Abstract
Mycosis fungoides and its leukemic variant, Sezary syndrome, are the most common primary cutaneous T-cell lymphomas (CTCLs). In an ex vivo study, we investigated the percentage, phenotype, and suppressive function of CD4+CD25+ regulatory T cells (Tregs) from peripheral blood of CTCL patients. The percentage of Tregs did not differ significantly between patients and controls. Functional assays demonstrated a dichotomy in Treg function: in four out of 10 patients CD4+CD25+ T cells were incapable of suppressing autologous CD4+CD25- T-cell proliferation, whereas suppressive function was intact in the other six patients. Suppressive activity of Tregs inversely correlated with the peripheral blood tumor burden. T-plastin gene expression, used as a Sezary cell marker, confirmed that Sezary cells were heterogeneous for CD25 expression. Mixed lymphocyte reactions demonstrated that CD4+CD25- T cells from patients who lacked functional Tregs were susceptible to suppression by Tregs from healthy controls, and had not become suppressive themselves. Furthermore, we found reduced expression of Foxp3 in the CD4+CD25+ Tregs of these patients relative to the other six CTCL patients and controls. Our findings thus indicate a dysfunction of peripheral Tregs in certain CTCL patients, which correlates with tumor burden.
INTRODUCTION
Primary cutaneous T-cell lymphoma (CTCL) comprises a heterogeneous group of extranodal non-Hodgkin’s lymphomas, representing 2% of all lymphomas and occurs at an annual incidence of 0.3-1 per 100,000 persons (Weinstock and Gardstein, 1999). Mycosis fungoides and its leukemic variant, Sezary syndrome, are the most frequent CTCLs involving the skin. Mycosis fungoides presents with a skin-restricted infiltration of clonal T cells and an indolent course. Sezary syndrome represents an aggressive form of CTCL and is characterized by a diffuse pruritic erythroderma, lymphadenopathy, and the presence of a clonal proliferation of malignant T cells in the peripheral blood, skin, and lymph nodes. Malignant T cells in Sezary syndrome represent a clonal expansion of mature helper T cells with a memory phenotype (Kim et al., 2005). Generally, CTCL cells appear to proliferate poorly in vitro and it is still unclear what mechanisms are adopted by CTCLs in vivo to allow expansion and escape immune surveillance. The dysregulation of the STAT proteins, Stat3 and Stat5, in CTCL may be one mechanism adopted by malignant T cells to promote survival (Brender et al., 2001; Mitchell et al., 2003). Recently, in vitro proliferation of CD4+ T cells from CTCL patients, for up to 3 months, was demonstrated by co-culture of these cells with autologous immature dendritic cells loaded with apoptotic CD4+ T cells. The patient T cells cultured in this manner were shown to adopt a CD4+CD25+ T-regulatory (Treg) phenotype (Berger et al., 2005).
Naturally occurring CD4+CD25+ Tregs specialized CD4+ T cells, which express high levels of CD25, and whose main function is to suppress the activity of other immune cells, thus maintaining immunological tolerance (Shevach, 2002). In humans, like in mice, these Tregs comprise 5-10% of CD4+ T cells in peripheral blood (Taams et al., 2001), are identified by the selective expression of a transcription factor, Foxp3 (Ramsdell, 2003), and express high levels of the inhibitory receptor, CTLA-4 (Read et al., 2000; Taams et al., 2001). In vitro studies have shown that CD4+CD25+ Tregs are anergic and do not proliferate in response to anti-CD3 mAb or antigenic stimulation (Levings et al., 2001; Taams et al., 2001, 2002). Whereas the presence of Tregs is clearly beneficial for the prevention of auto-immunity, in the case of tumor biology, the presence of these cells could diminish effective natural tumor surveillance and clearance. Indeed, several studies have suggested that tumor progression might be associated with an increase in CD4+CD25+ Tregs (reviewed by Terabe and Berzofsky, 2004). Based on the study by Berger et al. (2005) and our own observations that malignant CD4+ T cells in CTCLs are generally anergic, we investigated whether this unresponsiveness was associated with an increase in CD4+CD25+ Treg percentage or function.
RESULTS
Anergy in CD4+ T cells from CTCL patients is not associated with an overall increase in the percentage of CD4+CD25+ T cells
We first investigated the proliferative capacity of CD4+ CTCL T cells. CD4+ T cells from CTCL patients displayed significantly less proliferation in response to anti-CD3 mAb and irradiated antigen-presenting cells (APCs) than CD4+ T cells isolated from healthy controls (HCs) (Figure 1a). We hypothesized that this observed anergy may be caused by the conversion of malignant CD4+ T cells into CD4+CD25+ Tregs, as suggested by a recent study (Berger et al., 2005). Therefore, we investigated the percentage of CD4+CD25+ T cells in the CD4+ T-cell population of patients by immunophenotyping for CD4 and CD25 expression. Figure 1b shows a representative dot plot for CD4 and CD25 staining in peripheral blood lymphocytes from an HC and a CTCL patient. The boxed region indicates the percentage of CD4+CD25bright cells, which have been suggested to contain the most effective regulatory T cells (Baecher-Allan et al., 2001). As expected, CTCL patients had a dramatic expansion of CD4+ T cells in the peripheral blood (Figure 1b and Table 1). However, no significant difference was observed between the mean percentage of CD4+CD25+ or CD4+CD25bright T cells present in the CD4+ T-cell population: 9.2±0.5 vs 9.7±4% of CD4+CD25+ T cells and 1.4±0.3 vs 1.9±0.9% of CD4+CD25+bright T cells in HCs and CTCLs, respectively (Figure 1c). Together, these data show that in CTCL patients the anergic phenotype of CD4+ T cells is not accompanied by an increased percentage of CD4+CD25+total or CD4+CD25+bright T cells.
Figure 1. The anergic phenotype of CD4+ T cells from CTCL patients is not owing to an overall increase in CD4+CD25+ T cells.
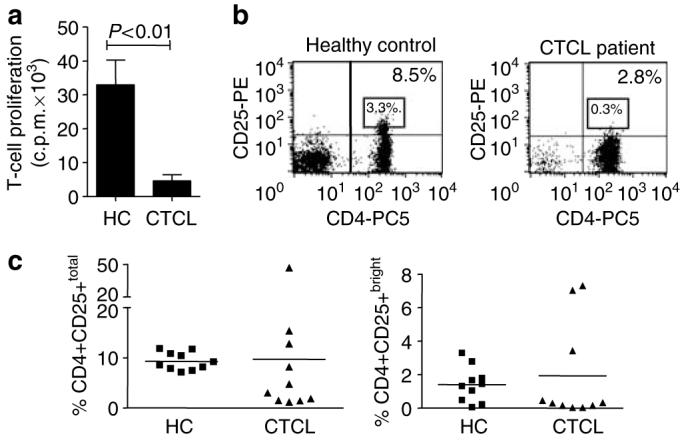
CD4+ T cells were isolated from PBMC of HC and CTCL patients via MACS. (a) CD4+ T cells were stimulated with anti-CD3 mAb (50 ng/ml) and APC, and T-cell proliferation was measured by 3H-thymidine incorporation. Data are depicted as mean c.p.m.±SEM, n = 10 independent experiments. (b) Representative dot plots of peripheral blood lymphocytes from HC versus a CTCL patient showing the percentage of CD4+CD25+total and CD4+CD25+bright T cells in CD4+ T cells; the square region indicates CD4+CD25bright T cells. (c) The percentages of CD4+CD25+total and CD4+CD25+bright T cells in the CD4+ T-cell fraction are shown for each individual HC and CTCL patient.
Table 1. CD4+CD25+ Treg activity and markers of peripheral blood tumor burden in CTCL patients.
| CTCL |
HC (n=10) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| CTCL | SzS | SzS | SzS | SzS | SzS | SzS | SzS | SzS | MF | MF | — |
| Months since diagnosis1 | 21 | 1 | 5 | 1 | 36 | 6 | 1 | 4 | 1 | 16 | — |
| Treatment2 | ECP | ECP | ECP | ECP | ECP | ECP | ECP | ECP | ECP | ECP | — |
| Tar | Tar/IFN | ||||||||||
| Suppression by Tregs (%)3 | 0 | 0 | 0 | 0 | 65 | 89 | 66 | 76 | 92 | 99 | 57±7 |
| Sezary cell count (%)4 | 71 | 43 | 20 | 63 | 12 | 9 | 7 | 10 | 0 | 0 | — |
| CD4+ T-cell count (%)5 | 88 | 81 | 54 | 85 | 62 | 56 | 27 | 70 | 39 | 42 | 30±3 |
| Total lymphocyte count (109/l)6 | 23 | 30 | 11 | 18 | 4.1 | 4.3 | 6.2 | 4.5 | 1.5 | 5.3 | — |
The table shows clinical data and Treg function for each CTCL patient (no. 1-10). Patients were diagnosed as either SzS or MFs.
Patients samples were taken at the indicated number of months after diagnosis.
Patients were treated with extracorporeal photopheresis (ECP), in some cases in combination with Targretin (Tar), or Interferon alpha2B (IFN).
Percentage suppression by CD4+CD25+ Tregs was measured by calculating the inhibition of CD4+CD25- T-cell proliferation in the presence of CD4+CD25+ Tregs.
Sezary cell count in the lymphocyte population was estimated by counting the number of atypical lymphocytes with cerebriform nuclei (Sezary cells) in blood smears.
The percentage CD4+ T cells in PB lymphocytes was measured via flow cytometry by gating on lymphocytes and CD4+ T cells.
Total lymphocyte count was assessed flow cytometry.
Data on the healthy controls are averages±SEM of 10 independent experiments.
CTCL patients with a high tumor burden contain dysfunctional CD4+CD25+ regulatory T cells
We next investigated whether the anergic phenotype of CD4+ T cells in CTCL patients was owing to an overall increase in suppressive function of the CD4+CD25+ Tregs. Therefore, we examined the suppressive effects of CD4+CD25+ T cells on CD4+CD25- T-cell proliferation upon stimulation with anti-CD3 mAb and autologous APC. CD4+CD25+ and CD4+CD25- T cells from HC and CTCL patients were isolated with similar efficiency and purity (Figure 2a and b). Surprisingly, when tested functionally, in four of the 10 CTCL patients, the isolated CD4+CD25+ T cells did not suppress the CD4+CD25- T-cell proliferation (representative graph in Figure 2c and Table 1, patients 1-4), whereas intact suppressive activity of the CD4+CD25+ T cells was observed in the other six CTCL patients (Figure 2c and Table 1, patients 5-10). Thus, functional characterization of Tregs revealed a distinct classification of CTCL patients into those with suppressive and those with non-suppressive CD4+CD25+ Tregs. Interestingly, we observed that the four CTCL patients with non-functional CD4+CD25+ T cells had a significantly enhanced Sezary cell count (49±11 vs 7±2%, P=0.0061) and total lymphocyte count (20±4×109/l vs 4±1×109/l, P=0.0095) compared to the patients with intact Treg-suppressive capacity (see also Table 1). Indeed, we found a significant negative correlation between markers of tumor burden and the level of suppression by CD4+CD25+ Tregs (R=-0.757, -0.935, and -0.650 for the total lymphocyte count, Sezary count, and percentage CD4+ T cells, respectively, P<0.05, data not shown). Thus, a loss of Treg activity is significantly associated with increasing peripheral blood tumor burden.
Figure 2. Dichotomy in suppressive activity of CD4+CD25+ Tregs from CTCL patients.
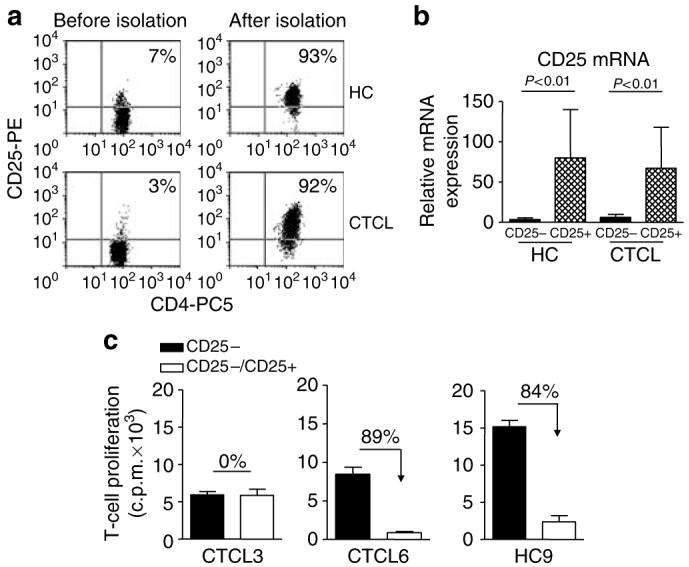
(a) CD4+CD25+ T cells were isolated from phosphate buffer from HC and CTCL patients by MACS. Representative dot plots show the percentage CD4+CD25+ T cells from HC and CTCL patients before and after purification. (b) The relative mRNA expression of CD25 in purified CD4+CD25- and CD4+CD25+ T-cell subsets from HC and CTCL patients was determined by real-time PCR. Results are shown as the mean±SEM of eight independent experiments. (c) Isolated CD4+CD25- T cells were cultured with anti-CD3 mAb and irradiated autologous PBMC in the absence (black bars, 1:0 ratio) or presence (open bars, 1:1 ratio) of autologous CD4+CD25+ T cells. Results of three representative independent experiments are shown: one CTCL patient with non-suppressive CD4+CD25+ T cells (CTCL3), one CTCL patient with suppressive CD4+CD25+ T cells (CTCL6), and one representative HC (HC9).
Non-functional CD4+CD25+ Tregs display a significantly lower expression of Foxp3 mRNA expression in comparison to functional CD4+CD25+ Tregs
The four CTCL patients who contained the non-suppressive CD4+CD25+ T cells (patients 1-4) also contained a significantly lower percentage of CD4+CD25+total and CD4+CD25bright T cells as compared to the HCs (2.4±70.8% of CD4+CD25+ and 0.13±0.06% of CD4+CD25bright cells, P<0.05). As both the presence of Foxp3 and CTLA-4 have been associated with CD4+CD25+ Treg function, we next evaluated the expression of these genes in CD4+CD25- and CD4+CD25+ T cell subsets by real-time PCR analysis. In HC, Foxp3 and CTLA-4 mRNA expression was significantly enhanced in CD4+CD25+ T cells compared to CD4+CD25- T cells (Figure 3). In CTCL patients with dysfunctional CD4+CD25+ Tregs (non-suppressive), the relative expression of Foxp3 mRNA was significantly decreased in the purified CD4+CD25+ T-cell subset as compared to CD4+CD25+ T cells from HC and CTCL patients with intact Treg activity (suppressive) (Figure 3). The relative expression of CTLA-4 was also decreased in CD4+CD25+ T cells from the non-suppressive CTCL patients as compared to HC and suppressive CTCL patients, but this was not significant (Figure 3). We used the Sezary cell-specific marker T-plastin (Su et al., 2003; Nebozhyn et al., 2006) to identify the cell populations containing tumor cell-derived mRNA (Figure 3c). Consistent with previously published data (Lin et al., 1999; Su et al., 2003; Nebozhyn et al., 2006), no T-plastin expression was observed in RNA isolated from the T cells of HC. However, T-plastin was detected in 8/10 CTCL patients both in the suppressive and non-suppressive group and in both T-cell populations. These data demonstrate that the tumor-derived cells display a distinct heterogeneity in CD25 expression. Together, these results indicate that the dysfunctional CD4+CD25+ Tregs from CTCL patients showed a concomitant decrease in Foxp3 expression and that the tumor cells were not exclusively CD25+.
Figure 3. Lack of suppressive function in CD4+CD25+ Tregs in CTCL patients is associated with significantly lower Foxp3 mRNA expression.
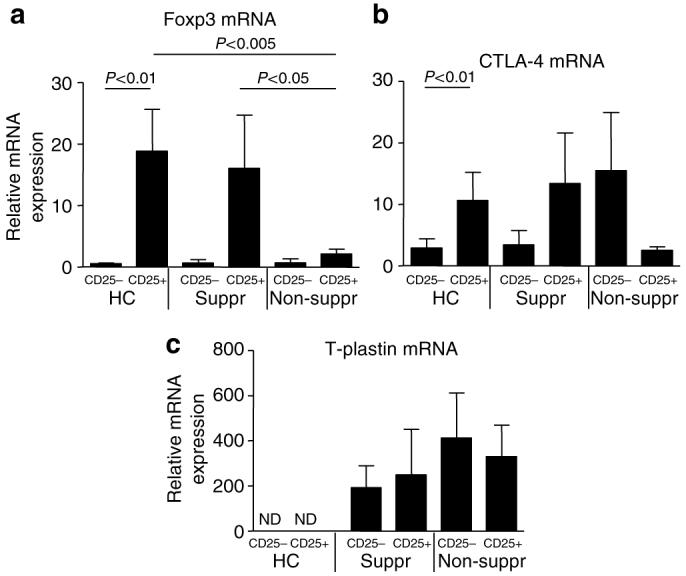
(a) The relative mRNA expression levels of Foxp3, (b) CTLA-4, and (c) T-plastin in CD4+CD25- and CD4+CD25+ T cells were assessed by real-time PCR. The mean±SEM of the mRNA expression is shown for HCs (n=8), suppressive CTCL patients (n=4), and non-suppressive CTCL patients (n=4). Significant differences are indicated in the figures; ND, not detected.
The defect in Treg-mediated suppression lies within the CD4+CD25+ T-cell subset
We investigated whether the defective suppression was owing to a defect in the CD4+CD25+ or in the CD4+CD25- T-cell population. Therefore, mixed lymphocyte reactions were performed in which responder T cells from a healthy donor were co-cultured with APC derived from a CTCL patient, in the absence or presence of CD4+CD25- or CD4+CD25+ T cells from the same CTCL patient. In this allogeneic setting, the CD4+CD25+ T cells from the non-suppressive CTCL patients induced substantially lower suppression (30%, Figure 4a) compared to CD4+CD25+ T cells from HC (80%, Figure 4c) or from “suppressive” CTCL patients (60%, Figure 4b). The CD4+CD25- T cells from both non-suppressive and suppressive CTCL patients showed only weak, if any, capacity to suppress T-cell proliferation (17 and 2%, Figure 4a and b), indicating that the CD4+CD25- T-cell population had not acquired suppressive activity. Figure 4c demonstrates that in contrast to the anergy observed in Figure 1, CD4+CD25- T cells from CTCL patients were able to proliferate under strong stimulatory conditions as provided by allogeneic stimulation. In addition, Figure 4c shows that the CD4+CD25- T cells from CTCL patients who lacked Treg-mediated suppression are susceptible to suppression by CD4+CD25+ T cells from HCs. Together, these data indicate that the defect in Treg-mediated regulation in the non-suppressive CTCL patients is not owing to an intrinsic defect in the susceptibility of the CD4+CD25- T-cell population to suppression, but rather that the defect lies in the CD4+CD25+ T cells.
Figure 4. The defect in Treg-mediated regulation lies within the CD4+CD25+ T-cell population.
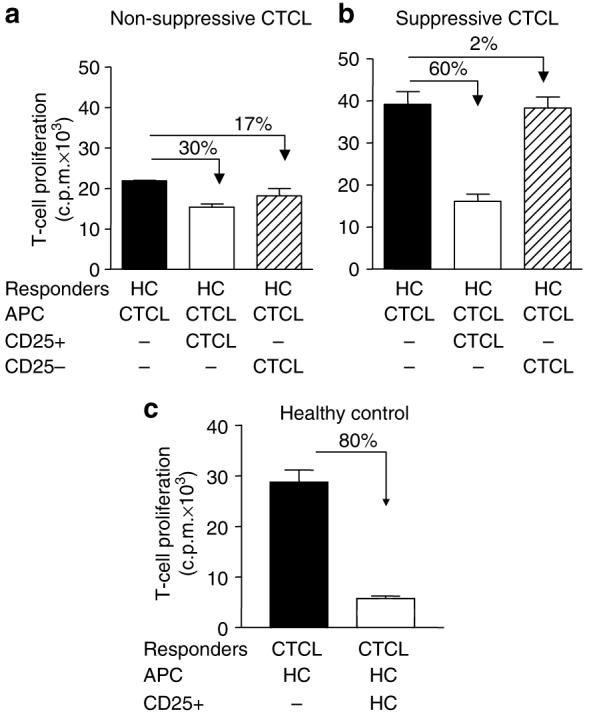
CD4+CD25+ or CD4+CD25- T cells from (a) non-suppressive or (b) suppressive CTCL patients were tested for their capacity to suppress the proliferation of allogeneic T cells derived from HC. CD4+CD25- HC T cells were cultured alone (black bars, 1:0), in the presence of CD4+CD25+ CTCL T cells (white bars, 1:1 ratio) or CD4+CD25- CTCL T cells (hatched bars, 1:1 ratio). (c) CD4+CD25- T-cells from a non-suppressive patient were cultured in the absence (black bar, 1:0) or presence of allogeneic CD4+CD25+ T cells derived from HC (white bar, 1:1 ratio). The suppressive activity is indicated by percentage inhibition in each graph. The intrinsic suppressive activity of the CD4+CD25+ T cells in the corresponding autologous system were 0, 89, and 40% for non-suppressive CTCLs, suppressive CTCLs, and HCs, respectively. One of the two independent experiments is shown.
DISCUSSION
Disease progression in advanced stages of CTCL is characterized by a marked shift in the host immune system to one that is more suppressive and less cytotoxic (Kim et al., 2005). A recent study suggested that tumor cells in CTCL can be induced to adopt a Treg phenotype and function using an in vitro photopheresis model (Berger et al., 2005). Therefore, it seemed plausible that there may be an increase in the relative number and/or activity of peripheral Tregs in CTCL patients. Our results on 10 CTCL patients, however, demonstrate no significant increase in the percentage of CD4+CD25+ or CD4+CD25bright T cells in the peripheral blood as compared to HCs. These data indicate that the significantly reduced proliferation of malignant CD4+ T cells in CTCL patients to TCR-activating stimuli is not attributed to the conversion of these cells into classical CD4+CD25+ Tregs in the periphery.
Interestingly, our functional assays indicate a striking dichotomy in our patient cohort: one group that does not contain suppressive CD4+CD25+ Tregs, called “non-suppressive CTCL”, and one group that has CD4+CD25+ Tregs with suppressive capacity (denominated “suppressive CTCL”). Importantly, this dichotomy in Treg function correlates with typical markers of peripheral blood tumor burden: that is, the non-suppressive CTCL patients have significantly increased counts of CD4+ T cells, total lymphocytes, and Sezary cells as compared to the suppressive CTCL group. Consistent with the significant correlation between tumor burden and loss of functional Tregs, we observed that the two erythrodermic mycosis fungoides patients (CTCLs 9 and 10) were extremely potent in the suppression assay, resulting in 92 and 99% suppression of T-cell proliferation, respectively. The dichotomy in Treg function furthermore illustrates that the lack of suppressive capacity in the severe CTCL patients was not owing to their higher average age (66±4 years) compared to controls (38±3 years), as suggested recently (Tsaknaridis et al., 2003), as intact suppression was observed in the other six CTCL patients who were of similar age (65±3 years). Also, other recent studies demonstrate intact CD4+CD25+ Treg function in the elderly (>70 years) and the young (<35 years), with similar expression of CTLA-4 (Gregg et al., 2005) and Foxp3 (AN Akbar, personal communication).
Sezary cells can be identified by the expression of cell surface markers such as CD40, CD40 ligand (Storz et al., 2001), and SC5 (Nikolova et al., 2001, 2002). However, the potential use for these molecules to isolate Sezary cells is limited by the fact that they are also found on some normal cells in the peripheral blood. The use of specific TCRVβ chain antibodies as an alternative approach is limited by the lack of available mAb reacting with some TCRVDJβ gene products in the blood (Bigler et al., 1996; Langerak et al., 2001). Although it was not possible to isolate directly intact tumor cells based on cell surface marker expression for functional evaluation, we were able to demonstrate the presence of Sezary cells in both CD4+CD25- and CD4+CD25+ T-cell subsets using the recently described Sezary cell marker T-plastin (Su et al., 2003; Nebozhyn et al., 2006), which is predominantly a cytoplasmic protein that functions to regulate actin assembly and cellular motility. The detection of T-plastin mRNA expression confirmed that peripheral blood tumor cells display a distinct heterogeneity in terms of T-cell phenotypic cell surface marker expression, as both the CD25- and CD25+ cells expressed T-plastin regardless of Treg function.
We found that the lack of function of CD4+CD25+ Tregs in the non-suppressive CTCL group was not owing to decreased susceptibility to suppression in the CD4+CD25- responder T cells, nor had suppressive activity shifted towards these cells. Instead, the dysfunction appears to lie in the CD4+CD25+ Treg subset, which coincides with a significantly lower expression of Foxp3 compared to HCs. Foxp3 gene expression has been detected in adult T-cell lymphoma, which is also characterized by a clonal proliferation of CD4+CD45RO+ tumor cells, albeit with consistently higher CD25 expression and increased numbers of CD4+CD25+ Tregs as compared to CTCLs (Karube et al., 2004; Walsh et al., 2006). Interestingly, a recent study in multiple myeloma patients described similar findings to our study, that is, a decrease in the percentage of CD4+CD25+FOXP3+ T cells with a concomitant decrease in suppressive activity (Prabhala et al., 2006), suggesting that this phenomenon might contribute to dysfunctional T-cell responses in other hematological malignancies as well. The same paper showed that increasing the ratio of CD4+CD25+ T cells to responder cells did not compensate for the lack of suppression.
Besides a lack of Foxp3, other mechanisms might contribute to the observed functional dysregulation of CD4+CD25+ T cells in the non-suppressive patients. As survival and function of CD4+CD25+ T cells is highly dependent on the production of IL-2 by neighboring T cells (Malek and Bayer, 2004), a low level of IL-2, as reported in CTCL patients (Dummer et al., 1992) might trigger apoptosis of CD4+CD25+ T cells (Taams et al., 2001). Notably, we did observe a high percentage of non-viable CD4+CD25+ T cells (30-50%, as measured by trypan blue stain, data not shown) in three out of the four non-suppressive CTCL patients, a feature that was found in only one out of six suppressive patients. Additionally, a lack of efficient IL-2 signalling in CTCL patients (Mitchell et al., 2003) may contribute to lower relative numbers of peripheral Tregs.
Surprisingly, CTLA-4 gene expression appeared increased in the CD4+CD25- population of non-suppressive patients as compared to suppressive CTCL patients (Figure 3b). This is consistent with a recent study showing a direct correlation between disease stage/progression in CTCL and increased expression of CTLA-4 (Wong et al., 2006). In view of the observed T-plastin expression in both CD25- and CD25+ T-cell subsets, it would seem likely that this represents increased tumor cell expression of CTLA-4 with increasing peripheral blood tumor burden, albeit with an unexplained restriction to the CD25- population. The mechanism and functional consequences remain unclear, although it has been postulated that tumor-derived CTLA-4 might suppress the proliferation of normal T cells, whereas defective CTLA-4 signalling pathways in the tumor cell, notably owing to a dysregulation of AP1 detected previously in CTCL (Mao et al., 2003) would prevent growth suppression of the tumor cell itself.
At first glance, our data may seem in contrast to a recent study by Berger et al. (2005), which proposed that CTCL is owing to a malignant proliferation of CD4+CD25+ regulatory T cells. The main difference between our studies is that we investigated the phenotype and suppressive function of peripheral CD4+CD25+ T cells ex vivo, whereas Berger et al. used an in vitro model to mimic potential interactions between epidermal Sezary cells and immature dendritic cellsin the skin. It is possible though to reconcile the findings of these two studies: Berger et al. postulated that in the early stages of CTCL, CD4+ T cells in Pautrier microabscesses within the epidermis encounter Langerhans cells presenting apoptotic cell fragments, and that this continuous stimulation leads to the induction and expansion of tumor cells as Tregs. These tumor-derived Tregs then prevent the induction of effective antitumor immunity by inhibiting cytotoxicity by CD8+ T cells (Piccirillo and Shevach, 2001). This allows the malignant CD4+ T cells to persist in the skin and migrate into the lymph nodes and peripheral blood (Bunn et al., 1981). Subsequently, with increasing tumor burden, additional changes develop such as decreased TCR complexity in the normal T cells and reduced tumor IL-2 responsiveness. Further disease progression may then occur owing to dysregulation of Treg function in the periphery, as shown in this current study. Nevertheless, it may be possible that these peripheral blood tumor cells can still retain the capacity to develop functional Treg activity if they receive the correct in vitro signals as suggested by Berger et al.
Although we have shown that CD4+CD25+FOXP3+ T-cell presence and function may be dysregulated in CTCL patients with a high tumor burden, it is still unclear whether or not these dysfunctional Tregs are tumor-derived and exactly what the pathogenic consequences are. There are an increasing number of targeted therapies being used in CTCL, such as Denileukin Difitox (diptheria-IL-2 fusion toxin) and HuMaxCD4, but the consequences of the dysregulation of Tregs in late stages of CTCL for therapeutic intervention remain to be established.
MATERIALS AND METHODS
Patients
Ten patients (mean age 64±2 years, men/women 7/3) fulfilled the criteria for a diagnosis of CTCL. Clinical and immunophenotypic features included erythroderma, pruritus, and lymphadenopathy. All patients were treated with extracorporeal photopheresis, some in combination with Targretin (Bexarotene) or Interferon alpha2B (see Table 1). The percentage of Sezary cells in the lymphocyte population was estimated by counting the number of atypical lymphocytes with cerebriform nuclei (Sezary cells) in blood smears, by one expert individual as described (Russell-Jones and Whittaker, 2000). All patients were human T-lymphotropic virus type I-negative. The study was conducted according to the Declaration of Helsinki Principles. The study was approved by the Guy’s and St Thomas Ethics Committee, and participants were recruited to the study with informed written consent. Ethical approval for the use of phosphate buffer from sex-matched HCs (mean age 38±3 years) was obtained from the King’s College London Research Ethical Committee.
Cell isolation and purification
Peripheral blood mononuclear cells (PBMCs) were obtained from CTCL patients and HC using density gradient centrifugation (Lymphoprep, Norway). CD4+ T cells were isolated by magnetic cell sorting (>95% purity) using a T-cell isolation kit (Miltenyi Biotec, Germany) and subsequently the CD4+CD25+ T cells were separated from the CD4+CD25- T cells with anti-CD25 microbeads (Miltenyi Biotec) (Taams et al., 2001, 2002). Purity of the cell subsets was confirmed by flow cytometry (representative example shown in Figure 2a) and real-time PCR (see Figure 2b). The mean fluorescence intensity of CD25 in the purified CD4+CD25+ T-cell subsets was 21±3 and 26±11 in HCs and CTCLs, respectively.
Flow cytometry
Total lymphocyte counts were determined by flow cytometry by the hospital immunology services. For phenotypic analysis, PBMCs were stained with mAbs to CD4 (PC5-labelled, Beckman Coulter, UK) and CD25 (PE-labelled, Miltenyi Biotec). Viable CD4+ T cells were gated on forward scatter/side scatter profile and high expression of CD4. The percentage of CD4+CD25+total and CD4+CD25+bright T cells was determined as shown in Figure 1b.
Proliferation and suppression assays
Cells were cultured in RPMI-1640 medium, supplemented with 1% penicillin/streptomycin, 1% glutamine, and 10% heat-inactivated fetal calf serum. To assess anergy, CD4+CD25- or CD4+CD25+ T cells (1×105/well) were cultured with irradiated autologous PBMC as APC (1×105/well) and 50 ng/ml anti-CD3 mAb (OKT3, Ortho Biotech, Bridgewater, VA). To assess suppressive activity, CD4+CD25-T cells were cultured with or without CD4+CD25+ T cells (1×105/well). For allogeneic assays, a similar experimental setup was used, but responder cells were stimulated with allogeneic APC and anti-CD3 (2 ng/ml) mAb in the absence or presence of allogeneic suppressor cells. Cells were cultured for 3 days (autologous assay) or 7 days (allogeneic assay), and during the last 18 hours of culture, [3H]-TdR was added at 1 μCi/well (Amersham, UK). Proliferative responses are expressed as the mean [3H]-TdR incorporation (c.p.m.) of triplicate wells±SEM. Percent suppression was calculated as (proliferation of CD4+CD25- T cells in the presence of CD4+CD25+ T cells/proliferation of CD4+CD25- T cells in the absence of CD4+CD25+ T cells)×100%. Functional analysis of samples from patients and HCs was performed in parallel.
Quantitative real-time reverse transcription-PCR
RNA extraction and real-time PCR analysis was performed as described previously (Mitchell et al., 2003). PCR primers and fluorogenic probes for the target genes and endogenous controls were purchased as TaqMan® Gene Expression Assay™ (ABI). The assay numbers for the endogenous control (cyclophilin) and target genes were as follows: Hs99999904_ml (cyclophilin), Hs00166229_m1 (CD25), Hs00203958_m1 (FoxP3), Hs00175480_m1 (CTLA-4), and Hs00192406_m1 (T-plastin).
Statistics
A Mann-Whitney U-test was used to investigate functional or phenotypic differences between cells from HCs and non-suppressive or suppressive CTCL patients. The Wilcoxon test was used to test differences in the relative mRNA expression of the various genes between the CD25- and CD25+ T-cell subsets. Correlations between disease severity markers and suppressive activity by CD4+CD25+ T cells were calculated with the Spearman’s ρ method.
ACKNOWLEDGMENTS
We thank Hayley Wright, Bjorn Schuster, and Jennifer Lawson for assistance in some experiments. M.T. is supported by a grant from the BBSRC. Work in S.J.’s lab is supported by the Wellcome Trust and the Medical Research Council of UK.
Abbreviations
- APC
antigen-presenting cell
- CTCL
cutaneous T-cell lymphoma
- HC
healthy control
- PBMC
peripheral blood mononuclear cell
- Treg
regulatory T cell
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory T cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Berger CL, Tigelaar R, Cohen J, Mariwalla K, Trinh J, Wang N, et al. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105:1640–7. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- Bigler R, Boselli C, Foley B, Vonderheid E. Failure of anti-T-cell receptor V beta antibodies to consistently identify a malignant T-cell clone in Sezary syndrome. Am J Pathol. 1996;149:1477–83. [PMC free article] [PubMed] [Google Scholar]
- Brender C, Nielsen M, Kaltoft K, Mikkelsen G, Zhang Q, Wasik M, et al. Stat3-mediated constitutive expression of SOCS-3 in cutaneous T-cell lymphoma. Blood. 2001;97:1056–62. doi: 10.1182/blood.v97.4.1056. [DOI] [PubMed] [Google Scholar]
- Bunn PJ, Edelson R, Ford S, Shackney S. Patterns of cell proliferation and cell migration in the Sezary syndrome. Blood. 1981;57:452–63. [PubMed] [Google Scholar]
- Dummer R, Posseckert G, Nestle F, Witzgall R, Burger M, Becker JC, et al. Soluble interleukin-2 receptors inhibit interleukin 2-dependent proliferation and cytotoxicity: explanation for diminished natural killer cell activity in cutaneous T-cell lymphomas in vivo? J Invest Dermatol. 1992;98:50–4. doi: 10.1111/1523-1747.ep12494223. [DOI] [PubMed] [Google Scholar]
- Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, et al. The number of human peripheral blood CD4+CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–6. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karube K, Ohshima K, Tsuchiya T, Yamaguchi T, Kawano R, Suzumiya J, et al. Expression of Foxp3, a key molecule in CD4+CD25+ regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol. 2004;126:81–4. doi: 10.1111/j.1365-2141.2004.04999.x. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langerak AW, van den Beemd R, Wolvers-Tettero ILM, Boor PPC, van Lochem EG, Hooijkaas H, et al. Molecular and flow cytometric analysis of the Vβ repertoire for clonality assessment in mature TCRαβ T-cell proliferations. Blood. 2001;98:165–73. doi: 10.1182/blood.v98.1.165. [DOI] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Roncarolo M-G. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–301. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Lau A, Huynh T, Lue T. Differential regulation of human T-plastin gene in leukocytes and non-leukocytes: identification of the promoter, enhancer, and CpG island. DNA Cell Biol. 1999;18:27–37. doi: 10.1089/104454999315592. [DOI] [PubMed] [Google Scholar]
- Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–74. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- Mao X, Orchard G, Lillington DM, Russell-Jones R, Young BD, Whittaker SJ. Amplification and overexpression of JUNB is associated with primary cutaneous T-cell lymphomas. Blood. 2003;101:1513–9. doi: 10.1182/blood-2002-08-2434. [DOI] [PubMed] [Google Scholar]
- Mitchell TJ, Whittaker SJ, John S. Dysregulated expression of COOH-terminally truncated Stat5 and loss of IL2-inducible Stat5-dependent gene expression in Sezary syndrome. Cancer Res. 2003;63:9048–54. [PubMed] [Google Scholar]
- Nebozhyn M, Loboda A, Kari L, Rook AH, Vonderheid EC, Lessin S, et al. Quantitative pcr on 5 genes reliably identifies CTCL patients with 5-99% circulating tumor cells with 90% accuracy. Blood. 2006;107:3189–96. doi: 10.1182/blood-2005-07-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova M, Bagot M, Boumsell L, Bensussan A. Identification of cell surface molecules characterizing human cutaneous T-cell lymphomas. Leukemia Lymph. 2002;43:741–6. doi: 10.1080/10428190290016836. [DOI] [PubMed] [Google Scholar]
- Nikolova M, Tawab A, Marie-Cardine A, Bagot M, Boumsell L, Bensussan A. Increased expression of a novel early activation surface membrane receptor in cutaneous T cell lymphoma cells. J Invest Dermatol. 2001;116:731–8. doi: 10.1046/j.0022-202x.2001.doc.x. [DOI] [PubMed] [Google Scholar]
- Piccirillo CA, Shevach EM. Control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- Prabhala RH, Neri P, Bae JE, Tassone P, Shammas MA, Allam CK, et al. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006;107:301–304. doi: 10.1182/blood-2005-08-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–8. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell-Jones R, Whittaker S. Sezary syndrome: diagnostic criteria and therapeutic options. Semin Cutan Med Surg. 2000;19:100–8. doi: 10.1016/s1085-5629(00)80006-1. [DOI] [PubMed] [Google Scholar]
- Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Storz M, Zepter K, Kamarashev J, Dummer R, Burg G, Haffner AC. Coexpression of CD40 and CD40 ligand in cutaneous T-cell lymphoma (mycosis fungoides) Cancer Res. 2001;61:452–4. [PubMed] [Google Scholar]
- Su M-W, Dorocicz I, Dragowska WH, Ho V, Li G, Voss N, et al. Aberrant expression of T-plastin in Sezary cells. Cancer Res. 2003;63:7122–7127. [PubMed] [Google Scholar]
- Taams LS, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol. 2001;31:1122–31. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Taams LS, Vukmanovic-Stejic M, Smith J, Dunne PJ, Fletcher JM, Plunkett FJ, et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–30. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16:157–62. doi: 10.1016/j.coi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Tsaknaridis L, Spencer L, Culbertson N, Hicks K, LaTocha D, Chou YK, et al. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- Walsh PT, Benoit BM, Wysocka M, Dalton NM, Turka LA, Rook AH. A role for regulatory T cells in cutaneous T-cell lymphoma; induction of a CD4+CD25+Foxp3+ T-cell phenotype associated with HTLV-1 infection. J Invest Dermatol. 2006;126:690–2. doi: 10.1038/sj.jid.5700121. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Gardstein B. Twenty-year trends in the reported incidence of mycosis fungoides and associated mortality. Am J Public Health. 1999;89:1240–4. doi: 10.2105/ajph.89.8.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Wilson AJ, Gibson HM, Hafner MS, Hedgcock CJ, Berger CL, et al. Increased expression of CTLA-4 in malignant T-cells from patients with mycosis fungoides - cutaneous T cell lymphoma. J Invest Dermatol. 2006;126:212–9. doi: 10.1038/sj.jid.5700029. [DOI] [PubMed] [Google Scholar]


