Abstract
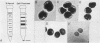
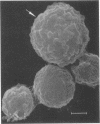
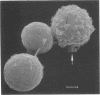
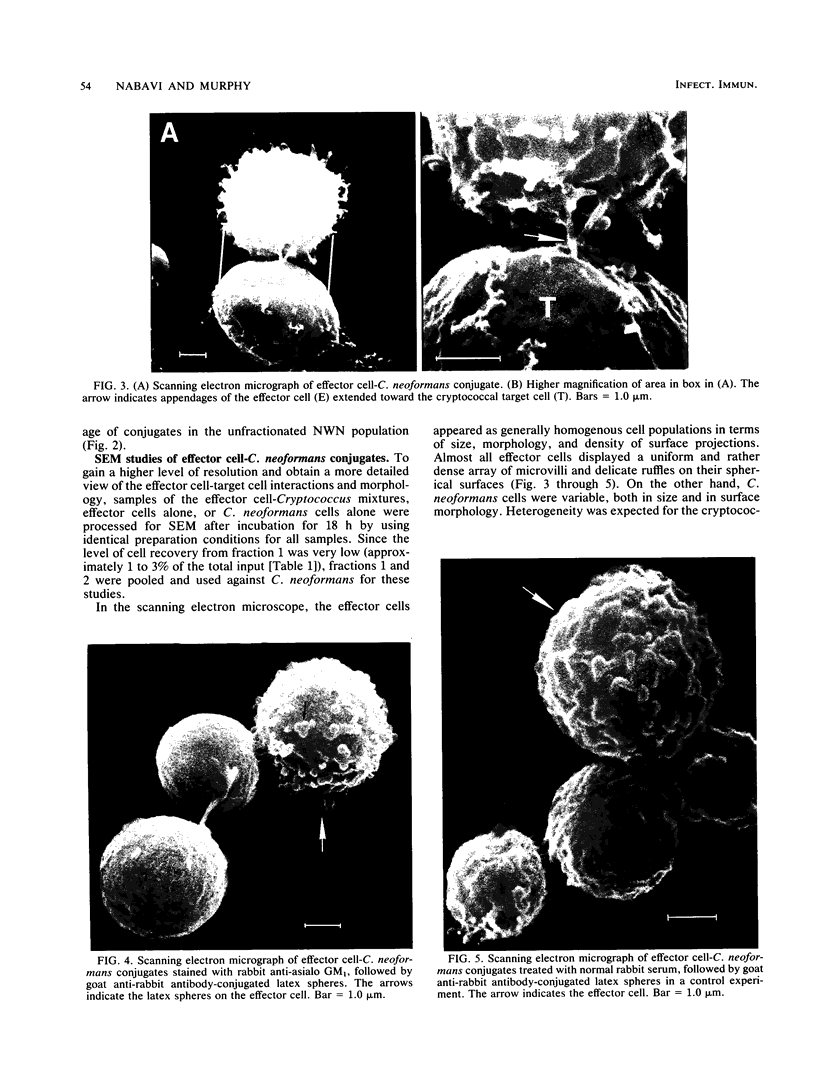
Nylon wool-nonadherent splenic cells from 7- to 8-week-old CBA mice were further fractionated on discontinuous Percoll gradients. Enrichment of natural killer (NK) cells in Percoll fractions 1 and 2 was confirmed by morphological examination, by immunofluorescent staining, and by assessing the cytolytic activity of each Percoll cell fraction against YAC-1 targets in the 4-h51Cr release assay. Cells isolated from each Percoll fraction were tested for growth-inhibitory activity against Cryptococcus neoformans, a pathogenic yeastlike organism, by using an in vitro 18-h growth inhibition assay. The results showed that NK cell enrichment was concomitant with enrichment of anti-Cryptococcus activity in Percoll fractions 1 and 2. Cells from NK cell-rich fractions formed conjugates with the mycotic targets similar to the conjugates reported in NK cell-tumor systems. In addition, the percentage of effector cell-Cryptococcus conjugates was directly proportional to the level of the C. neoformans growth-inhibitory activity of the effector cells used. Scanning electron microscopy of the effector cell-Cryptococcus conjugates showed direct contact between the effector cells and the cryptococcal targets. An immunolabeling method combined with scanning electron microscopy was used to demonstrate that the effector cells attached to C. neoformans were asialo GM1 positive and, therefore, had NK cell characteristics.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bulmer G. S., Tacker J. R. Phagocytosis of Cryptococcus neoformans by alveolar macrophages. Infect Immun. 1975 Jan;11(1):73–79. doi: 10.1128/iai.11.1.73-79.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpen O., Virtanen I., Saksela E. Ultrastructure of human natural killer cells: nature of the cytolytic contacts in relation to cellular secretion. J Immunol. 1982 Jun;128(6):2691–2697. [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973 Jun;127(6):694–697. doi: 10.1093/infdis/127.6.694. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Farram E., Targan S. R. Identification of human natural killer soluble cytotoxic factors (NKCF) derived from NK-enriched lymphocyte populations: specificity of generation and killing. J Immunol. 1983 Mar;130(3):1252–1256. [PubMed] [Google Scholar]
- Frey T., Petty H. R., McConnell H. M. Electron microscopic study of natural killer cell-tumor cell conjugates. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5317–5321. doi: 10.1073/pnas.79.17.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamliel H., Leizerowitz R., Gurfel D., Polliack A. Scanning immuno-electron microscopy of human leukaemia and lymphoma cells: a comparative study of techniques using immunolatex spheres as marker. J Microsc. 1981 Aug;123(Pt 2):189–199. doi: 10.1111/j.1365-2818.1981.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Henkart P. A., Millard P. J., Reynolds C. W., Henkart M. P. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984 Jul 1;160(1):75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Itoh K., Suzuki R., Umezu Y., Hanaumi K., Kumagai K. Studies of murine large granular lymphocytes. II. Tissue, strain, and age distributions of LGL and LAL. J Immunol. 1982 Jul;129(1):395–405. [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Kawase I., Urdal D. L., Brooks C. G., Henney C. S. Selective depletion of NK cell activity in vivo and its effect on the growth of NK-sensitive and NK-resistant tumor cell variants. Int J Cancer. 1982 May 15;29(5):567–574. doi: 10.1002/ijc.2910290513. [DOI] [PubMed] [Google Scholar]
- Keller R., Bächi T., Okumura K. Discrimination between macrophage-and NK-type tumoricidal activities via anti-asialo GM1 antibody. Exp Cell Biol. 1983;51(3):158–164. [PubMed] [Google Scholar]
- Kozel T. R., Highison B., Stratton C. J. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun. 1984 Feb;43(2):574–579. doi: 10.1128/iai.43.2.574-579.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Suzuki R., Hinuma S., Saitoh F. Studies of murine large granular lymphocytes. I. Identification as effector cells in NK and K cytotoxicities. J Immunol. 1982 Jul;129(1):388–394. [PubMed] [Google Scholar]
- Luini W., Boraschi D., Alberti S., Aleotti A., Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981 Aug;43(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Mattes M. J., Sharrow S. O., Herberman R. B., Holden H. T. Identification and separation of Thy-1 positive mouse spleen cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Immunol. 1979 Dec;123(6):2851–2860. [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Murphy J. W., Pahlavan N. Cryptococcal culture filtrate antigen for detection of delayed-type hypersensitivity in cryptococcosis. Infect Immun. 1979 Jul;25(1):284–292. doi: 10.1128/iai.25.1.284-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Kiessling R., Biberfeld P., Andersson B. Target-effector interaction in the natural killer (NK) cell system. II. The isolation of NK cells and studies on the mechanism of killing. J Immunol. 1978 Dec;121(6):2509–2517. [PubMed] [Google Scholar]
- Rubin P., Pross H. F., Roder J. C. Studies of human natural killer cells. II. Analysis at the single cell level. J Immunol. 1982 Jun;128(6):2553–2558. [PubMed] [Google Scholar]
- Schimpff S. C., Bennett J. E. Abnormalities in cell-mediated immunity in patients with Cryptococcus neoformans infection. J Allergy Clin Immunol. 1975 Jun;55(6):430–441. doi: 10.1016/0091-6749(75)90082-2. [DOI] [PubMed] [Google Scholar]
- Swenson F. J., Kozel T. R. Phagocytosis of Cryptococcus neoformans by normal and thioglycolate-activated macrophages. Infect Immun. 1978 Sep;21(3):714–720. doi: 10.1128/iai.21.3.714-720.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. C., Weitzen M. L., Kahle R., Granger G. A., Bonavida B. Studies on the mechanism of natural killer cytotoxicity. II. coculture of human PBL with NK-sensitive or resistant cell lines stimulates release of natural killer cytotoxic factors (NKCF) selectively cytotoxic to NK-sensitive target cells. J Immunol. 1983 May;130(5):2479–2483. [PubMed] [Google Scholar]