Abstract
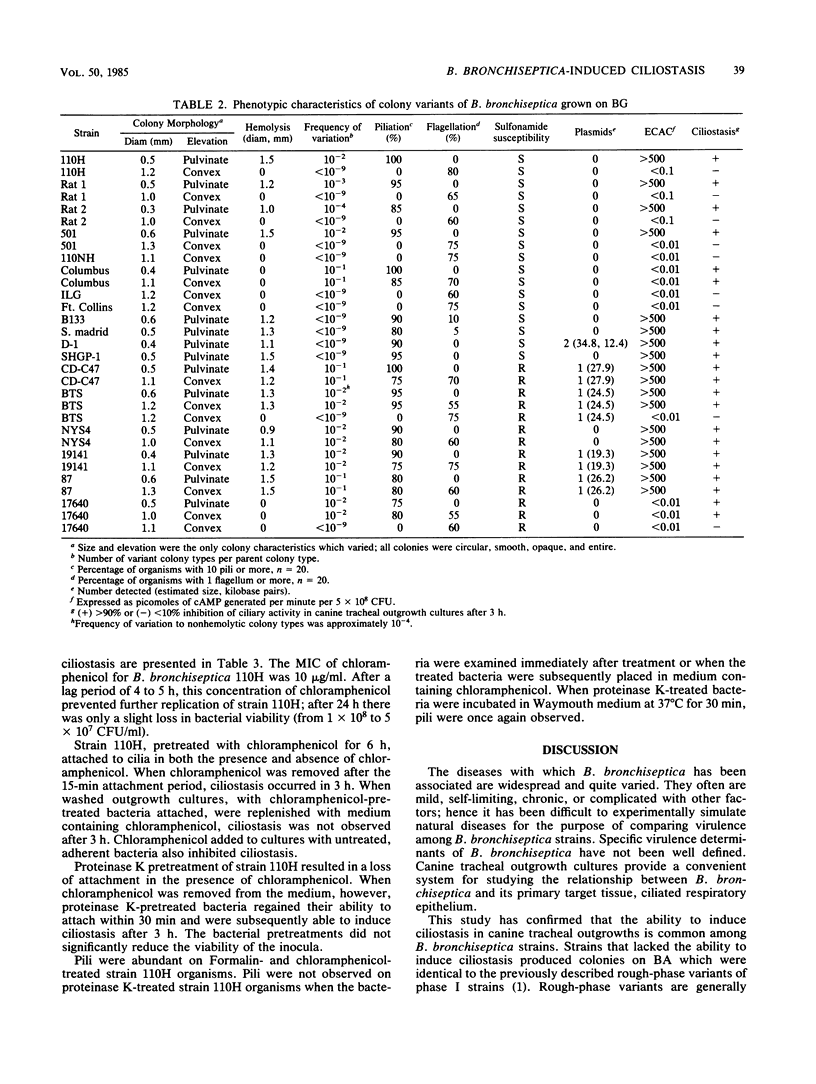
Eighteen strains of Bordetella bronchiseptica, selected on the basis of previously determined phenotypic characteristics, were examined for their ability to induce ciliostasis in canine tracheal outgrowth cultures. Fifteen strains grown on brucella agar caused ciliostasis. Strains that did not cause ciliostasis were stable, nonpiliated, and nonhemolytic, they did not produce extracellular adenylate cyclase, and were morphologically indistinguishable from rough-phase variants on brucella agar. Plasmids were detected in only five of the strains which induced ciliostasis, transfer of plasmids from four of these strains to one which did not induce ciliostasis did not alter its virulence or colony morphology. All strains which were hemolytic on Bordet-Gengou agar produced extracellular adenylate cyclase. Two nonhemolytic strains, one which produced only rough colonies on brucella agar, also induced ciliostasis. Two types of colony (phase) variation were observed: one recognizable on both brucella agar and Bordet-Gengou agar at frequencies of less than or equal to 10(-2), associated with multiple loss of virulence determinants, and the other recognizable only on Bordet-Gengou agar at frequencies of greater than or equal to 10(-2), associated with flagellum expression. The possession of readily detectable somatic pili was the only phenotypic characteristic consistently associated with the ability to induce ciliostasis. Formalin-killed and chloramphenicol-inhibited B. bronchiseptica strain 110H organisms had detectable pili and attached to cilia, but did not cause ciliostasis. Protease-treated B. bronchiseptica strain 110H organisms did not have detectable pili and in the presence of chloramphenicol did not attach to cilia. Attachment to cilia, although not in itself sufficient to cause ciliostasis, is intimately associated with and may be required for the induction of ciliostasis by B. bronchiseptica strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bemis D. A., Greisen H. A., Appel M. J. Bacteriological variation among Bordetella bronchiseptica isolates from dogs and other species. J Clin Microbiol. 1977 Apr;5(4):471–480. doi: 10.1128/jcm.5.4.471-480.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis D. A., Greisen H. A., Appel M. J. Pathogenesis of canine bordetellosis. J Infect Dis. 1977 May;135(5):753–762. doi: 10.1093/infdis/135.5.753. [DOI] [PubMed] [Google Scholar]
- Bemis D. A., Kennedy J. R. An improved system for studying the effect of Bordetella bronchiseptica on the ciliary activity of canine tracheal epithelial cells. J Infect Dis. 1981 Oct;144(4):349–357. doi: 10.1093/infdis/144.4.349. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Drummond J. G., Curtis S. E., Meyer R. C., Simon J., Norton H. W. Effects of atmospheric ammonia on young pigs experimentally infected with Bordetella bronchiseptica. Am J Vet Res. 1981 Jun;42(6):963–968. [PubMed] [Google Scholar]
- Ezzell J. W., Dobrogosz W. J., Kloos W. E., Manclark C. R. Phase-shift markers in Bordetella: alterations in envelope proteins. J Infect Dis. 1981 Apr;143(4):562–569. doi: 10.1093/infdis/143.4.562. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow R. A., Causey S. C., Geary S. J., Wren W. S. Comparison of an infective avirulent and canine virulent Bordetella bronchiseptica. Am J Vet Res. 1983 Feb;44(2):207–211. [PubMed] [Google Scholar]
- Graham A. C., Abruzzo G. K. Occurrence and characterization of plasmids in field isolates of Bordetella bronchiseptica. Am J Vet Res. 1982 Oct;43(10):1852–1855. [PubMed] [Google Scholar]
- Hanada M., Shimoda K., Tomita S., Nakase Y., Nishiyama Y. Production of lesions similar to naturally occurring swine atrophic rhinitis by cell-free sonicated extract of Bordetella bronchiseptica. Nihon Juigaku Zasshi. 1979 Feb;41(1):1–8. doi: 10.1292/jvms1939.41.1. [DOI] [PubMed] [Google Scholar]
- Harris D. L., Switzer W. P., Harris R. A. A suggested mechanism for the pathogenesis of infectious atrophic rhinitis. Can J Comp Med. 1971 Oct;35(4):318–323. [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little T. W. Respiratory disease in pigs: a study. Vet Rec. 1975 Jun 21;96(25):540–544. doi: 10.1136/vr.96.25.540. [DOI] [PubMed] [Google Scholar]
- Nogimori K., Tamura M., Yajima M., Ito K., Nakamura T., Kajikawa N., Maruyama Y., Ui M. Dual mechanisms involved in development of diverse biological activities of islet-activating protein, pertussis toxin, as revealed by chemical modification of lysine residues in the toxin molecule. Biochim Biophys Acta. 1984 Sep 28;801(2):232–243. doi: 10.1016/0304-4165(84)90072-2. [DOI] [PubMed] [Google Scholar]
- Peppler M. S. Isolation and characterization of isogenic pairs of domed hemolytic and flat nonhemolytic colony types of Bordetella pertussis. Infect Immun. 1982 Mar;35(3):840–851. doi: 10.1128/iai.35.3.840-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
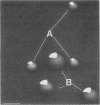
- Peppler M. S., Schrumpf M. E. Phenotypic variation and modulation in Bordetella bronchiseptica. Infect Immun. 1984 Jun;44(3):681–687. doi: 10.1128/iai.44.3.681-687.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D. R., Parker C. D. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect Immun. 1982 Nov;38(2):548–553. doi: 10.1128/iai.38.2.548-553.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
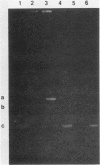
- Shimizu M., Kuninori K., Inoue M., Mitsuhashi S. Drug resistance and R plasmids in Bordetella bronchiseptica isolates from pigs. Microbiol Immunol. 1981;25(8):773–786. doi: 10.1111/j.1348-0421.1981.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Terakado N., Araki S., Mori Y., Sekizaki T., Hashimoto K. Non-conjugative R plasmid with five drug resistance from Bordetella bronchiseptica of pig origin. Nihon Juigaku Zasshi. 1981 Dec;43(6):971–974. doi: 10.1292/jvms1939.43.971. [DOI] [PubMed] [Google Scholar]
- Terakado N., Mitsuhashi S. Properties of R factors from Bordetella bronchiseptica. Antimicrob Agents Chemother. 1974 Dec;6(6):836–840. doi: 10.1128/aac.6.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. I., Nedelman J., Hendley J. O., Hewlett E. L. Species specificity of Bordetella adherence to human and animal ciliated respiratory epithelial cells. Infect Immun. 1983 Nov;42(2):692–695. doi: 10.1128/iai.42.2.692-695.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo Y., Shimizu T. Adherence of Bordetella bronchiseptica to swine nasal epithelial cells and its possible role in virulence. Res Vet Sci. 1979 Jul;27(1):15–21. [PubMed] [Google Scholar]