrocarbon receptor (AhR) to AhR-interacting protein - The role of hsp90. J Biol Chem. 2000;275:36407–14. doi: 10.1074/jbc.M004236200. [DOI] [PubMed] [Google Scholar]
- 24.Yao G, Craven M, Drinkwater N, Bradfield CA. Interaction networks in yeast define and enumerate the signaling steps of the vertebrate aryl hydrocarbon receptor. PLoS Biol. 2004;2:e65. doi: 10.1371/journal.pbio.0020065. art. no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning And Expression Of A Human Ah Receptor Cdna. Mol Pharmacol. 1993;44:911–7. [PubMed] [Google Scholar]
- 26.Cox MB, Miller CA. The p23 co-chaperone facilitates dioxin receptor signaling in a yeast model system. Toxicol Lett. 2002;129:13–21. doi: 10.1016/s0378-4274(01)00465-9. [DOI] [PubMed] [Google Scholar]
- 27.Miller CA. Two tetratricopeptide repeat proteins facilitate human aryl hydrocarbon receptor signalling in yeast. Cell Signal. 2002;14:615–23. doi: 10.1016/s0898-6568(02)00002-5. [DOI] [PubMed] [Google Scholar]
- 28.Whitelaw ML, McGuire J, Picard D, Gustafsson JA, Poellinger L. Heat shock protein hsp90 regulates dioxin receptor function in vivo. Proc Natl Acad Sci U S A. 1995;92:4437–41. doi: 10.1073/pnas.92.10.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shetty PV, Bhagwat BY, Chan WK. p23 enhances the formation of the aryl hydrocarbon receptor-DNA complex. Biochem Pharmacol. 2003;65:941–8. doi: 10.1016/s0006-2952(02)01650-7. [DOI] [PubMed] [Google Scholar]
- 30.Pohjanvirta R, Viluksela M, Tuomisto JT, Unkila M, Karasinska J, Franc MA, et al. Physicochemical differences in the AH receptors of the most TCDD-susceptible and the most TCDD-resistant rat strains. Toxicol Appl Pharmacol. 1999;155:82–95. doi: 10.1006/taap.1998.8565. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzen A, Okey AB. Detection and characterisation of Ah Receptor in tissue and cells from human tonsils. Toxicol Appl Pharmacol. 1991;107:203–14. doi: 10.1016/0041-008x(91)90203-q. [DOI] [PubMed] [Google Scholar]
- 32.Okey AB, Giannone JV, Smart W, Wong JMY, Manchester DK, Parker NB, et al. Binding of 2,3,7-8-tetrachlorodibenzo-p-dioxin to AH receptor in placentas from normal versus abnormal pregnancy outcomes. Chemosphere. 1997;34:1535–47. doi: 10.1016/s0045-6535(97)00449-9. [DOI] [PubMed] [Google Scholar]
- 33.Butler RA, Kelley ML, Powell WH, Hahn ME, Van Beneden RJ. An aryl hydrocarbon receptor (AHR) homologue from the soft-shell clam, Mya arenaria: evidence that invertebrate AHR homologues lack 2,3,7,8-tetrachlorodibenzo-p-dioxin and beta-naphthoflavone binding. Gene. 2001;278:223–34. doi: 10.1016/s0378-1119(01)00724-7. [DOI] [PubMed] [Google Scholar]
- 34.Duncan DM, Burgess EA, Duncan I. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 1998;12:1290–303. doi: 10.1101/gad.12.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell-Coffman JA, Bradfield CA, Wood WB. Caenorhabditis elegans orthologs of the aryl hydrocarbon receptor and its heterodimerization partner the aryl hydrocarbon receptor nuclear translocator. Proc Natl Acad Sci U S A. 1998;95:2844–9. doi: 10.1073/pnas.95.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes A, White S, D'Silva K, Rose M. Simultaneous determination of PCDDs, PCDFs, PCBs and PBDEs in food. Talanta. 2004;63:1147–55. doi: 10.1016/j.talanta.2004.05.039. [DOI] [PubMed] [Google Scholar]