Abstract
Induction of COX-2 activity in cerebral ischemia results in increased neuronal injury and infarct size. Recent studies investigating neurotoxic mechanisms of COX-2 demonstrate both toxic and paradoxically protective effects of downstream prostaglandin receptor signaling pathways. We tested whether misoprostol, a PGE2 receptor agonist that is utilized clinically as an anti-ulcer agent and signals through the protective PGE2 EP2, EP3, and EP4 receptors, would reduce brain injury in the murine middle cerebral artery occlusion–reperfusion (MCAO-RP) model. Administration of misoprostol, at the time of MCAO or 2 h after MCAO, resulted in significant rescue of infarct volume at 24 and 72 h. Immunocytochemistry demonstrated dynamic regulation of the EP2 and EP4 receptors during reperfusion in neurons and endothelial cells of cerebral cortex and striatum, with limited expression of EP3 receptor. EP3−/− mice had no significant changes in infarct volume compared to control littermates. Moreover, administration of misoprostol to EP3+/+ and EP3−/− mice showed similar levels of infarct rescue, indicating that misoprostol protection was not mediated through the EP3 receptor. Taken together, these findings suggest a novel function for misoprostol as a protective agent in cerebral ischemia acting via the PGE2 EP2 and/or EP4 receptors.
Keywords: Stroke, Misoprostol, Prostaglandin, PGE2, Cerebral ischemia
A neurotoxic effect of cyclooxygenase-2 (COX-2) has been demonstrated in rodent models of focal cerebral ischemia [10,16,21,22]. The cyclooxygenases COX-1 and COX-2 catalyze the first committed step in the synthesis of prostaglandins and thromboxane, which are lipid signaling molecules that participate in a broad range of physiologic and pathologic processes, including synaptic plasticity, neuronal injury, and neuroinflammation (reviewed in [15]). The five prostanoids PGE2, PGD2, PGI2, PGF2α, and TXA2 bind to distinct G-protein coupled receptors (GPCRs), designated as the EP, DP, IP, FP, and TP receptors, respectively (reviewed in [8]). Activation of prostaglandin (PG) receptors leads to changes in the production of cAMP and/or phosphoinositol turnover and intracellular Ca2+ mobilization. Further complexity occurs in the case of PGE2, which can bind four receptor subtypes (EP1, EP2, EP3, and EP4) and PGD2, which can bind to two different receptors (DP1 and DP2), with distinct and potentially antagonistic signaling cascades.
Recent studies examining the mechanisms of COX-2 neurotoxicity have focused on the roles of individual prostaglandin signaling pathways downstream of COX-2. Although at least two prostaglandin signaling pathways, the PGE2 EP1 [2,17] and the PGD2 DP2 receptors [18] have been found to be neurotoxic, an important finding has been that a larger group of prostaglandin receptors can mediate a paradoxical and significant protective effect [6,18-20,26]. EP2 and DP1-mediated neuroprotection are dependent on PKA activation [18,20], and EP3 neuroprotection is associated with increases in the pro-survival phospho-AKT [6]. In vivo, the PGE2 EP2 receptor exerts a significant neuroprotective effect in both focal and permanent MCAO models [19,20] and the EP4 receptor exerts a protective effect against NMDA excitotoxicity in vivo [1]. The PGD2 DP1 receptor mediates significant protection in vivo in neonatal hypoxic ischemic injury [25] and transient focal ischemia [24]. Thus, selected prostaglandin signaling systems downstream of COX-2 exert potent beneficial effects in the setting of ischemia.
Misoprostol is a widely used anti-ulcer agent in patients who are at risk for non-steroidal anti-inflammatory drug (NSAID)-mediated gastritis and ulcer disease [23] and repletes cytoprotective levels of PGE2 necessary for maintaining integrity of the gastric mucosa. Misoprostol binds the PGE2 EP2, EP3, and EP4 receptors (Ki: 34, 7.9, and 23 nM for EP2, EP3, and EP4, respectively [8]) increases cAMP production, indicating activation of the EP2 and EP4 receptors, which are positively coupled to adenylate cyclase. Based on our recent data demonstrating a protective role of the EP2 receptor in cerebral ischemia [19,20], we tested whether misoprostol, an agonist at this receptor, would reduce infarct volume in the MCAO model of transient focal ischemia. Results of this study indicate that misoprostol exerts a pronounced protective effect in MCAO followed by reperfusion, and that it exerts this effect through the EP2 and/or EP4 receptors in vivo.
This study was conducted in accordance with the National Institutes of Health guidelines for the use of experimental animals and protocols were approved by the Institutional Animal Care and Use Committee. For MCAO-RP studies, male mice aged 10–12 weeks (20–25 g) were used. For the EP3−/− and +/+ MCAO-RP studies (see Supplementary data), male 12-week-old mice were used.
Focal cerebral ischemia was induced by 90 min of reversible right MCA occlusion (MCAO) under isofluorane anesthesia followed by 22.5 or 72 h of reperfusion and terminal histopathology, as previously described [20]. Rectal temperature and intraischemic and 22.5-h neurological deficits were assessed and did not show differences between genotypes or treatments. Monitoring of physiological variables and laser Doppler flowmetry (LDF) were performed in companion cohorts (n = 4/group) [20]. Brain tissue was also harvested from sham and MCAO-RP mice for immunocytochemistry (see Supplementary data).
Mice were given three subcutaneous injections of either vehicle (hydroxypropyl-methyl cellulose; HPMC) or 1 mg/kg misoprostol in HPMC (1:100 misoprostol:HPMC; Pfizer, Groton, CT) [5] either at onset of MCAO or 2 h after MCAO, followed by two subsequent doses 6 and 12 h later (see Supplementary data).
Statistical analysis was performed by Student's t-test or one-way analysis of variance (ANOVA), followed by Tukey post hoc analysis. All data are reported as mean ± standard error of the mean (SEM). p values <0.05 were considered significant.
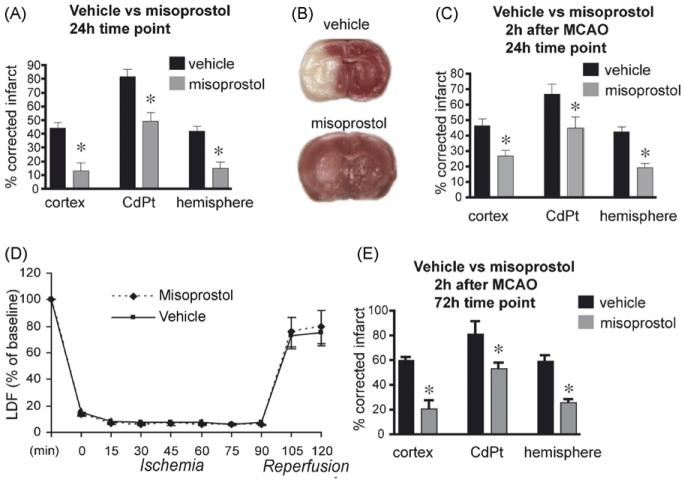
Misoprostol binds to the EP2-4 receptors. From previous studies demonstrating protective effects of EP2, EP3, and EP4 receptor activation in models of excitotoxicity or ischemia [1,19,20,26], we hypothesized that misoprostol, which can bind all three EP receptors, might also mediate pharmacological protection. Administration of 1 mg/kg of misoprostol at the onset of 90 min of MCAO, followed by subsequent injections at 6 and 12 h of reperfusion resulted in a significant rescue of tissue at 24 h (Fig. 1A and B). Neurological scores were significantly improved in the misoprostol-treated cohort (2.4 ± 0.2 vs. 1.8±0.2; p < 0.05). A second cohort of mice was subjected to MCAO-RP, with misoprostol administered 2 h after MCAO, and two subsequent doses at 8 and 14 h. Here, post-MCAO administration of misoprostol again resulted in a significant rescue in cerebral cortex, striatum and hemisphere (Fig. 1C). Neurological scores were also significantly improved in the misoprostol-treated group in this cohort (2.6±0.3 vs. 1.7±0.3, p < 0.05). Laser Doppler flow measurements did not demonstrate any differences in flow between vehicle and misoprostol groups (Fig. 1D). A final cohort received misoprostol 2 h after MCAO and again at 6 and 12 h, and brains were examined at 72 h. Comparable protection was seen at this delayed time point (Fig. 1E). Neurological scores were also significantly improved with misoprostol treatment (2.6±0.24 vs. 1.0±0.22 vs. p < 0.05). These studies indicate that subcutaneous administration of misoprostol significantly reduced stroke volume and improved neurological scores if given at the time of MCAO or 2 h after onset of MCAO out to 72 h of reperfusion.
Fig. 1.
Misoprostol is protective in MCAO-RP. (A) Infarct volumes measured in cerebral cortex, caudate-putamen (CdPt), and hemisphere in mice treated with vehicle or misoprostol (1 mg/kg) and subjected to 90 min MCAO with 22.5 h survival (n = 10–11 per group; *p < 0.05). (B) Representative coronal sections stained with TTC at 24 h showing preservation of tissue with misoprostol administration. (C) Misoprostol protected against ischemic infarction in cerebral cortex, CdPt, and hemisphere when given 2h after MCAO (n = 10–11 per group; *p < 0.05). (D) CBF was measured using laser Doppler flowmetry during ischemia and reperfusion and did not show differences between vehicle and misoprostol (n = 4 per condition). LDF data was the average of values at 15 min intervals during ischemia/reperfusion. (E) Infarct volumes remained significantly reduced at 72 h after MCAO (n = 5 and 7 per group; *p < 0.05) when misoprostol was given 2 h after MCAO, and again at 6 and 12 h after MCAO. Physiological parameters did not differ between vehicle and misoprostol treatments (see Supplementary Table 1).
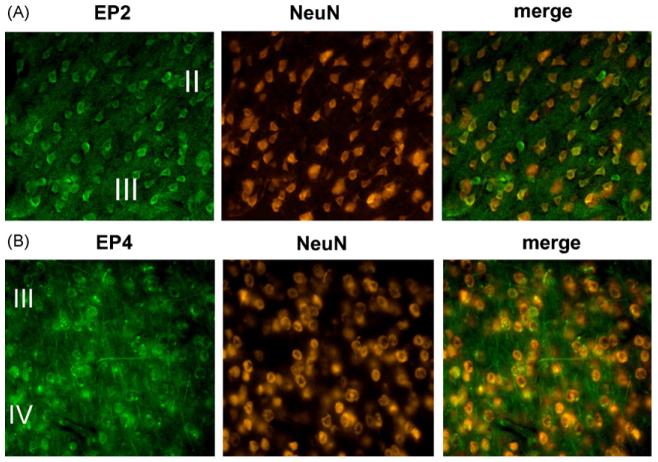
To determine the cellular substrates of misoprostol signaling and protection, the temporal dynamics of EP2, EP3, and EP4 receptor expression were investigated in sham and MCAO-RP mice at 4 and 24 h after MCAO (see Supplementary data). Immunostaining for EP3 was in accord with previous in situ hybridization studies [11] and showed no significant expression in striatum and cerebral cortex in sham and ischemic brains (data not shown). In sham treated mice, EP2 and EP4 receptors colocalized with NeuN in neurons (Fig. 2A and B; layers II–IV of frontal cortex), and also colocalized with Factor VIII, a marker of endothelial cells (not shown) indicating that the EP2 and EP4 receptors are basally expressed in neurons and in endothelial cells. At 4 h of reperfusion, neuronal EP2 staining decreased in parallel with NeuN in neurons in the ischemic core, but EP2 expression persisted in peri-infarct NeuN-positive neurons (data not shown). In addition, at 4 h of reperfusion, EP2 expression was markedly induced in endothelium in the peri-infarct area and ischemic zones (Fig. 3A), colocalizing with ICAM-1, which is induced in endothelial cells as well as microglia following MCAO-RP. EP4 showed a similar downregulation in neurons at 4 h of reperfusion and a marked induction in endothelial cells (Fig. 3B). At 24 h after MCAO, EP2 but not EP4 receptor expression persisted in endothelium in the peri-infarct zone (Fig. 3C). Colocalization studies with GFAP and Iba1, markers of astrocytes and microglia, respectively, did not demonstrate localization of the EP2 or EP4 receptors in these cell types during reperfusion (not shown). These data indicate that EP2 and EP4 are dynamically regulated selectively in neurons and endothelium during the course of reperfusion. Neuronal expression of EP2 and EP4 is lost in the ischemic core by 4 h of reperfusion, but remains in the peri-infarct zone. In endothelium, levels of EP2 and EP4 receptor expression are upregulated early in reperfusion at 4 h in the ischemic core and peri-infarct areas; by 24 h of reperfusion, vascular expression of EP4 has disappeared, but EP2 expression remains. The temporal dynamics of EP2 and EP4 cellular expression suggest that the pharmacological protection by misoprostol may be mediated by neurons and/or endothelial cells.
Fig. 2.
EP2 and EP4 receptors are expressed in neurons under basal conditions (400× magnification). Colocalization studies with the neuron-specific marker NeuN demonstrates staining of (A) EP2 and (B) EP4 in cortical neurons in frontal cortex. Both EP2 and EP4 were broadly expressed in neurons of cerebral cortex, striatum, and hippocampus.
Fig. 3.
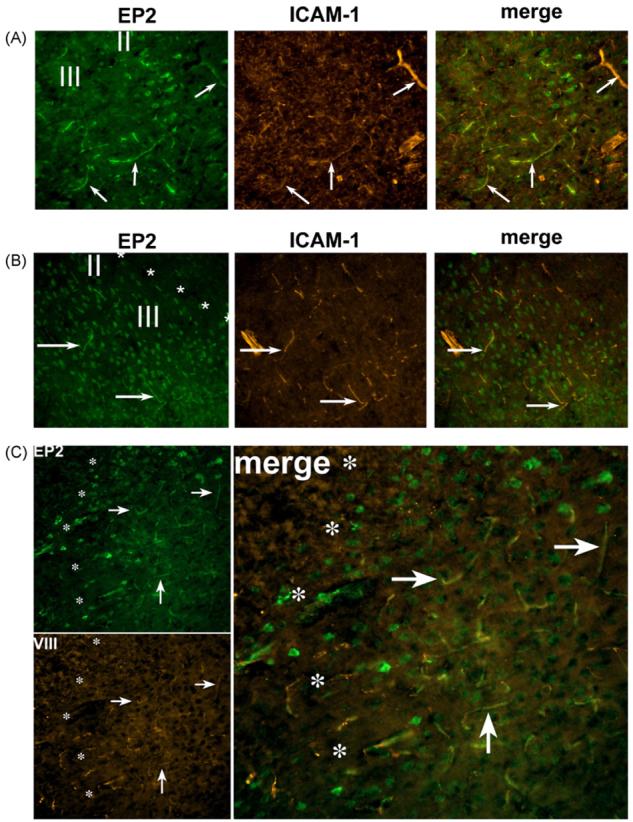
EP2 and EP4 are dynamically regulated in neurons and endothelial cells during reperfusion after MCAO (200× magnification). In frontal cortex, layers II and III, at 4 h of reperfusion, EP2 (A) and EP4 (B) colocalize with ICAM-1 in endothelium (arrows) in the infarct and peri-infarct areas. (A) EP2 is expressed in microvessels and scattered neurons in the infarcted zone as well as the peri-infarct area (not shown). Note scattered clusters of EP2 stained neurons remaining in infarcted zone in layer II. (B) EP4 is expressed only in the peri-infarct zone at 4 h of reperfusion in neurons and endothelium. Asterisks delineate the boundary between infarct zone (upper right corner) and the peri-infarct zone (lower left portion of image). (C) At 24 h after MCAO, only EP2 expression persists in endothelium in the peri-infarct area (to the right of asterisks; arrows). There is scattered neuronal staining in the peri-infarct zone. For all sections, the boundaries between peri-infarct and infarcted zones were defined in adjacent sections with NeuN staining.
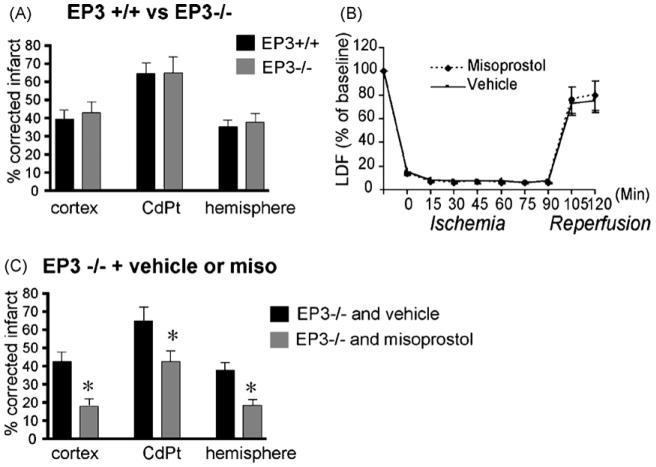
To further establish whether misoprostol is acting selectively via the EP2 and/or EP4 receptors, we tested whether the EP3 receptor mediated any protective effect by misoprostol. From previous studies, it has been demonstrated that the EP2 receptor confers significant protection in the MCAO-RP model [19,20] and that pharmacological activation of EP4 protects in a striatal model of excitotoxicity [1]. We first determined whether the EP3 receptor functioned in MCAO-RP. C57B6 male EP3−/− and +/+ mice were subjected to MCAO-RP. Interestingly, infarct size and neurological scores did not show differences between genotypes (Fig. 4A; neurological scores for EP3+/+: 2.5±0.3 vs. EP3−/− 2.0±0.2), indicating that the EP3 receptor did not influence infarct size in vivo at the 24-h time point. Moreover, administration of misoprostol to EP3−/− mice did not diminish the protective effect of misoprostol, and resulted in similar decreases in infarct volume compared to EP3+/+ mice given misoprostol. These findings indicate that the EP3 receptor does not influence outcome in MCAO, and does not transduce the protective effect of misoprostol (Fig. 4B). Thus, these data, in combination with the immunostaining demonstrating a dynamic regulation of EP2 and EP4 in penumbra with reperfusion suggest that the positive effects of misoprostol are likely mediated via the EP2 and/or the EP4 receptors in this model.
Fig. 4.
The EP3 receptor does not transduce the protective effect of misoprostol in MCAO-RP. (A) EP3−/− and +/+ mice do not show differences in infarct volumes at 24 h (n = 10 per genotype). (B) CBF as measured by Laser Doppler flowmetry during ischemia and reperfusion did not show differences between EP3+/+ and −/− mice (n = 4 per condition). (C) Administration of misoprostol to EP3−/− mice results in similar in infarct volume in EP3−/− and +/+ mice (n = 10–11 per group; *p < 0.05). Physiological measurements did not differ between genotypes (see Supplementary Table 2).
Recent efforts to understand mechanisms of COX-2 neurotoxicity have uncovered both toxic and paradoxically protective effects of selected prostaglandin signaling pathways in brain. In this study, we have examined the effects of a commonly used anti-ulcer agent that binds the PGE2 EP2, EP3, and EP4 receptors in a rodent model of MCAO-RP, and tested the hypothesis that this agonist would protect against ischemia. Subcutaneous injection of misoprostol resulted in significant reductions in infarct size when given at the time of MCAO and 2 h after the onset of MCAO, with comparable protection at 24 and 72 h after MCAO. There were no differences in blood flow as measured by LDF or physiological measurements between treatments.
In vivo genetic and pharmacologic studies have demonstrated the protective effects of EP2 and EP4 signaling in cerebral ischemia and excitotoxicity, respectively [1,19,20]. To test whether misoprostol exerted a protective effect via the EP3 receptor, we first had to determine the function of this receptor in vivo in MCAO-RP. Deletion of the EP3 receptor had no effect on infarct volume in the present study. Previous pharmacological examinations of EP3 function have shown a neuroprotective effect of this receptor in acute NMDA toxicity in organotypic hippocampal slices [26] and in spinal cord slices subjected to chronic glutamate toxicity from blockade of astrocytic glutamate transporters; this latter EP3-mediated neuroprotection was associated with an increase in levels of the pro-survival phospho-AKT [6]. The difference observed in vivo may be due to a low abundance of EP3 receptor in brain regions supplied by the middle cerebral artery, in particular the striatum and cerebral cortex as compared to the hippocampus [11], where there are modest levels of EP3 expression in CA1-4 regions and dentate gyrus. We also cannot exclude potential differences in species used in organotypic and in vivo paradigms (rat vs. mouse) or brain maturities (postnatal vs. 2-month brain) resulting in divergent effects of EP3 signaling. The lack of effect of EP3 deletion is also in contrast to a pharmacological study in which pre-administration of the EP3 agonist ONO-AE-248 increased infarct volume in a MCAO-RP [3]. Several possible interpretations may be relevant here. ONO-AE-248 binds selectively to EP3 over EP1, EP2, and EP4 in CHO cells expressing these receptors [9], however its specificity and selectivity in vivo are not known. In addition, genetic deletion and pharmacologic activation may not be completely complementary in the case of the EP3 receptor. The EP3 receptor has three distinct isoforms derived by alternative splicing that vary at the carboxy terminus that result in differential downstream signaling, desensitization, and constitutive activity [4,7,12-14]. Genetic deletion of EP3 would result in ablation of all three isoforms whereas ICV administration of EP3 agonist may activate one or more isoforms depending on the expression patterns of the EP3 isoforms in the setting of MCAO-RP, the brain penetration of the EP3 agonist into brain, and the constitutive signaling properties and desensitization kinetics. For the purposes of this study, however, administration of misoprostol to EP3−/− and +/+ mice showed similar levels of protection, indicating that the protective effect of misoprostol in vivo was not mediated by EP3 signaling, but rather by the EP2 and/or EP4 receptors.
Immunostaining experiments revealed a dynamic regulation of EP2 and EP4 expression during reperfusion. In early reperfusion (4 h), neuronal EP2 and EP4 expression disappeared entirely in the infarcted zone in parallel with NeuN, but endothelial EP2 and EP4 expression levels were markedly induced in the microvasculature, as evidenced by colocalization of EP2 and EP4 with the endothelial cell markers ICAM-1 (Fig. 3) or Factor VIII (data not shown). By 24 h, EP2 but not EP4 expression persisted in endothelium. This temporal expression pattern suggests that the cellular targets of misoprostol during early and later phases of reperfusion may be endothelial cells. In support of this hypothesis are negative findings from experiments in primary neuronal cultures and organotypic hippocampal slices treated with either NMDA (10 μM) or oxygen–glucose deprivation (OGD) in which misoprostol (10 nM to 10 μM) did not elicit any protective response (Supplementary Fig. 1).
In this study, we have identified a novel function for the PGE2 receptor agonist misoprostol, a commonly used anti-ulcer agent that promotes a significant rescue of brain tissue in the MCAO-RP model of transient focal ischemia. Misoprostol exerted significant protection against ischemia when given at the time of or 2 h after stroke onset. These data indicate that misoprostol may have a potential role as a protective agent in stroke patients, particularly as it is currently approved for clinical use and well tolerated by patients. The present study suggests that the protective effect of misoprostol is mediated through the protective EP2 and/or EP4 receptors in vivo.
Acknowledgments
Supported by NS045727 and the American Federation for Aging Research (KA), DK46205 and DK37097 (RMB), and NS050505 and 055215 (LM).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neulet.2008.04.054.
References
- 1.Ahmad AS, Ahmad M, de Brum-Fernandes AJ, Dore S. Prostaglandin EP4 receptor agonist protects against acute neurotoxicity. Brain Res. 2005;1066:71–77. doi: 10.1016/j.brainres.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad AS, Saleem S, Ahmad M, Dore S. Prostaglandin EP1 receptor contributes to excitotoxicity and focal ischemic brain damage. Toxicol. Sci. 2006;89:265–270. doi: 10.1093/toxsci/kfj022. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad M, Ahmad AS, Zhuang H, Maruyama T, Narumiya S, Dore S. Stimulation of prostaglandin E2-EP3 receptors exacerbates stroke and excitotoxic injury. J. Neuroimmunol. 2007;184:172–179. doi: 10.1016/j.jneuroim.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An S, Yang J, So SW, Zeng L, Goetzl EJ. Isoforms of the EP3 subtype of human prostaglandin E2 receptor transduce both intracellular calcium and cAMP signals. Biochemistry. 1994;33:14496–14502. doi: 10.1021/bi00252a016. [DOI] [PubMed] [Google Scholar]
- 5.Best V, Ruiz P, Spurney RF. The Prostaglandin E(1) (PGE(1)) analog misoprostol ameliorates autoimmune disease and depletes T lymphocytes in MRL-lpr/lpr Mice. Am. J. Ther. 1995;2:943–948. doi: 10.1097/00045391-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bilak M, Wu L, Wang Q, Haughey N, Conant K, Hillaire C, Andreasson K. PGE2 receptors rescue motor neurons in a model of amyotrophic lateral sclerosis. Ann. Neurol. 2004;56:240–248. doi: 10.1002/ana.20179. [DOI] [PubMed] [Google Scholar]
- 7.Bilson HA, Mitchell DL, Ashby B. Human prostaglandin EP3 receptor isoforms show different agonist-induced internalization patterns. FEBS Lett. 2004;572:271–275. doi: 10.1016/j.febslet.2004.06.089. [DOI] [PubMed] [Google Scholar]
- 8.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 9.Cao J, Shayibuzhati M, Tajima T, Kitazawa T, Taneike T. In vitro pharmacological characterization of the prostanoid receptor population in the non-pregnant porcine myometrium. Eur. J. Pharmacol. 2002;442:115–123. doi: 10.1016/s0014-2999(02)01489-9. [DOI] [PubMed] [Google Scholar]
- 10.Dore S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, Hurn PD, Traystman RJ, Andreasson K. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann. Neurol. 2003;54:155–162. doi: 10.1002/ana.10612. [DOI] [PubMed] [Google Scholar]
- 11.Ek M, Arias C, Sawchenko P, Ericsson-Dahlstrand A. Distribution of the EP3 prostaglandin E(2) receptor subtype in the rat brain: relationship to sites of interleukin-1-induced cellular responsiveness. J. Comp. Neurol. 2000;428:5–20. doi: 10.1002/1096-9861(20001204)428:1<5::aid-cne2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa H, Katoh H, Yamaguchi Y, Nakamura K, Futakawa S, Negishi M. Different membrane targeting of prostaglandin EP3 receptor isoforms dependent on their carboxy-terminal tail structures. FEBS Lett. 2000;473:76–80. doi: 10.1016/s0014-5793(00)01508-8. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa H, Negishi M, Ichikawa A. Two isoforms of the prostaglandin E receptor EP3 subtype different in agonist-independent constitutive activity. J. Biol. Chem. 1996;271:1857–1860. doi: 10.1074/jbc.271.4.1857. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa H, Negishi M, Katoh H, Ichikawa A. Two isoforms of prostaglandin EP3 receptor exhibiting constitutive activity and agonist-dependent activity in rho-mediated stress fiber formation. Biochem. Biophys. Res. Commun. 1997;234:631–636. doi: 10.1006/bbrc.1997.6655. [DOI] [PubMed] [Google Scholar]
- 15.Hewett SJ, Bell SC, Hewett JA. Contributions of cyclooxygenase-2 to neuroplasticity and neuropathology of the central nervous system. Pharmacol. Therapeut. 2006;112:335–357. doi: 10.1016/j.pharmthera.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. Reduced susceptibility to ischemic brain injury and N-methyl-d-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nature medicine. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 18.Liang X, Wu L, Hand T, Andreasson K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J. Neurochem. 2005;92:477–486. doi: 10.1111/j.1471-4159.2004.02870.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Wu L, Breyer R, Mattson MP, Andreasson K. Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann. Neurol. 2005;57:758–761. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- 20.McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J. Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagayama M, Niwa K, Nagayama T, Ross ME, Iadecola C. The cyclooxygenase-2 inhibitor NS-398 ameliorates ischemic brain injury in wild-type mice but not in mice with deletion of the inducible nitric oxide synthase gene. J. Cereb. Blood Flow Metab. 1999;19:1213–1219. doi: 10.1097/00004647-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Nogawa S, Forster C, Zhang F, Nagayama M, Ross ME, Iadecola C. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10966–10971. doi: 10.1073/pnas.95.18.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Numo R. Prevention of NSAID-induced ulcers by the coadministration of misoprostol: implications in clinical practice. Scand. J. Rheum. 1992;92:25–29. doi: 10.3109/03009749209101386. [DOI] [PubMed] [Google Scholar]
- 24.Saleem S, Zhuang H, de Brum-Fernandes AJ, Maruyama T, Narumiya S, Dore S. PGD(2) DP1 receptor protects brain from ischemia-reperfusion injury. Eur. J. Neurosci. 2007;26:73–78. doi: 10.1111/j.1460-9568.2007.05627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniguchi H, Mohri I, Okabe-Arahori H, Aritake K, Wada K, Kanekiyo T, Narumiya S, Nakayama M, Ozono K, Urade Y, Taniike M. Prostaglandin D2 protects neonatal mouse brain from hypoxic ischemic injury. J. Neurosci. 2007:4303–4312. doi: 10.1523/JNEUROSCI.0321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu L, Wang Q, Liang X, Andreasson K. Divergent effects of prostaglandin receptor signaling on neuronal survival. Neurosci. Lett. 2007;421:253–258. doi: 10.1016/j.neulet.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]