Abstract
The Gnomoniaceae are characterised by ascomata that are generally immersed, solitary, without a stroma, or aggregated with a rudimentary stroma, in herbaceous plant material especially in leaves, twigs or stems, but also in bark or wood. The ascomata are black, soft-textured, thin-walled, and pseudoparenchymatous with one or more central or eccentric necks. The asci usually have a distinct apical ring. The Gnomoniaceae includes species having ascospores that are small, mostly less than 25 μm long, although some are longer, and range in septation from non-septate to one-septate, rarely multi-septate. Molecular studies of the Gnomoniaceae suggest that the traditional classification of genera based on characteristics of the ascomata such as position of the neck and ascospores such as septation have resulted in genera that are not monophyletic. In this paper the concepts of the leaf-inhabiting genera in the Gnomoniaceae are reevaluated using multiple genes, specifically nrLSU, translation elongation factor 1-alpha (tef1-α), and RNA polymerase II second largest subunit (rpb2) for 64 isolates. ITS sequences were generated for 322 isolates. Six genera of leaf-inhabiting Gnomoniaceae are defined based on placement of their type species within the multigene phylogeny. The new monotypic genus Ambarignomonia is established for an unusual species, A. petiolorum. A key to 59 species of leaf-inhabiting Gnomoniaceae is presented and 22 species of Gnomoniaceae are described and illustrated.
Keywords: Foliicolous fungi, multilocus phylogenetics, polyphasic taxonomy, species identification, species recognition
INTRODUCTION
The ascomycete order Diaporthales includes a number of plant pathogenic fungi. The most notorious of these is the chestnut blight fungus [Cryphonectria parasitica (Murrill) M.E. Barr] that killed all of the American chestnut trees [Castanea dentata (Marsh.) Borkh.] in a few decades and thus altered the landscape of eastern North America (Anagnostakis 1987). Additional tree diseases are caused by members of the Diaporthales particularly in the Gnomoniaceae G. Winter. These include oak anthracnose [Apiognomonia errabunda (Roberge ex Desm.) Höhn.], cherry leaf scorch [A. erythrostoma (Pers.) Höhn.], sycamore canker [A. veneta (Sacc. & Speg.) Höhn.] (Sinclair & Lyon 2005) and ash anthracnose [Gnomoniella fraxini Redlin & Stack, now Plagiostoma fraxini (Redlin & Stack) Sogonov, anamorph Discula fraxinea Redlin & Stack]. Dogwood anthracnose, a disease that has killed dogwood trees (Cornus florida L., C. nuttallii Audubon ex Torr. & A. Gray) on both the east and west coasts of North America, is caused by Discula destructiva Redlin (1991), an asexually reproducing species in the Gnomoniaceae for which no sexual state is known (Zhang & Blackwell 2001, Castlebury et al. 2002). Recently it was discovered that the cause of butternut canker (Sirococcus clavigignenti-juglandacearum Nair et al.), a fungus that threatens to destroy another North American tree species (Juglans cinerea L.) belongs in the Gnomoniaceae (Ostry 1996, Mejia et al. 2008).
The Diaporthales are a well-defined order of the Sordariomycetes, Sordariomycetidae, as demonstrated using a four-gene phylogeny (Zhang et al. 2006). Diaporthalean fungi are characterised morphologically by brown to black perithecial fruiting bodies immersed in a stroma or the substrate, lack of true paraphyses at maturity, and unitunicate asci that float free within the centrum at maturity and often have a conspicuous ring in the apex (Barr 1978, Samuels & Blackwell 2001). The ascospores vary from non-septate to multi-septate or muriform, ellipsoidal to elongate, and hyaline or pale yellow to dark brown, rarely black. The asexual states of Diaporthales are generally coelomycetous, producing phialidic, often annellidic conidiogenous cells, and mostly non- or one-septate conidia in acervuli or pycnidia with or without a well-developed stroma, although some anamorphic states produce dark brown, multi-septate conidia.
Within the Diaporthales up to eight variously conceived families have been included over the past 30 years. These familial classifications of the Diaporthales were summarised by Zhang & Blackwell (2001) comparing Wehmeyer (1975), Barr (1978, 1990), and Kirk et al. (2001). In a molecular study Castlebury et al. (2002) analysed nuclear large subunit ribosomal DNA sequence data and outlined six major lineages, mostly recognised as families, within the Diaporthales. Since then three families have been added. A recent review discusses the definition of the nine currently accepted families included in the Diaporthales (Rossman et al. 2007,Rossman et al. 2007).
The family Gnomoniaceae based on the genus Gnomonia has been variously conceived since it was established by Winter (1886). This name was proposed for conservation by Hawksworth & Eriksson (1988) against Obryzaceae Körber and the proposal was accepted (McNeill et al. 2006). The concept of the Gnomoniaceae as monographed by Monod (1983) is in general agreement with results of molecular studies that include Gnomonia and its many segregate genera (Castlebury et al. 2002, DeSilva et al. 2008, Mejia et al. 2008). Other concepts of the family such as those proposed by Kobayashi (1970), Barr (1978, 1990), Vasilyeva (1998), and Eriksson et al. (2001) differ significantly from these results. The most commonly accepted concept of the Gnomoniaceae prior to the molecular studies cited above was that of Barr (1978). She recognised the suborder Gnomoniineae with the two families Gnomoniaceae and Valsaceae Tul. & C. Tul. distinguished by the placement of the neck. The Gnomoniaceae was defined as having “perithecia upright; necks central, rarely eccentric, erumpent separately” and included three subfamilies, one of which was the Gnomonioideae that included four genera now recognised within the Gnomoniaceae, i.e. Apiognomonia Höhn., Gnomonia, Gnomoniella Sacc., and Ophiognomonia (Sacc.) Sacc. The Valsaceae was defined as having “perithecia oblique or horizontal; necks oblique or lateral, erumpent separately or converging through stromatic disc” with the subfamily Plagiostomoideae that included four genera now recognised in the Gnomoniaceae, i.e. Apioplagiostoma M.E. Barr, Plagiosphaera Petr., Plagiostoma Fuckel, and Pleuroceras Riess. Kobayashi (1970) followed Höhnel (1917) in placing all genera of the Diaporthales in one family, Diaporthaceae Höhn. The family Cryptosporellaceae Arx & E. Müll. (Von Arx & Müller 1954) was established for the genus Cryptosporella Sacc. but this family name is considered invalid because of the lack of a Latin description (ICBN Art. 36.1). Mejia et al. (2008) demonstrated that Cryptosporella belongs to the Gnomoniaceae as outlined by Castlebury et al. (2002), thus the name Cryptosporellaceae is a synonym of the much older Gnomoniaceae.
Species in the Gnomoniaceae are characterised by ascomata that are immersed, rarely erumpent or superficial, solitary, without a stroma, or aggregated with a rudimentary stroma, in herbaceous plant material, especially in leaves, twigs or stems, but also in bark or wood. The ascomata are dark brown to black, generally soft-textured, thin-walled, and pseudoparenchymatous with either central or eccentric necks. Generally the asci have a distinct apical ring although this is not the case for species having long ascospores as in Crytosporella. The ascospores are generally small, mostly less than 25 μm long, although some are longer especially those of Cryptosporella, and range in septation from non-septate to one-septate, either in median or eccentric position. The asexual states of members of the Gnomoniaceae are acervular or pycnidial with a broad opening; conidiogenous cells are phialidic, and conidia are pallid, non-septate (Monod, 1983).
The Gnomoniaceae sensu Monod (1983) included 22 genera, some of which were excluded from this family by Castlebury et al. (2002). According to the latter authors, the family comprised the teleomorph genera Apiognomonia, Apioplagiostoma, Ditopella De Not., Gnomonia, Gnomoniella, Gnomoniopsis (Sacc.) Berl, Linospora Fuckel, Ophiognomonia, Phragmoporthe Petr., Plagiostoma, and Pleuroceras as well as species of the anamorph genera Discula Sacc. and Sirococcus Preus. Some genera previously placed in the Gnomoniaceae sensu Monod (1983) have been removed such as Mazzantia Mont., now placed within the Diaporthaceae, and Sydowiella Petr., type of the Sydowiellaceae Lar.N. Vassiljeva (Rossman et al. 2007,Rossman et al. 2007). Two genera, namely Cryptodiaporthe and Cryptosporella with its synonym Ophiovalsa on woody substrates, were placed in the Valsaceae by Barr (1978) and not considered by Monod (1983); however, Castlebury et al. (2002) determined that these genera belong in the Gnomoniaceae.
Considerable confusion exists about the generic concepts in the Diaporthales including the Gnomoniaceae such that one species may have been placed in several different genera. For example, Ophiognomonia melanostyla, originally described in Sphaeria, was transferred to Cryptoderis Auersw., Gnomonia, Gnomoniella, and Pleuroceras, all before 1899 when it was designated the type species of the genus Ophiognomonia.
The genus Gnomonia includes nearly 280 specific and subspecific names. The type species, Gnomonia gnomon, and G. setacea (Pers.: Fr.) Ces. & De Not. were recently re-described by Sogonov et al. (2005). Species of Gnomonia typically have solitary, thin-walled, immersed perithecia with long necks and lack any stroma. In most species ascospores have one median septum. Species of Gnomonia generally occur on overwintered leaves and are relatively commonly collected in temperate regions. Recent data show that the genus Gnomonia is not monophyletic (Sogonov et al. 2005); some species have been transferred to the Sydowiellaceae (Moročko & Fatehi 2007, Rossman et al. 2007,Rossman et al. 2007).
The genus Apiognomonia has been distinguished from Gnomonia by unequally septate ascospores (Barr 1978, Monod 1983). Most of the 28 species and subspecific names placed in Apiognomonia were originally described in Gnomonia. Results of a molecular study demonstrated that the type species, A. veneta, is closely related but distinct from A. errabunda (Sogonov et al. 2007). Both have a Discula asexual state. In molecular studies A. errabunda has previously grouped with Cryptodiaporthe aesculi and Plagiostoma (Mejia et al. 2008).
Cryptodiaporthe Petr. is based on C. aesculi (Fuckel) Petr. that occurs on branches of Aesculus hippocastanum. Unlike typical members of the Gnomoniaceae, this genus occurs on woody plant parts as do species of Cryptosporella. Both genera were placed in the Valsaceae by Barr (1978) and Monod (1983) based on the presence of stromatic tissues. Castlebury et al. (2002) demonstrated that C. aesculi belongs in the Gnomoniaceae. At present 56 species names have been placed in Cryptodiaporthe. Pathogenic species in Cryptodiaporthe include C. populi (Sacc.) Butin, cause of Cryptodiaporthe canker of poplar, and C. salicella (Fr.) Petr., cause of Cryptodiaporthe canker of willow (Sinclair & Lyon 2005). Cryptodiaporthe corni, cause of golden canker of alternate leaf dogwood, Cornus alternifolia L. f. (Redlin & Rossman 1991) has been excluded from the Gnomoniaceae and shown to belong in the Cryphonectriaceae (Castlebury et al. 2002, Gryzenhout et al. 2006).
The genus Plagiostoma was established for Gnomonia-like fungi having eccentric necks that result in horizontal or oblique ascomata and one-septate ascospores. Barr (1978) included this genus in the Valsaceae based on these characteristics of the ascomata, while Monod (1983) placed Plagiostoma in the Gnomoniaceae. The type species, P. euphorbiae (Fuckel) Fuckel, is known from dead stems of Euphorbia in Europe and has been included in molecular studies (Castlebury et al. 2002). At present about 32 additional species are included in Plagiostoma, most of which occur on overwintered herbaceous and woody plant parts of diverse dicotyledonous plants including hardwood trees.
The genus Cryptosporella based on C. hypodermia (Fr.) Sacc., now includes the genus Ophiovalsa Petr., type species O. suffusa (Fr.) Petr., and occurs exclusively on woody substrates as recently monographed by Mejia et al. (2008). Species of Cryptodiaporthe have traditionally been defined as having one-septate ascospores. At present, Cryptosporella is a distinct genus within the Gnomoniaceae and includes nine species (Mejia et al. 2008). Unlike most other members of the Gnomoniaceae, Cryptosporella is characterised by a distinctly valsoid arrangement of ascomata. However, Cryptosporella is similar to other members of the Gnomoniaceae in having stromatal tissues that are prosenchymatous, forming small ectostromatic discs between the erumpent cluster of necks. This genus is not considered in detail here.
The type species of Ditopella, D. ditopa (Fr.) J. Schröt., is common on woody branches of Alnus. In addition to being morphologically similar to the phragmosporous Phragmoporthe conformis (Berk. & Broome) Petr., Castlebury et al. (2002) showed their close phylogenetic relationship using LSU sequences. Species of Ditopella and Phragmoporthe are morphologically similar to Gnomonia except that their necks are individually surrounded by a rudimentary stroma and thus were placed in the tribe Ditopelleae of the Pseudovalsaceae M.E. Barr (Barr 1978). Thirteen species were described in Ditopella, of which two were excluded from the Diaporthales by Barr (1978). Ditopella is characterised by having one-septate, rarely non-septate ascospores in polysporous asci, while Phragmopothe differs from Ditopella by ascosporses having more than one septum in eight-spored asci. In addition to the type, two other species are placed in Phragmoporthe, P. ploettneriana (Henn.) Petr. and P. pseudotsugae A. Funk. Two species placed in Phragmoporthe by Monod (1983) belong in Magnaporthe outside the Diaporthales (Kraus & Webster 1972, Barr 1978).
The genus Gnomoniella was established for Gnomonia-like species having non-septate ascospores. The type species, G. tubaeformis (Fr.) Sacc., occurs on overwintered leaves and petioles of Alnus in Europe and North America (Barr 1978). Gnomoniella fraxini was recognised as a member of the Gnomoniaceae by Castlebury et al. (2002). At present 85 species and subspecific names are included in Gnomoniella, most of which are poorly known.
Gnomoniopsis was originally described as a subgenus within Gnomonia for species having ascospores that develop additional septa. The type species is Gnomoniopsis chamaemori (Fr.) Berl. Barr (1978) suggested that the development of additional septa was “of only occasional occurrence” and thus considered Gnomoniopsis to be a synonym of Gnomonia. The only other species in Gnomoniopsis, G. devexa (Desm.) Moesz & Smarods, was recognised as Plagiostoma devexum (Desm.) Fuckel by Barr 1978.
The genus Ophiognomonia was based on Gnomoniella subgenus Ophiognomonia Sacc. for species having elongate, often septate ascospores. The type species, O. melanostyla (DC.: Fr.) Sacc., occurs on overwintered leaves and petioles of Tilia spp. in temperate regions. About 15 additional species are currently included in this genus but most of these are obscure. Two of these species are known as endophytes of woody plants, O. cryptica D. Wilson & M.E. Barr isolated from leaves of Quercus emoryi (Wilson et al. 1997) and O. elasticae (Koord.) M. Monod on Ficus (Paulus et al. 2007). Although O. cryptica is a dominant endophyte with interesting ecological implications, no living isolates of this species have been preserved (Wilson et al. 1997).
With collection and culturing of fresh specimens it has become possible to re-evaluate the generic concepts in the Gnomoniaceae by analyzing the phylogenetic relationship of many species using multiple genes. Phylogenetic affinities of uncultureable species can be determined by sequencing multicopy genes and analyzing these sequences in relation to phylogenetically circumscribed genera. This study was undertaken to accurately define the leaf-inhabiting genera of the Gnomoniaceae including the type and additional species of as many genera as possible. In the course of this project many new species were collected and are described herein.
MATERIALS AND METHODS
Collection and observation of herbarium specimens
Fresh specimens were collected by the first author in Canada (British Columbia, Ontario), Russia (Novgorod, Nizhniy Novgorod, Tver oblasts), Switzerland, and the United States (District of Columbia, Georgia, Hawaii, Louisiana, Maine, Maryland, Mississippi, New Jersey, New York, North Carolina, Pennsylvania, Tennessee, Virginia, Washington) in 2004–2007. Living and dead, attached or fallen, overwintered leaves, and overwintered dead parts of herbaceous plants were examined for the presence of ascomata or conidiomata. Those containing seemingly gnomoniaceous fungi were air dried and stored in paper bags or envelopes. Additional fresh material was collected by others and sent for use in this study from Austria, Bulgaria, Finland, Lithuania, Russia (Primorsky Kray), and the United Kingdom (Scotland). All specimens were deposited in the U.S. National Fungus Collections (BPI).
Additional herbarium specimens were examined from the U.S. National Fungus Collections (BPI) as well as the Museum Botanicum Berolinense (B), Centraalbureau voor Schimmelcultures (CBS), Farlow Reference Library and Herbarium of Cryptogamic Botany in Harvard University (FH), Conservatoire et Jardin botaniques de la Ville de Genève (G), Royal Botanic Gardens at Kew (K), Leiden University branch of the Nationaal Herbarium Nederland (L), Musée et Jardins Botanique Cantonaux in Lausanne (LAU), Botanische Staatssammlung München (M), New York State Museum Mycological Collections Herbarium (NYS), Muséum National d'Histoire Naturelle (PC), Mycology Herbarium of Royal Ontario Museum (TRTC), Uppsala University (UPS), and Eidgenössische Technische Hochschule in Zürich (ZT).
Fresh and herbarium specimens were first examined on natural substrates using a Wild M5A (Wild Heerbrugg Ltd., Heerbrugg, Switzerland) or Leica MZ APO (Leica Microsystems AG, Weitzlar, Germany) dissecting microscope and photographed with a DXM 1200 digital camera (Nikon Instruments Inc., Melville, NY, U.S.A.). Perithecia and pycnidia-like conidiomata were extracted from leaf tissue using a sterile surgical scalpel under a dissecting microscope, placed into a drop of 3 % aqueous KOH, 7 % aqueous sodium acetate solution or water on a clean microscope slide. After rehydration, perithecia were examined and measured. Perithecia and pycnidia-like conidiomata were crushed to release their contents, which were transferred with an attenuated glass capillary, a scalpel or a micropipette to a clean area of the slide. For acervular conidiomata, a small part of the conidial mass with the underlying hyphal mat intermixed with leaf tissue was extracted to a slide. The material was covered with a cover slip and examined under Nomarski differential interference contrast (DIC) with an Axioplan2 microscope (Carl Zeiss, New York, NY, U.S.A.) and photographed.
Culture preparation and morphology
For preparation of pure cultures, fresh material was rehydrated and crushed in sterile 7 % sodium acetate solution or water. Ascospores and asci or conidia were removed by means of an attenuated glass capillary or a micropipette and transferred to cornmeal agar (CMA, Sigma®, Sigma Chemical Co., St. Louis, MO, U.S.A.) plates containing 1 % (v/v) of an antibiotics solution (0.2 % streptomycin sulfate and 0.2 % neomycin sulfate in sterile distilled water). Plates were incubated at room temperature and periodically examined for germination of ascospores or conidia with a dissecting microscope in transmitted light or the Axioplan2 microscope with low-magnification (×2.5–20) objectives. Germinated ascospores or conidia were transferred to fresh CMA or potato dextrose agar (PDA, Difco™, Becton, Dickinson & Co., Sparks, MD, U.S.A.) and incubated at room temperature. Most cultures obtained in this study were deposited at the Centraalbureau voor Schimmelcultures (CBS, Utrecht, The Netherlands). For macroscopic descriptions of colonies, strains were grown on PDA, malt extract agar (MEA) containing 3 % malt extract (Bacto™) and 1.5 % agar (Bacto™), and malt yeast agar (MYA) containing MEA supplemented with 0.3 % yeast extract (Bacto™). Cultures were placed in an incubator with a 12 h light/dark cycle with blacklight (near UV) and cool white fluorescent light at 23 °C presented as (23 °C l/d) in the descriptions. In order to stimulate sporulation and/or perithecial formation by imitating natural conditions, some cultures were incubated on the same media as follows: 4 h blacklight/white fluorescent light at 2 °C, 10 h same light at 10 °C, 1 h darkness at 10 °C, and 9 h darkness at 2 °C. This cycle is presented as 2/10 °C l/d in the descriptions. Cultures were observed for up to five mo. Colours were determined according to Kornerup & Wanscher (1978) with only colour names used herein.
Measurements and data management
Measurements in descriptions are given as minimum and maximum values in parentheses and ranges as intervals between the first and third quartile. Arithmetic means, standard deviations and number of measurements are given in parentheses. Thus, measurements are provided as length × width = (min–)Q1–Q3(–max) × (min–)Q1–Q3(–max) μm (mean1 × mean2, SD1, SD2, n). Measurement of microstructures are rounded to the nearest 0.5 μm. Images were processed with Adobe Photoshop 5.0 (Adobe Systems, Inc., San Jose, CA, U.S.A.). Original software (Sogonov 2005) built on MS Access 2000 (Microsoft Corporation, Bellevue, WA, U.S.A.) was used for collecting and storing data and images of the samples and for statistical evaluations.
DNA amplification and sequencing
Genomic DNA was extracted directly from actively growing surface mycelium scraped from PDA plates with the PUREGENE Cell and Tissue kit (Gentra Systems, Minneapolis, MN, U.S.A.) according to the manufacturer's instructions using approximately 50 mg fresh mycelium. For some collections, ribosomal genes were amplified directly from perithecial or conidiomatal contents in one of two ways. A small amount of ascal or conidial masses was extracted from a perithecium or conidioma with a sterile scalpel under the dissecting microscope and placed on the inner sidewall of a 0.2 mL PCR tube cap. Approximately 5 μL of PCR-grade water were added to the mass of spores with a micropipette. Alternatively, a perithecium or conidioma was placed in a drop of PCR-grade water on a fresh microscope slide and squeezed using a scalpel. Then approximately 5 μL of the water containing a cloud of asci or conidia was transferred either with a micropipette to the inner sidewall of a 0.2 mL PCR tube as above. PCR tubes containing spore suspensions were stored at -18 °C until amplification. The spore suspension was then spun to the bottom of the tube in a microcentrifuge (∼30 s) after the PCR mix had been added to the tube. Before amplification, the spore suspensions were incubated for 5 min at 95 °C.
The genes coding for the internal transcribed spacer regions 1 and 2, including the 5.8S rDNA (ITS) and a region of the large ribosomal subunit (nrLSU), a fragment of the translation elongation factor 1-alpha (tef1-α) containing introns 4 and 5,and RNA polymerase II (rpb2) were amplified in 25 or 50 μL reactions on a GeneAmp 9700 thermal cycler (Applied Biosystems, Foster City, CA, U.S.A.) under the following conditions: 0.2–0.3 ng/μL of genomic DNA, 4 mM/μL each dNTP, 0.05 units/μL DNA polymerase (AmpliTaq®, Applied Biosystems, Foster City, CA, U.S.A. or GeneChoice®, Cat. No. T-12, GeneChoice, Inc., Frederick, MD, U.S.A.), 0.5 pmoles/μL each primer and 10 % vol. of the manufacturer's supplied 10× PCR buffer containing 15 mM MgCl2. The thermal cycler program was as follows: 2 min at 95 °C followed by 35 cycles of 30 s at 94 °C, 30 s at 55 °C, 1 min at 72 °C, with a final extension period of 10 min at 72 °C. If no amplicon was obtained from a reaction under these conditions, the annealing temperature was decreased to 50 or 52 °C and/or 4 % of DMSO (v/v) was added to the reaction mix. Following amplification, the PCR products were purified with ExoSAP-IT (USB Corporation, Cleveland, OH, U.S.A.) according to the manufacturer's instructions. Internal transcribed spacer regions 1 and 2, including the 5.8S rDNA, were amplified and sequenced using the primers ITS5 and ITS4 (White et al. 1990). A region of the tef1-α gene was amplified using primers EF1–728F designed by Carbone & Kohn (1999) and EF1–1567R designed by Rehner (2001). The tef1-α fragment was sequenced using primers EF1–983F and EF1–1567R (Rehner 2001).
Phylogenetic analyses
Sequences were edited using Sequencher v. 4.2 for Windows (Gene Codes Corporation, Ann Arbor, MI, U.S.A.). Alignments were manually adjusted using BioEdit v. 7.0.5.2 (Hall, http://www.mbio.ncsu.edu/BioEdit/) or JalView (Clamp et al. 2004). Sequences were deposited in GenBank and listed in Table 1 or as specimens sequenced for those not used in the phylogenetic analysis.
Table 1.
Specimens and cultures of Gnomoniaceae sequenced for this study.*
|
|
|
|
|
|
|
GenBank Accession Numbers
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Taxon | Specimen | Culture | Country | Host | Collector | tef1-α | ITS | nrLSU | rpb2 |
| Ambarignomonia petiolorum | BPI 844274 | CBS 121227 | U.S.A. : VA | Liquidambar styraciflua | M.V. Sogonov | EU221898 | EU254748 | EU255070 | EU219307 |
| Amphiporthe hranicensis | BPI 843515 | CBS 119289 | Austria | Tilia platyphylla | W. Jaklitsch | EU221890 | EU199178 | EU199122 | EU199137 |
| Apiognomonia borealis | NA | CBS 799.79 | Norway | Geranium sylvaticum | M. Monod | EU221971 | EU255000 | EU255169 | EU219275 |
| Apiognomonia errabunda | NA | CBS 109747 | Switzerland | Fagus sylvatica | M. Monod | EU221914 | DQ313525 | AF408334 | EU219261 |
| Apiognomonia hystrix | CBSH 11343 | CBS 911.79 | Switzerland | Acer pseudoplatanus | M. Monod | EU221986 | DQ313549 | EU255180 | EU219260 |
| Apiognomonia veneta | NA | CBS 897.79 | Switzerland | Platanus orientalis | M. Monod | EU221910 | DQ313532 | EU255195 | EU219259 |
| “Apioplagiostoma” aceriferum | NA | CBS 778.79 | Switzerland | Acer campestre | M. Monod | EU221970 | EU254750 | EU255072 | EU219316 |
| Cryphonectria cubensis | BPI 841768 | CBS 101281 | Cameroon | Eucalyptus urophylla | I. Gibson | EU222012 | NS | AF408338 | DQ862016 |
| Cryphonectria nitschkei | BPI 747935 | CBS 109758 | Russia | Quercus mongolica | L. Vasilyeva | DQ862031 | NS | AF408335 | DQ862015 |
| Cryphonectria parasitica | NA | ATCC 38755 | U.S.A.: CT | Castanea dentata | N. DePalma | EU222014 | NS | EU199123 | DQ862017 |
| Cryptosporella alnicola | NA | CBS 121074 | U.S.A.: MN | Corylus cornuta | L. Vasilyeva | EU221960 | EU199204 | EU255076 | EU199160 |
| Cryptosporella betulae | BPI 748448 | CBS 109763 | Austria | Betula alba | W. Jaklitsch | EU221884 | EU199180 | AF408375 | EU199139 |
| Cryptosporella confusa | BPI 843580 | CBS 121063 | U.S.A.: TN | Betula papyrifera | W. Jaklitsch | EU221958 | EU199219 | EU255079 | EU199175 |
| Cryptosporella femoralis | BPI 872326 | CBS 121076 | U.S.A.: NY | Alnus rugosa | L. Vasilyeva | EU221951 | EU199220 | EU255080 | EU199176 |
| Cryptosporella hypodermia | NA | CBS 171.69 | The Netherlands | Ulmus campestris | H.A. van der Aa | EU221881 | EU199225 | DQ862028 | DQ862018 |
| Cryptosporella suffusa | BPI 871231 | CBS 121077 | Austria | Alnus incana | W. Jaklitsch | EU221891 | EU199184 | EU199124 | EU199142 |
| Cryptosporella wehmeyeriana | BPI 843485 | CBS 121085 | U.S.A.: NC | Tilia sp. | L. Vasilyeva | EU221959 | EU199205 | EU255082 | EU199161 |
| Diaporthe phaseolorum | NA | ATCC 64802 | U.S.A.: MS | Stokesia laevis | F. Uecker | EU222020 | NS | EU255083 | EU219348 |
| Discula destructiva | BPI 1107757 | CBS 109771 | U.S.A.: WA | Cornus nuttallii | J. Ammirati | EU221897 | EU199186 | AF408359 | EU199144 |
| Ditopella ditopa | BPI 748439 | CBS 109748 | Austria | Alnus glutinosa | W. Jaklitsch | EU221943 | DQ323526 | AF408360 | EU219297 |
| Gnomonia amoena | BPI 877469 | CBS 121262 | U.S.A.: TN | Carpinus caroliniana | M.V. Sogonov | EU221983 | EU254771 | EU255091 | EU219293 |
| Gnomonia gnomon | NA | CBS 199.53 | Italy | Corylus avellana | M. Ribaldi? | EU221885 | AY818956 | AF408361 | EU219295 |
| Gnomonia neognomon | BPI 877526C | CBS 121265 | Canada: BC | Corylus californica | M.V. Sogonov | EU221982 | EU254787 | EU255098 | EU219336 |
| Gnomonia orcispora | BPI 877465C | CBS 121247 | U.S.A.: WA | Corylus californica | M.V. Sogonov | EU221922 | EU254788 | EU255099 | EU219314 |
| Gnomonia pseudoamoena | BPI 877518 | CBS 121261 | Canada: BC | Corylus californica | M.V. Sogonov | EU221984 | EU254795 | EU255102 | EU219305 |
| Gnomonia rodmanii | BPI 878211A | CBS 121909 | U.S.A.: GA | Carpinus caroliniana | M.V. Sogonov | NS | EU254796 | NS | EU219337 |
| Gnomonia skokomishica | BPI 877465B | CBS 121245 | U.S.A.: WA | Corylus californica | M.V. Sogonov | EU221929 | EU254797 | EU255103 | EU219291 |
| Gnomonia virginianae | BPI 844264 | CBS 121913 | U.S.A.: MD | Ostrya virginiana | M.V. Sogonov | EU221900 | EU254801 | EU255105 | EU219309 |
| Gnomoniopsis chamaemori | NA | CBS 803.79 | Finland | Rubus chamaemorus | M. Monod | NS | EU254808 | EU255107 | NS |
| Gnomoniopsis comari | CBSH 12997 | CBS 806.79 | Finland | Comarum palustre | M. Monod | NS | EU254821 | EU255114 | EU219286 |
| Gnomoniopsis fructicola | NA | CBS 208.34 | France | Fragaria sp. | G. Arnaud | EU221968 | EU254826 | EU255116 | EU219284 |
| Gnomoniopsis macounii | BPI 871008 | CBS 121468 | U.S.A.: NY | Spiraea sp. | L. Vasilyeva | EU221979 | EU254762 | EU255087 | EU219243 |
| Gnomoniopsis paraclavulata | BPI 877448 | CBS 121263 | U.S.A.: TN | Quercus alba | M.V. Sogonov | EU221939 | EU254839 | EU255120 | EU219248 |
| Gnomoniopsis racemula | BPI 871003 | CBS 121469 | U.S.A.: MN | Chamerion angustifolium | L. Vasilyeva | EU221889 | EU254841 | EU255122 | EU219241 |
| Gnomoniopsis tormentillae | NA | CBS 904.79 | Switzerland | Potentilla erecta | M. Monod | NS | EU254856 | EU255133 | NS |
| Leucostoma niveum | BPI 748232 | CBS 109489 | Russia | Populus sp. | L. Vasilyeva | EU222015 | NS | AF362558 | EU219343 |
| Mazzantia napelli | BPI 748443 | CBS 109769 | Austria | Aconitum vulparia | W. Jaklitsch | EU222017 | NS | AF408368 | NS |
| Melanconis alni | BPI 748444 | CBS 109773 | Austria | Alnus viridis | W. Jaklitsch | EU221896 | DQ323523 | AF408371 | EU219300 |
| Melanconis marginalis | BPI 748446 | CBS 109744 | Canada: BC | Alnus rubra | M.E. Barr | EU221991 | EU199197 | AF408373 | EU219301 |
| Melanconis stilbostoma | BPI 748447 | CBS 109778 | Austria | Betula alba | W. Jaklitsch | EU221886 | DQ323524 | AF408374 | EU219299 |
| Ophiognomonia alni-viridis | NA | CBS 782.79 | Switzerland | Alnus viridis | M. Monod | EU221974 | EU254864 | EU255138 | EU219333 |
| Ophiognomonia balsamiferae | BPI 877606 | CBS 121266 | Canada:BC | Populus balsamifera | M.V. Sogonov | EU221955 | EU254870 | EU255140 | EU219322 |
| Ophiognomonia intermedia | NA | CBS 119194 | United Kingdom | Betula pubescens | S. Green | EU222008 | EU254873 | DQ323520 | EU219321 |
| Ophiognomonia ischnostyla | NA | CBS 837.79 | Switzerland | Corylus avellana | M. Monod | EU221972 | EU254890 | EU255142 | EU219334 |
| Ophiognomonia leptostyla | NA | CBS 844.79 | Switzerland | Juglans regia | M. Monod | EU221996 | EU254910 | EU255149 | EU219338 |
| Ophiognomonia micromegala | BPI 877615A | CBS 121910 | U.S.A.: DC | Carya tomentosa | M.V. Sogonov | EU221944 | EU254918 | EU255150 | EU219332 |
| Ophiognomonia nana | NA | CBS 883.79 | Finland | Betula nana | M. Monod | EU221949 | DQ323534 | DQ323522 | EU219326 |
| Ophiognomonia nervisequa | BPI 877467B | CBS 121908 | U.S.A.: NC | Carpinus americana | M.V. Sogonov | EU221930 | EU254902 | EU255147 | EU219330 |
| Ophiognomonia padicola | NA | CBS 845.79 | Switzerland | Prunus padus | M. Monod | EU221946 | EU199192 | EU255152 | EU199150 |
| Ophiognomonia pseudoclavulata | BPI 844280 | CBS 121236 | U.S.A.: PA | Carya tomentosa | M.V. Sogonov | EU222004 | EU254923 | EU255153 | EU219317 |
| Ophiognomonia rosae | BPI 877636 | CBS 121267 | U.S.A.: ME | Rosa sp. | M.V. Sogonov | EU221956 | EU254936 | EU255158 | EU219319 |
| Ophiognomonia sassafras | BPI 877639 | CBS 121243 | U.S.A.: PA | Sassafras albidum | M.V. Sogonov | EU221941 | EU254941 | EU255159 | EU219327 |
| Ophiognomonia setacea | BPI 843499 | CBS 116850 | U.S.A.: TN | Quercus sp. | L. Vasilyeva | EU222007 | AY818953 | AY818959 | EU219339 |
| Ophiognomonia vasiljevae | BPI 877671 | CBS 121253 | U.S.A.: TN | Juglans nigra | M.V. Sogonov | EU221999 | EU254977 | EU255162 | EU219331 |
| Phragmoporthe conformis | BPI 748450 | CBS 109783 | Canada: BC | Alnus rubra | M.E. Barr | EU221993 | DQ323527 | AF408377 | NS |
| Plagiostoma aesculi | BPI 748430 | CBS 109765 | Austria | Aesculus hippocastanum | W. Jaklitsch | EU221913 | EU199179 | AF408342 | EU199138 |
| Plagiostoma amygdalinae | NA | CBS 791.79 | Switzerland | Euphorbia amygdaloides | M. Monod | NS | EU254995 | EU255165 | NS |
| Plagiostoma apiculatum | BPI 843527 | CBS 121466 | Austria | Salix alba | W. Jaklitsch | EU221957 | EU254996 | EU255166 | EU219278 |
| Plagiostoma barriae | BPI 877717B | CBS 121249 | U.S.A.: WA | Acer macrophyllum | M.V. Sogonov | EU221947 | EU254997 | EU255167 | EU219270 |
| Plagiostoma devexum | BPI 843489 | CBS 123201 | U.S.A.: NY | Polygonum sp. | L. Vasilyeva | EU221933 | EU255001 | EU255170 | EU219258 |
| Plagiostoma euphorbiae | NA | CBS 340.78 | The Netherlands | Euphorbia palustris | W. Gams | EU219234 | EU199198 | AF408382 | EU219292 |
| Plagiostoma fraxini | BPI 746412 | CBS 109498 | U.S.A.: MD | Fraxinus pennsylvanica | S. Redlin | EU221987 | AY455810 | AF362552 | EU219263 |
| Plagiostoma geranii | NA | CBS 824.79 | Switzerland | Geranium sylvaticum | M. Monod | NS | EU255009 | NS | EU219273 |
| “Plagiostoma” inclinatum | NA | CBS 772.79 | Switzerland | Acer campestre | M. Monod | NS | EU255034 | EU255183 | EU219315 |
| Plagiostoma petiolophilum | BPI 863769 | AR 3821 | U.S.A.: NY | Acer sp. | L. Vasilyeva | EU221988 | EU255039 | EU255185 | EU219257 |
| Plagiostoma rhododendri | NA | CBS 847.79 | Switzerland | Rhododendron hirsutum | M. Monod | NS | EU255044 | EU255187 | EU219272 |
| Plagiostoma robergeana | BPI 843593 | CBS 121472 | Austria | Staphylea pinnata | W. Jaklitsch | EU221908 | EU255046 | EU255188 | EU219262 |
| Plagiostoma salicellum | BPI 747938 | CBS 109775 | Austria | Salix sp. | W. Jaklitsch | EU221916 | DQ323529 | AF408345 | EU199141 |
| Pleuroceras oregonense | BPI 877719 | CBS 121260 | Canada: BC | Salix sitchensis | M.V. Sogonov | EU221931 | EU255060 | EU255196 | EU219313 |
| Pleuroceras pleurostylum | NA | CBS 906.79 | Switzerland | Salix helvetica | M. Monod | EU221962 | EU255061 | EU255197 | EU219311 |
| Pleuroceras tenellum | BPI 871059 | CBS 121082 | U.S.A.: NC | Acer rubrum | M.V. Sogonov | EU221907 | EU199199 | EU255202 | EU199155 |
| Sirococcus clavigignentijuglandacearum | NA | CBS 121081 | U.S.A.: MN | Juglans cinerea | M. Ostry | EU221998 | EU199200 | EU199133 | EU199156 |
| Sirococcus conigenus | BPI 871248 | CBS 101225 | Austria | Picea abies | R. Schneider | EU221927 | EU199201 | EU199134 | EU199157 |
| Valsa ceratosperma | BPI 748459 | CBS 109777 | Austria | Quercus robur | W. Jaklitsch | EU222016 | NS | EU255209 | EU219344 |
| Valsella salicis | BPI 748461 | CBS 109754 | Austria | Salix fragilis | W. Jaklitsch | EU222018 | NS | EU255210 | AF408389 |
ATCC = American Type Culture Collection, Manassas, VA U.S.A.; BPI = U.S. National Fungus Collections, USDA ARS, Beltsville, MD U.S.A.; CBS = Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; NA = None available; NS = Not Sequenced.
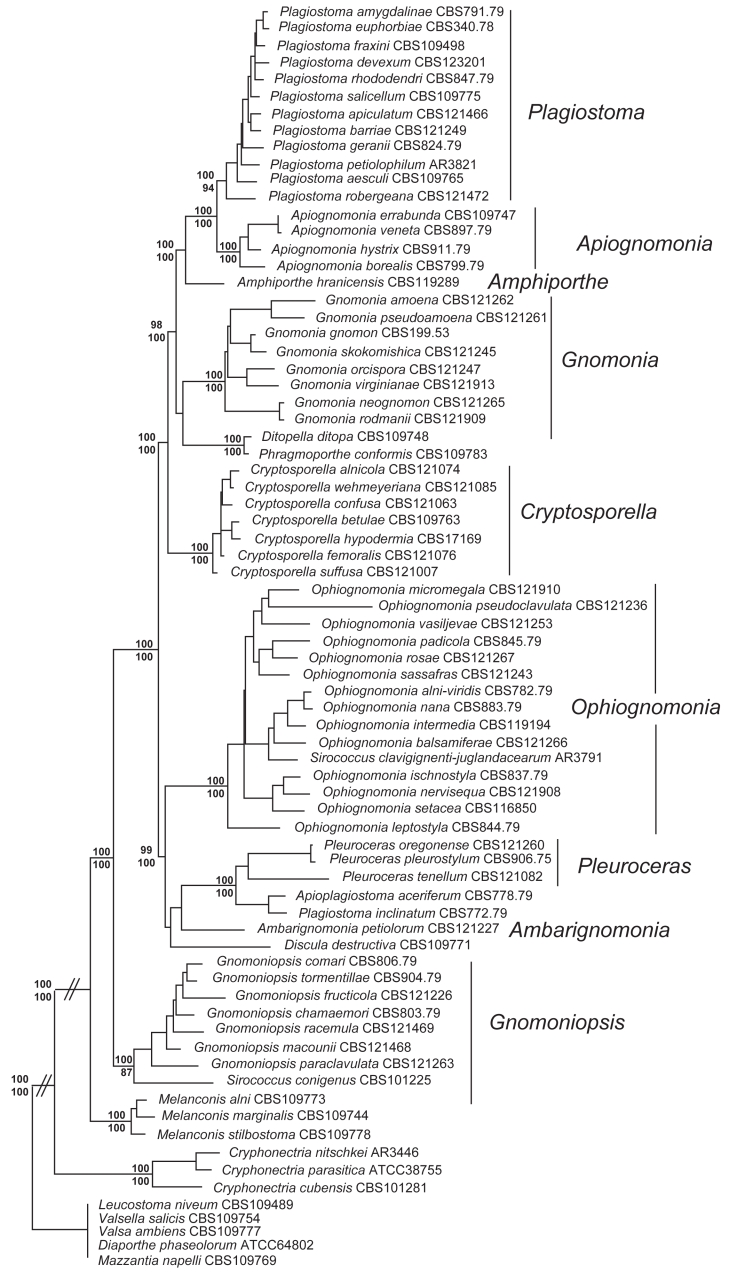
Genes were aligned individually and concatenated in a text editor. The alignment consisted of nrLSU (791 bp), tef1-α (470 bp), and rpb2 (1089 bp) sequences for a total of 2350 and 75 taxa. Of these, 64 belonged to the Gnomoniaceae, three to the Melanconidaceae, and eight to other representatives of the Diaporthales. The alignment was partitioned by gene and by codon position for tef1-α and rpb2. Partitions were analysed for conflict using the 70 % reciprocal NJ bootstrap analysis as in Reeb et al. (2004) using distance settings identified by ModelTest (Posada & Crandall 1998) for the maximum likelihood analysis detailed below. Trees were inferred by maximum parsimony (MP) using the heuristic search option with random sequence addition (1000 replications), MULTREES on and the branch swapping (tree bisection-reconnection) option of PAUP v. 4.0b10 (Swofford 2002). All characters were unordered and either given equal weight during the analysis or weighted according to a scheme of weight=3 for first and second codon positions, weight=1 for third codon positions and weight=2 for nrLSU. Gaps were treated as missing in the parsimony analysis. Relative support of branches was estimated with 1000 bootstrap replications (Felsenstein 1985) with MULTREES and TBR on and 10 random sequence additions for the MP bootstraps. Bootstrap values are indicated on Fig.1 under the respective branches.
Fig. 1.
ML phylogenetic analysis (ML score = -lnL 26702.18832) of sequences for the tef1-α, nrLSU and rpb2 multigene analysis of genera in the Gnomoniaceae for 64 gnomoniaceous taxa and 11 outgroup diaporthalean taxa. Bayesian posterior probabilities greater than 95 % are shown above each branch. MP bootstrap values greater than 70 % are shown below each branch.
Trees were also inferred using maximum likelihood as implemented in PAUP v. 4.0b10. ModelTest v. 3.7 (Posada & Crandall 1998) was used to determine the model used for the analysis. Likelihood settings were as follows: base=(0.2419 0.2900 0.2534), nst=6, rmat=(1.0000 3.8112 1.0000 1.0000 7.4314), rates=gamma, shape=0.7954 pinvar=0.5555. A heuristic search was performed with 10 random addition sequences using the MP tree as the starting tree. Maximum likelihood bootstrap analysis was not performed.
MrModeltest (v. 2.2) was used to estimate the model that best fit the data for the alignment. Each gene was analysed individually and the entire alignment was analysed unpartitioned. All analyses resulted in the same model. A Bayesian analysis using the resulting GTR+I+G model was applied to the three partitions (genes) was conducted. Three hot and one cold chain with Markov Chain Monte Carlo 2 million generations in MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001) was used for the analyses. Trees were sampled every 100 generations and the first 1 000 000 generations were eliminated (burn in period) after comparison in Excel when determining posterior probabilities (PP) for clades. Two separate runs were performed and posterior probabilities were pooled and indicated on Fig. 1 above the branches. Only probabilities greater than 95 % are shown.
In this study, 322 ITS sequences were obtained from specimens or cultures and deposited in GenBank as EU254748—EU255069 with host information, voucher specimen/culture and locality data. The ITS sequences could not be aligned across the whole family and were therefore not used in multigene phylogenetic analysis. However, ITS sequences were used to place taxa within genera through the use of a local BLAST server in BioEdit. The BLAST database contained 426 ITS sequences from taxa within the Gnomoniaceae and included previously sequenced isolates from GenBank. Taxa used in the multigene phylogenetic analysis were used as reference taxa for this database in determining generic placements. All genera are represented by their respective type species in the multigene analysis.
RESULTS AND DISCUSSION
Phylogenetic analyses
Results of the 70 % reciprocal NJ bootstrap analyses showed no conflict among the genes (trees not shown). However, tef1-α and nrLSU individually did not resolve all of the genera identified in the multigene analysis. Combined analysis of the tef1-α and nrLSU partitions did resolve all genera with >70 % support. The rpb2 individual NJ bootstrap analysis resolved all genera with >70 % support indicating that rpb2 is providing most of the signal for resolution of the genera.
Maximum parsimony analyses resulted in 24 equally parsimonious trees (score= 5095) and six equally parsimonious trees (score= 7047) for the unweighted and weighted analyses, respectively. Strict consensus trees calculated for each parsimony analysis did not differ in the identification of the clades at the genus level, with minor backbone differences (trees not shown). Maximum likelihood analysis resulted in one tree with a –lnL score of 26702.18332 (Fig.1) with Bayesian PP and MP bootstraps shown above and below the branches Seven genera with multiple species were strongly supported by the multi-gene phylogenetic analysis at a Bayesian PP level of 95 % or greater and MP bootstrap support of 70 % or greater: Apiognomonia, Cryptosporella, Gnomoniopsis, Gnomonia, Ophiognomonia, Plagiostoma, and Pleuroceras. Ambarignomonia is newly recognised with a single species, A. petiolorum, and Amphiporthe is recognised with the type species, A. hranicensis, in the Gnomoniaceae. The type species of Ditopella and Phragmoporthe are morphologically and biologically similar differing primarily in the number of ascospores in the asci and appear to be congeneric in this analysis, but are not further considered due to the small number of isolates sampled.
Bayesian analyis and MP boostrapping both supported a group containing Amphiporthe, Apiognomonia, Cryptosporella, Ditopella/Phragmoporthe, and Plagistoma, with Ambarignomonia, Ophiognomonia, and Pleuroceras forming a closely related second group of genera. The type species of Ophiognomonia, O. melanostyla, could not be sequenced for the multigene analysis. However, an ITS sequence was obtained directly from a specimen and was found by BLAST analysis to be very closely related to O. sassafras, which has been used as the reference taxon for Ophiognomonia in the multigene analysis. All other genera are represented by type species in the multigene analysis. In all analyses Gnomoniopsis is basal to other gnomoniaceaous taxa in this alignment with the Melanconidaceae forming a very closely related sister group to the Gmononiaceae. Sirococcus clavigignenti-juglandacearum, the butternut canker pathogen with no known sexual state, is strongly supported as within Ophiognomonia. However, Sirococcus conigenus, the type species of the anamorph genus Sirococcus, is placed with Gnomoniopsis in these analyses. The dogwood anthracnose pathogen, Discula destructiva, is not strongly supported as belonging to any of the genera present in this tree, but it forms a consistent relationship with Ambarignomonia and Pleuroceras. ITS data suggest a close relationship with Pleuroceras (trees not shown).
Revised concepts of accepted genera
Based on the molecular data presented here the previously established concepts for many of the genera in the Gnomoniaceae must be rejected. These were based primarily on characteristics of stromal development, perithecial neck orientation, and ascospore septation (Barr 1978, Monod 1983). The new concepts of the genera in the Gnomoniaceae presented here cannot be defined based on a single morphological characteristic; however, some generalisations can be made about the characteristics of each genus as presented in Table 2.
Table 2.
Characteristics of genera in the Gnomoniaceae.
| Gnomonia | Ambarignomonia | Apiognomonia | Cryptosporella | Gnomoniopsis | Ophiognomonia | Plagiostoma | |
|---|---|---|---|---|---|---|---|
| Habit of perithecia | Single on leaves of trees and shrubs. | Single on leaves of trees and shrubs. | Single on leaves of trees and shrubs and on herbaceous plants. In groups on twigs. | In groups on twigs. | Single on leaves of trees and shrubs. Single or in groups on herbaceous plants or on twigs. | Single on leaves of trees and shrubs and on herbaceous plants. | Single on leaves of trees and shrubs and on herbaceous plants. In groups on twigs. |
| Stroma | Without stroma. Some species with collar around neck. | Without stroma. With collar around neck. | Without stroma or with weak stroma if on twigs. | With weak stroma. | Without stroma. | Without stroma. | Without stroma or with weak stroma if on twigs. |
|
Perithecia
|
Erumpent, concave when dry; or remaining immersed but then with very short
necks or with collar around neck.
|
Perithecia remaining immersed, convex when dry.
|
|||||
| Two species may have some irregularly shrunk or concave perithecia when dry, partly erumpent. | |||||||
| Ascospores | One median or supramedian septum, rarely non-septate; ellipsoidal to fusiform or acerose, appendages short or long. | One median septum, fusiform, appendages medium. | One septum, variable from submedian, median to supramedian, ellipsoidal, appendages absent or present. | Usually non-septate, rarely with one median septum, ellipsoidal, fusiform, femoroid to vermiculate. | One submedian or median septum, ellipsoidal, slightly broader in their upper part with no apendages. | One median septum, rarely submedian, supramedian in filiform ascospores, or absent, ellipsoidal or fusiform (acerose), rarely filiform, appendages short or long but not stout. | One median septum, rarely submedian or absent, ellipsoidal, appendages absent or present. |
| Colony growth rate | Slow—moderate. | Slow. | Fast. | Slow—moderate. | Moderate—fast. | Moderate—fast. | Fast. |
| Conidiomata formation in culture | Rarely. | Never. | Often, sometimes abundant. | In some species none, in some abundant. | Usually abundant. | Rarely. | Often, sometimes abundant. |
| Host | Strictly family Betulaceae, mostly subfamily Coryloideae. | Known only from Liquidambar styraciflua (Hamamelidaceae). | Diverse taxonomic groups (mostly Aceraceae, Fagaceae, Geraniaceae, Platanaceae, occasionally Anacardiaceae, Hippocastanaceae, Juglandaceae, Onagraceae, Rosaceae, Tiliaceae). | Betulaceae, Tiliaceae, Ulmaceae. | Diverse taxonomic groups (Ericaceae, Fagaceae, Rosaceae, Tiliaceae). | Mostly Fagales (Betulaceae, Fagaceae, Juglandaceae), a few species on Lauraceae, Rosaceae, Salicaceae, Tiliaceae. | Diverse taxonomic groups (Aceraceae, Euphorbiaceae, Geraniaceae, Hippocastanaceae, Oleaceae, Polygonaceae, Salicaceae, Staphyleaceae). |
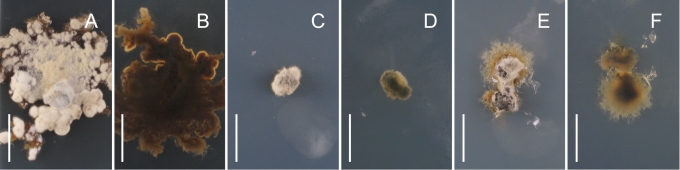
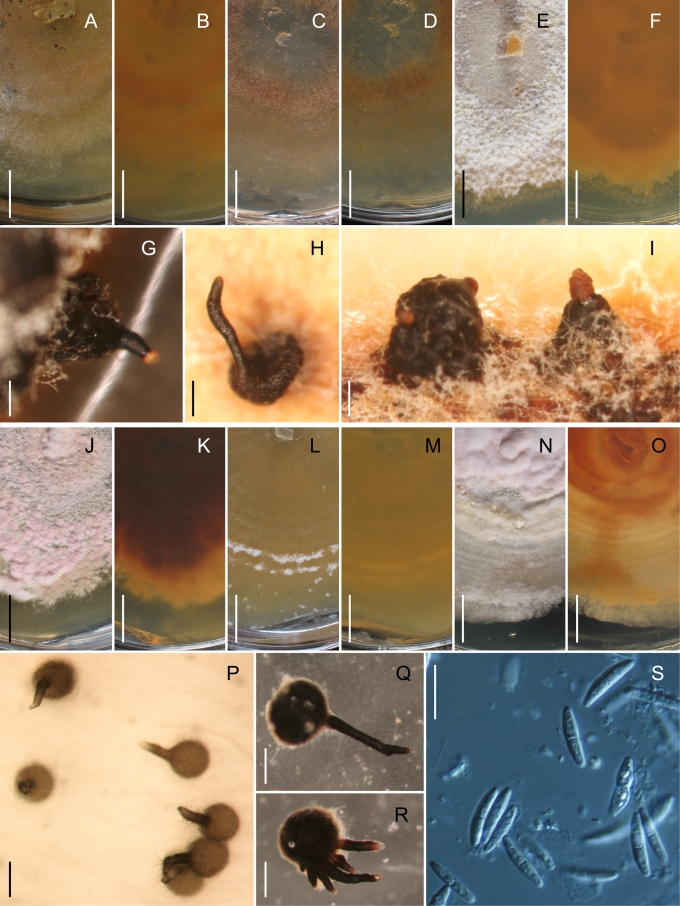
This study presents a revised concept of the genus Gnomonia. It includes relatively few species, some of which are newly described, that group with the type species G. gnomon in the multigene phylogeny (Fig. 1) or that ITS sequences show to be congeneric with G. gnomon. Gnomonina alnea, the type of the genus Gnomonina, is placed with Gnomonia based on ITS data and therefore Gnomonina is considered a synonym of Gnomonia. This revised concept of Gnomonia correlates with a number of morphological and host characteristics. All species occur on decaying leaves of woody trees and shrubs. The perithecia lack a stroma. Unlike most other species in the Gnomoniaceae except for a few species in Ophiognomonia that become partially erumpent, those species of Gnomonia without a neck become erumpent. If remaining immersed, the perithecia of species of Gnomonia have a short neck and lack a collar. A few species of Gnomonia have a collar, specifically G. amoena and G. pseudoamoena. The perithecia become concave or collapse from the top when dry, as illustrated in Figs 2, 5, 9, and 12, unlike other genera in the Gnomoniaceae that collapse from the base. The ascospores are ellipsoidal to fusiform, rarely acerose, bicellular with a median, occasionally supra-median, septum, or rarely non-septate, with short to long appendages. The colonies in culture grow at a slow to moderate rate and rarely form conidiomata in culture. Similar to the stromatic Cryptosporella, the genus Gnomonia occurs primarily on members of the Betulaceae.
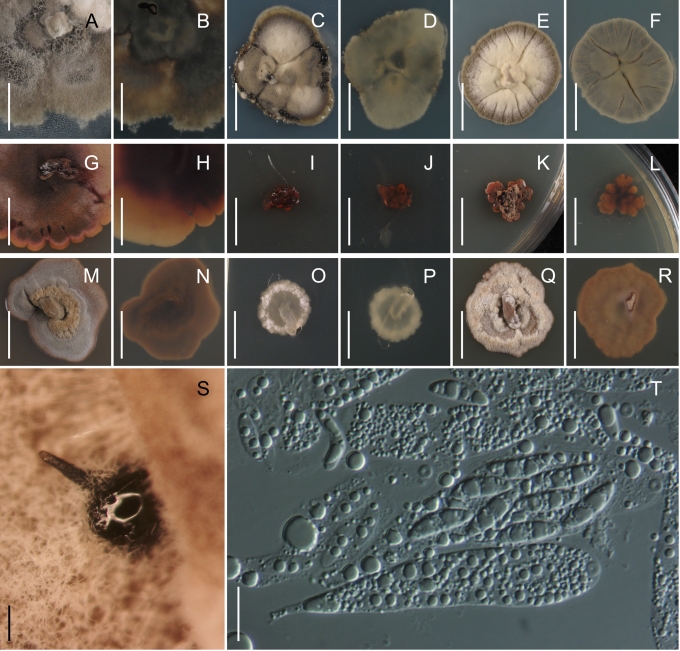
Fig. 2.
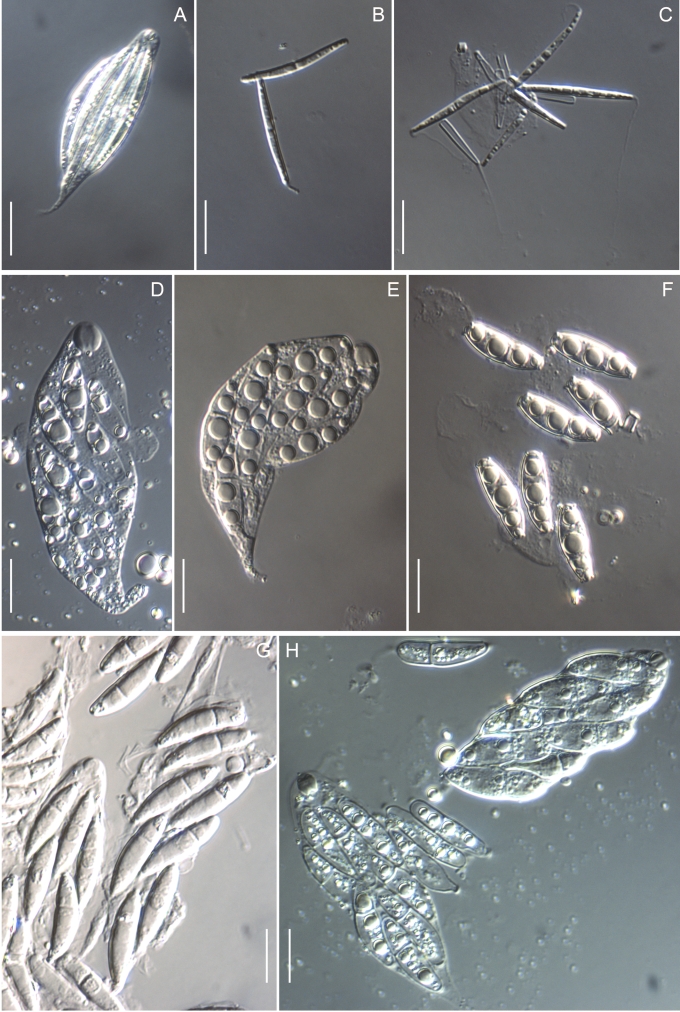
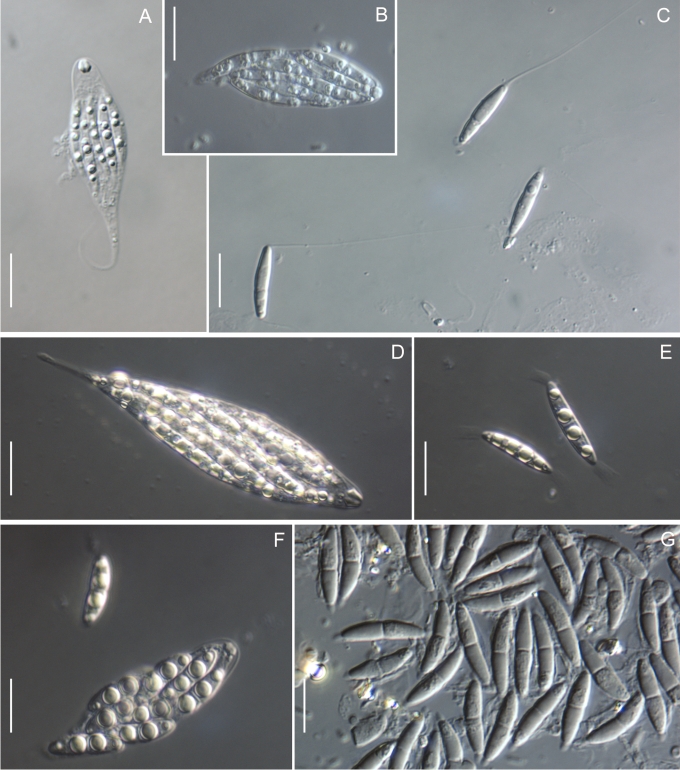
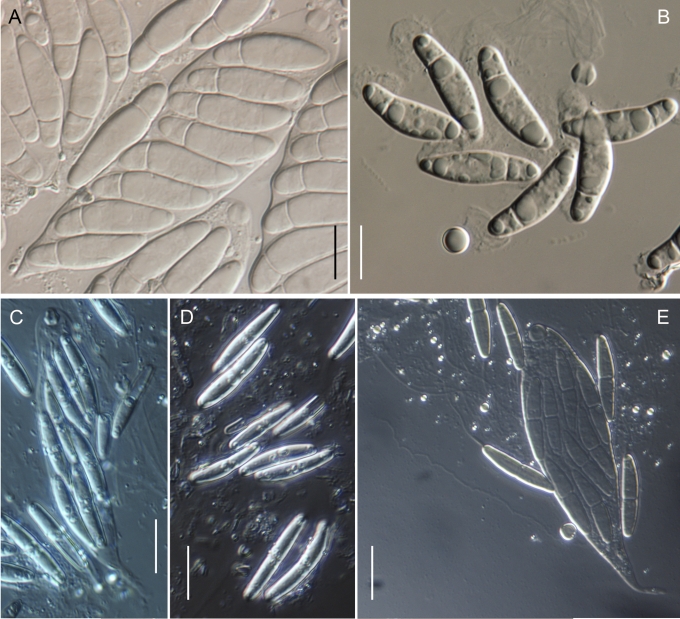
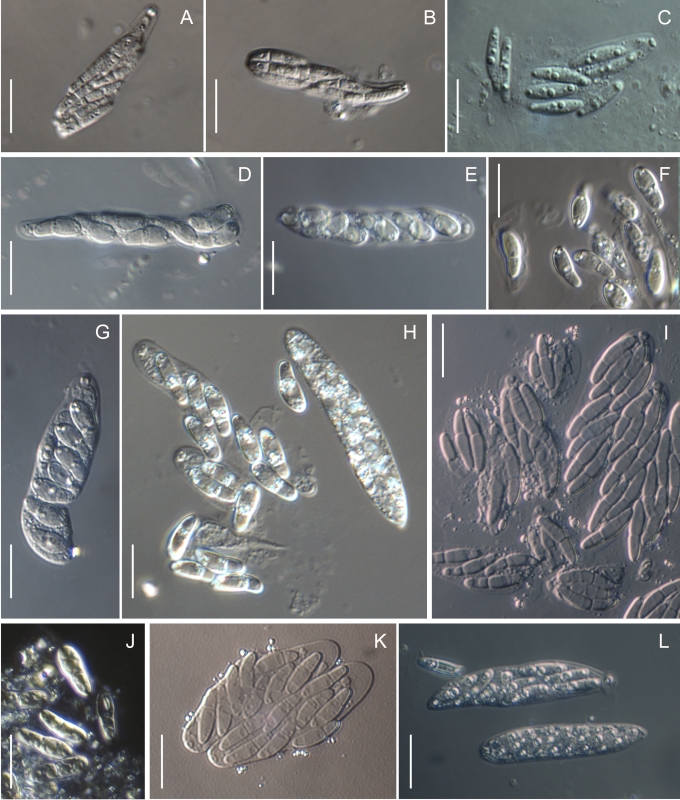
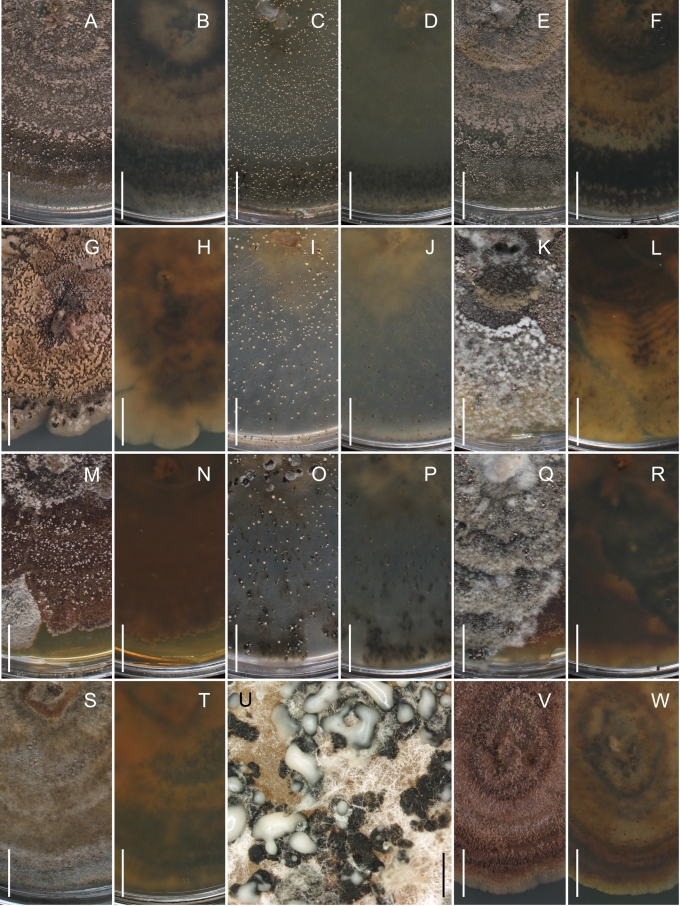
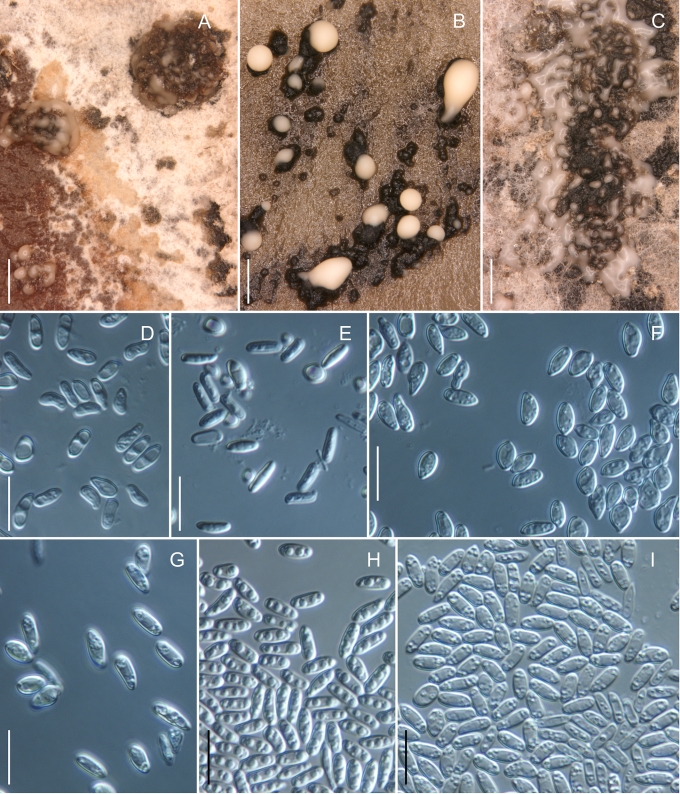
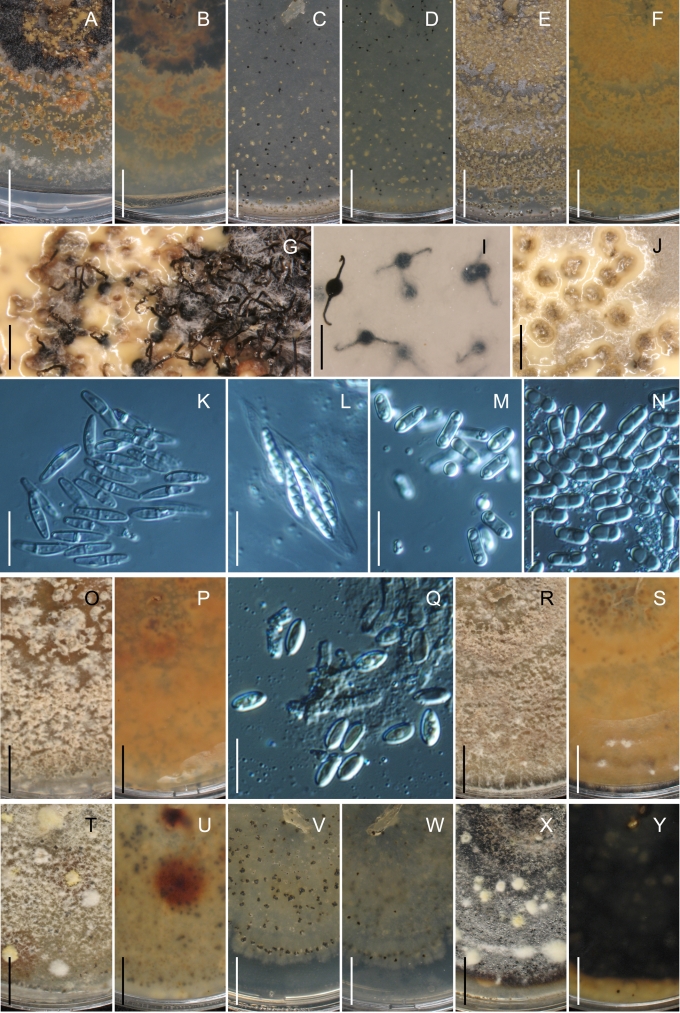
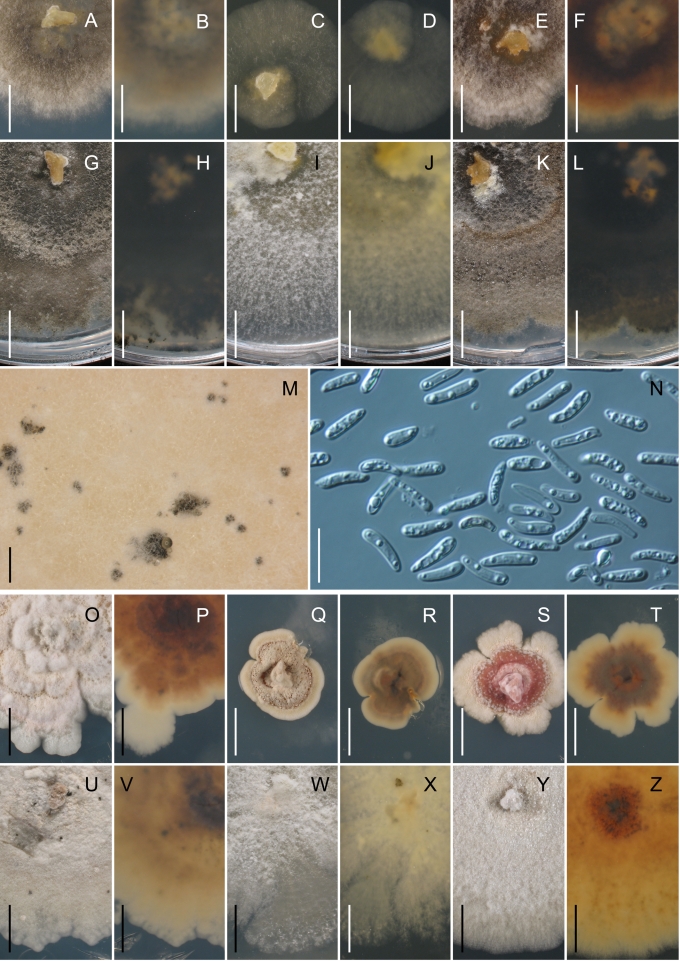
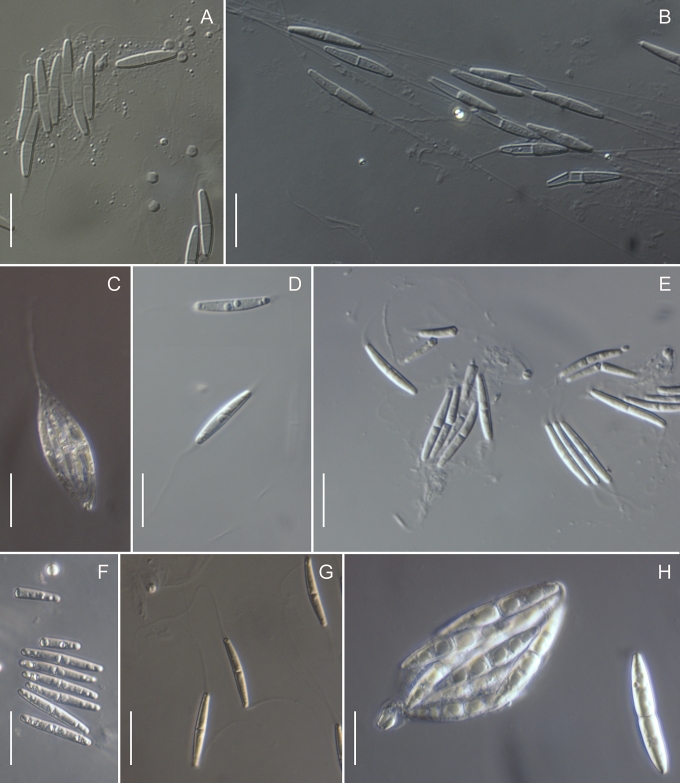
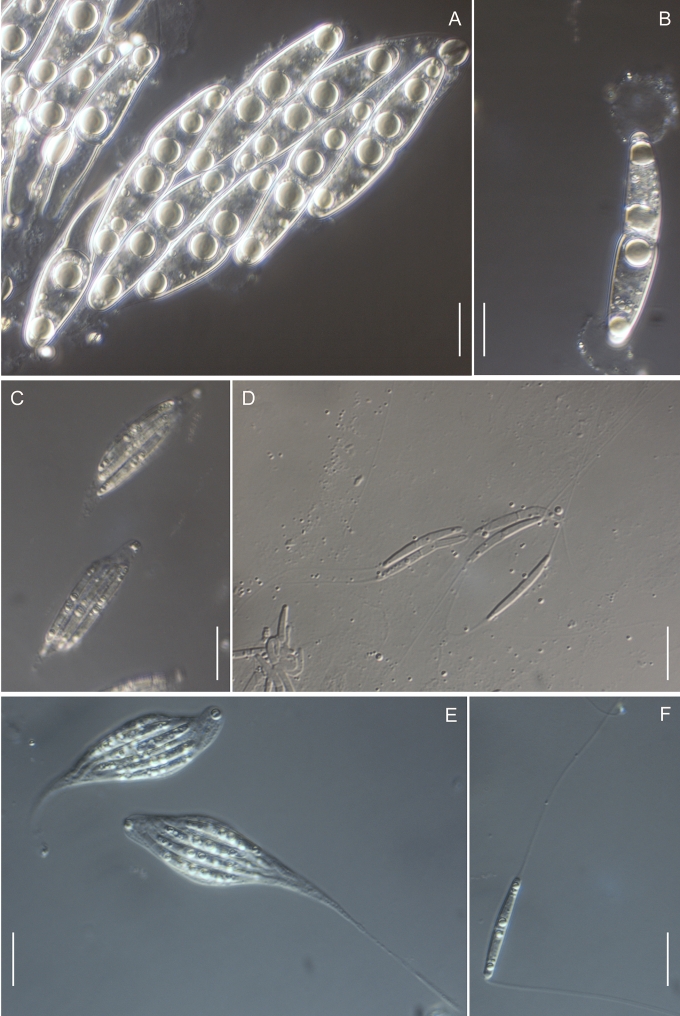
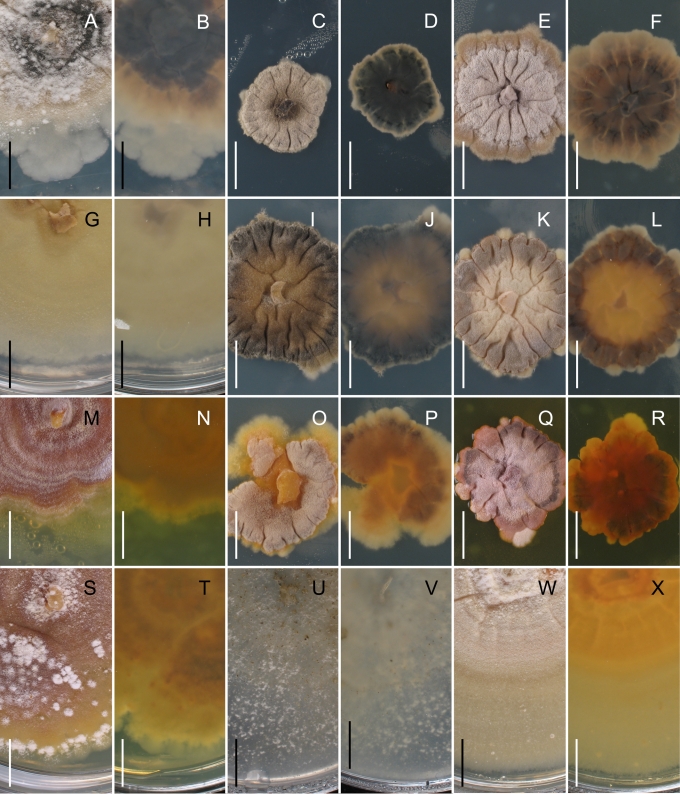
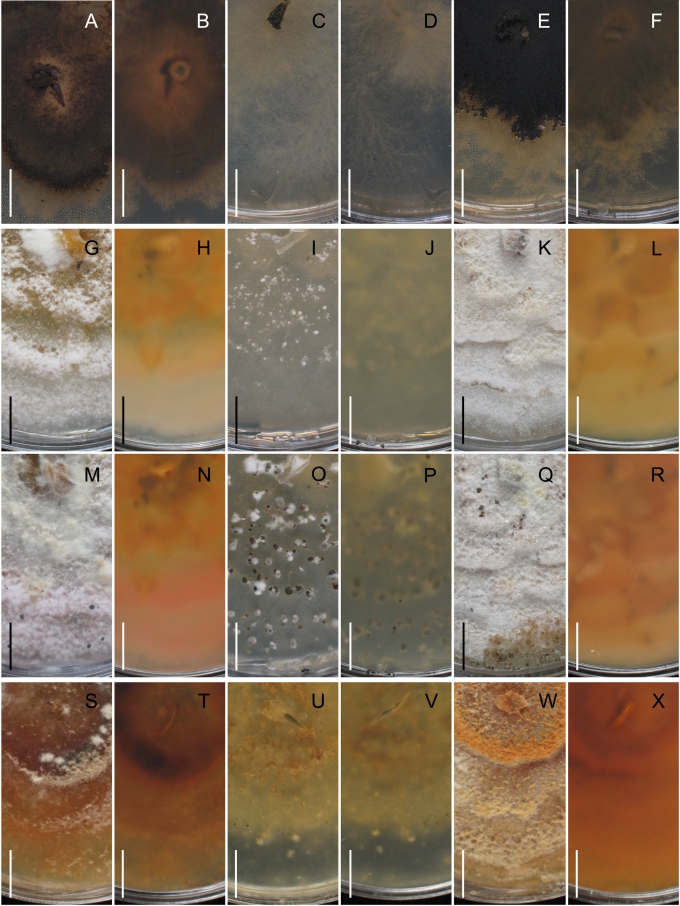
Morphology on natural substrates, perithecia. A, B. Gnomonia gnomon. A. Epitype BPI 844273. B. BPI 596632. C–E. G. alnea. C, E. Epitype BPI 877462A. D. BPI 799019. F, G. G. incrassata, holotype BPI 611818A. H, I. G. monodii, holotype BPI 877499A. A, C, D, F, H. Intact air-dry perithecia on leaves. B, E, G, I. Extracted and rehydrated perithecia. Scale 200 μm.
Fig. 5.
Morphology on natural substrates, perithecia. A, B. Gnomonia neognomon. A. BPI 877466A. B. BPI 877526C. C–H. G. orcispora. C, D. BPI 877526A. E–H. Holotype BPI 877465C. I–L. G. ostryae. I–K. BPI 611536. L. BPI 871051. A, C–F, I, J. Intact air-dry perithecia on leaves. B, H, K, L. Extracted and rehydrated perithecia. G. Semi-rehydrated perithecium on a small fragment of a leaf. Scale 200 μm.
Fig. 9.
Morphology on natural substrate, perithecia. A, B. Gnomonia pendulorum, holotype BPI 877526B. C, D. G. rodmanii, holotype BPI 878211A. E–G. G. skokomishica. E, G. Holotype BPI 877465B. F. BPI 877535. A, C, E, F. Intact air-dry perithecia on leaves. B, D, G. Extracted and rehydrated perithecia. Scale 200 μm.
Fig. 12.
Morphology on natural substrate, perithecia. A–C. Gnomonia virginianae. A. BPI 878210. B. BPI 877565A. C. BPI 878209. D–F. G. amoena. D, E. BPI 877469. F. BPI 877468. G. G. arnstadiensis, BPI 877470. H. G. pseudoamoena, BPI 877516. A, B, D, F–H. Intact air-dry perithecia on leaves and petioles. C. Extracted and rehydrated perithecia. E. Air-dry perithecium on fragment of petiole with removed outer tissue. Scale 200 μm.
The genus Ambarignomonia is established for the distinctive species, A. petiolorum, that is common on Liquidambar styraciflua (Hamamelidaceae), native to North America. Easy to recognise because of the white collar around the relatively long neck of the perithecia, Ambarignomonia is otherwise similar to members of the Gnomoniaceae in their occurrence on fallen leaves, lack of stromatic development, and perithecia that remain immersed in the substrate and collapse from the top when dry. The ascospores are fusiform, have one median septum, and bear appendages at both ends. The colonies are relatively slow-growing and do not produce conidiomata in cultures. In all analyses A. petiolorum appears to be unique among species in the Gnomoniaceae.
The type species of Apiognomonia, A. veneta, and a second species, A. errabunda, were redescribed by Sogonov et al. (2007). In the present work three additional species have been determined to be congeneric with these species including A. hystrix, on woody substrates. Apiognomonia includes species producing solitary perithecia without a stroma or with a weakly developed stroma on decaying leaves and twigs. The perithecia remain immersed and become convex or collapse from the base when dry. The ascospores have one septum that is variable in placement ranging from median to supramedian. They are ellipsoidal with or without appendages. In culture species of Apiognomonia are relatively fast-growing and often produce abundant conidiomata. Species of Apiognomonia occur on a wide variety of woody plant hosts in the Aceraceae, Fagaceae, and Plantanaceae as well as herbaceous families such as the Anacardiaceae, Geraniaceae, Onagraceae, and Rosaceae.
The concept of Gnomoniopsis is herein expanded to include the type, G. chamaemori, and six additional species. Perithecia are generally single, rarely in groups, on decaying leaves or twigs of woody trees, shrubs or herbaceous plants. No stromatic tissues are associated with the perithecia. The perithecia remain immersed in the substrate and become convex collapsing from the base when dry. The ascospores are ellipsoidal, slightly broader in the upper portion, have one submedian or median septum, and lack appendages. In cultures these fungi are moderately fast growing and usually produce abundant conidiomata on PDA. Species of Gnomoniopsis occur on a vast range of plant families including the Ericaceae, Fagaceae, Rosaceae, and Tiliaceae. Ditopellopsis racemula is herein placed in Gnomoniopsis.
Many species previously regarded as belonging to Gnomonia are now placed in Ophiognomonia. The perithecia occur singly on leaves of woody trees and shrubs as well as herbaceous plants. They lack a stroma and remain immersed becoming convex upon drying, although two species, O. balsamiferae and O. melanostyla, are partially erumpent and were found to collapse irregularly upon drying. The ascospores are ellipsoidal to fusiform with pointed ends, rarely filiform, have one median septum, with or without appendages of variable length. The cultures are moderately fast growing, rarely producing conidiomata. Most species of Ophiognomonia occur on members of the Fagales including the Betulaceae, Fagaceae, and Juglandaceae, but some also have been reported from other plant families.
The genus Plagiostoma is herein recognised to include the type species P. euphorbiae, one new species, and eleven additional species transferred from other genera. The type species of the genus Cryptodiaporthe, C. aesculi, groups with P. euphorbiae and its relatives, thus Cryptodiaporthe is considered a synonym of Plagiostoma. Perithecia of Plagiostoma occur singly or in groups on leaves and twigs of woody trees and shrubs as well as herbaceous plants. Often the perithecia lack a stroma. Like all genera of the Gnomoniaceae dealt with in this study except Ambarignomonia and Gnomonia, the perithecia remain immersed in the substrate becoming convex with the base collapsing upward when dry. The ascospores are ellipsoidal, have one median septum that is rarely submedian or absent, and may or may not bear appendages. The species grow relatively fast in culture and often produce abundant conidiomata on PDA. Species of Plagiostoma occur on a diverse range of woody and herbaceous hosts.
Evaluation of morphological and host characteristics
The groupings of species based on the multigene phylogeny presented here suggest that the morphological characters previously used to define genera must be re-evaluated. The generic classification proposed by Barr (1978) and Monod (1983) are presented as rectangular tables, referred to as a “pigeon-hole” system in which columns and rows show genera corresponding to ascospore and perithecial/stromatal characteristics. The phylogenies resulting from the analysis of multiple genes do not agree with such a rigid definition of genera based on one or two characteristics. Little congruence can be found between the newly defined genera based on molecular data and the genera based on a single morphological characteristic.
Host specificity is an important character in circumscription of genera and species of the Gnomoniaceae. The genus Gnomonia is almost strictly associated with plant hosts in the Betulaceae, mostly in the subfamily Coryloideae. Likewise, most of the species of Gnomonia are limited in their host range to a single genus and often to a single plant species. For example, the type species, G. gnomon, is restricted to species of Corylus except for one collection reported from a Populus seedling that may be an accidental colonisation of a non-specific host. The monotypic Ambarignomonia with A. petiolorum is likewise restricted to one plant host, Liquidambar styraciflua. The other genera of the Gnomoniaceae do not show such consistency in host associations. The genus Apiognomonia has the most diverse range of hosts that include hardwood trees as well as herbaceous plants. Species of Apiognomonia exhibit a diversity of host specificity with some species such as A. veneta occurring on plants in at least 10 different plant families while others such as A. acerina are restricted to one plant host. Species of Gnomoniopsis are mostly associated with either Fagaceae or Rosaceae also with the range of host specificity varying among species. While the type species G. chamaemori appears to be restricted to Rubus chamaemorus, other species are specific at the level of host genus such as G. clavulata on Quercus spp. Species of Ophiognomonia occur predominantly on members of the Fagales with some exceptions such as the type species O. melanostyla that infects overwintered leaves of Tilia spp. The genus Plagiostoma shows a broad host range with species occurring on a variety of hosts such as P. devexum on Persicaria, Polygonum, and Rumex (Polygonaceae) while others are species specific such as P. euphorbiae is known only from Euphorbia palustris. Few members of the Gnomoniaceae are known to infect hosts outside of the dicotyledonous plants; the asexual genus Sirococcus on conifers provides one exception (Rossman et al. 2007,Rossman et al. 2007).
Members of the Gnomoniaceae occur most commonly on fallen or still attached, overwintered leaves including petioles or herbaceous stems although some occur on woody substrates such as species of Cryptosporella but also, for example, Apiognomonia hystrix and Plagiostoma salicellum. When ascomata develop on woody substrates, these are often found on relatively small branches, one or two years old, that are dead but still attached to the host tree. A number of species of Gnomoniaceae have been reported as endophytes of woody plants (Viret & Petrini 1994, Cohen 1999, 2004, Danti et al. 2002, Vujanovic & Britton 2002, Green 2004, Moricca &Ragazzi 2008) but these are often not accurately identified. In addition, some species are pathogenic such as Apiognomonia veneta, cause of sycamore anthracnose, and Gnomoniopsis fructicola, cause of strawberry stem rot (Maas 1998).
One morphological character that was emphasised in earlier classification systems is the type and extent of stroma (Kobayashi 1970, Barr 1978, Monod 1980, Vasilyeva, 1998). No members of the Gnomoniaceae have a well-developed stroma. Species of Cryptosporella as well as Amphiporthe hranicensis, Apiognomonia hystrix, and Plagiostoma salicellum, i.e. species that occur on woody substrates, produce limited stromatic tissues.
These stromatic tissues may be associated with the rupture through the surface of the substrate. In addition, three species of Linospora, not considered in this study, produce a layer of tissue that covers the aggregated ascomata that are superficial or slightly immersed on leaves (Barr 1978, Monod 1983). In other families of the Diaporthales such as the Cryphonectriaceae, Diaporthaceae, Pseudovalsaceae, and Valsaceae, stromata are often well-developed (Castlebury et al., 2002, Gryzenhout et al. 2006, Voglmayer & Jaklitsch 2008).
Most members of the Gnomoniaceae including all species of Ambarignomonia, Gnomonia, and Ophiognomonia, produce ascomata singly, immersed, although some species of Gnomonia become erumpent. Similar to the development of a rudimentary stroma, the formation of grouped perithecia appears to be more common in species that develop on woody substrates. This is exemplified by Apiognomonia hystrix. Apiognomonia hystrix as Cryptodiaporthe hystrix was traditionally placed in the Valsaceae (Barr 1978) because of its occurrence on woody substrates with ascomata developing a rudimentary stroma. One of its synonyms, Gnomonia cerastis, was traditionally placed in the Gnomoniaceae due to its occurrence on overwintered leaves with ascomata lacking any stroma. As suggested by Monod (1983), specimens of A. hystrix are known to occur both on woody substrates as well as on overwintered leaves. Some species of Gnomoniopsis and Plagiostoma, i.e. P. salicellum, produce grouped perithecia on woody substrates. All species of Cryptosporella occur on woody substrates and produce ascomata in groups (Mejia et al. 2008).
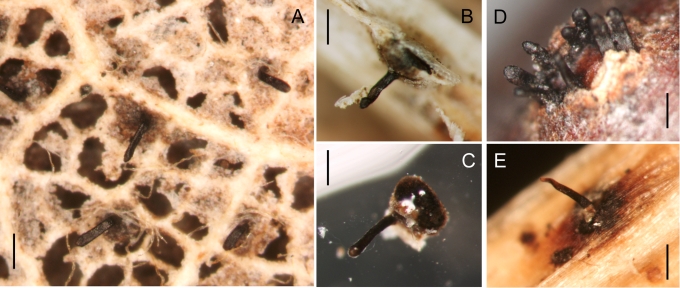
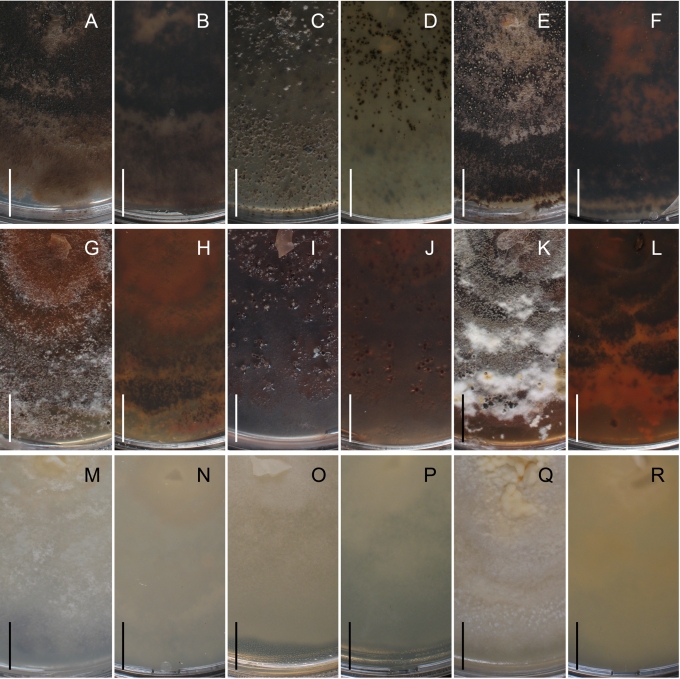
Ascomata of species of Gnomoniaceae are perithecial, i.e. no cleistothecial members are known, dark brown to black, smooth, with or without an elongated neck. In a few species, the neck is surrounded by a distinct, powdery collar. The perithecial walls are thin-walled, less than 30 μm diam, composed of only one or two regions. The outer region is composed of textura angularis with cell walls dark brown, slightly thickened, 1–2 μm. The inner region is composed of hyaline, elongate cells. The structure of the ascomata is relatively constant within the family. However, one characteristic of the ascomata that has some taxonomic significance is the collapse upon drying. In species of Ambarignomonia and Gnomonia the ascomata collapse from the top becoming concave when dry while in other members of the Gnomoniaceae the ascomata collapse from the base becoming convex when dry. This difference in collapse appears to be associated with the structure of the ascomata in which the basal wall is relatively thin compared to the side walls (Klebahn 1918).
One of the morphological characters formerly thought to be taxonomically significant at the generic level is the position of the neck categorised as either lateral or central. This character is sometimes difficult to assess in terms of discrete categories. Many species have necks that are centrally located. Necks may also be eccentric, e.g. not arising from the centre of the perithecium, in which case they can be either marginal and lateral. The term “lateral” is used herein only if the neck emerges from the margin of the perithecium and is oriented horizontal to the perithecium, at least at the base. Necks rising vertically from the margin of the perithecium are described as “marginal”. Necks often are eccentric i.e. not positioned in the exact centre of the perithecium, but neither are they truly central. We intentionally use the term “marginal” for the position of the neck in these genera to distinguish it from “lateral” in the narrow sense. This character may vary even within a single species e.g. Ophiognomonia setacea and does not correlate with the phylogenetically defined genera. Nevertheless, tendencies exist for some genera to have the neck in a certain position on the perithecium. For example, most species in Ophiognomonia have a central or slightly eccentric neck whereas eccentric to marginal necks are more common in other genera. Truly lateral necks do not occur in the genera treated in this paper although they are common in Pleuroceras (Barr 1978, Monod 1983). Most species have only one neck but one species, Gnomonia carpinicola, has perithecia each with 2–3 necks emerging from both sides of a leaf blade.
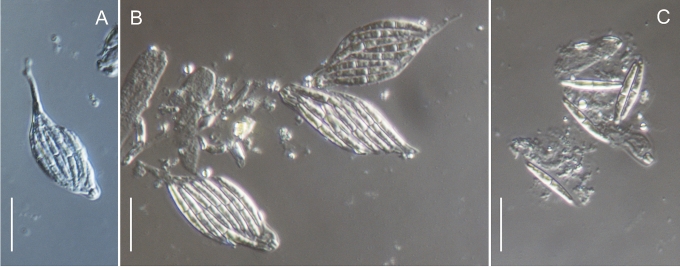
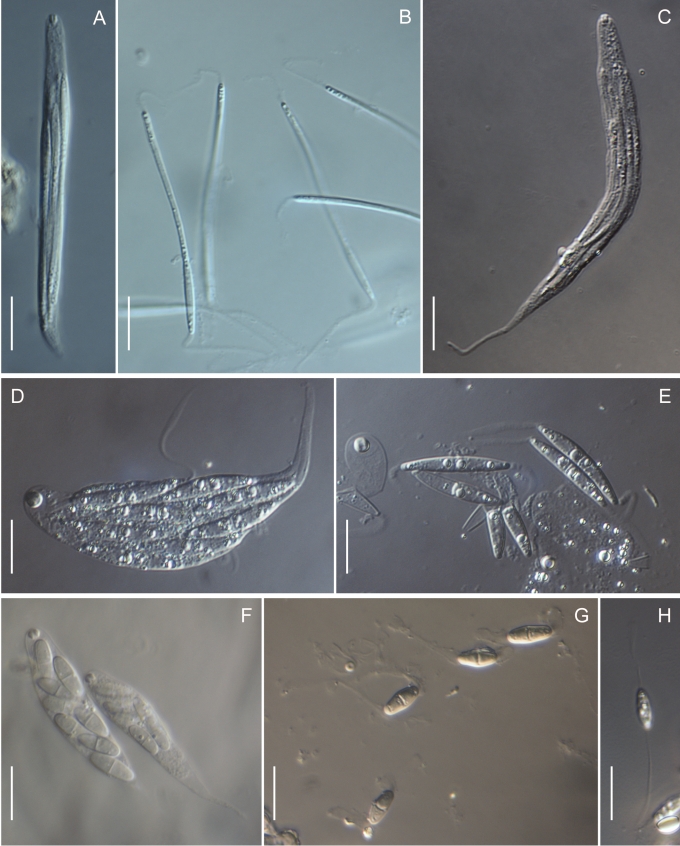
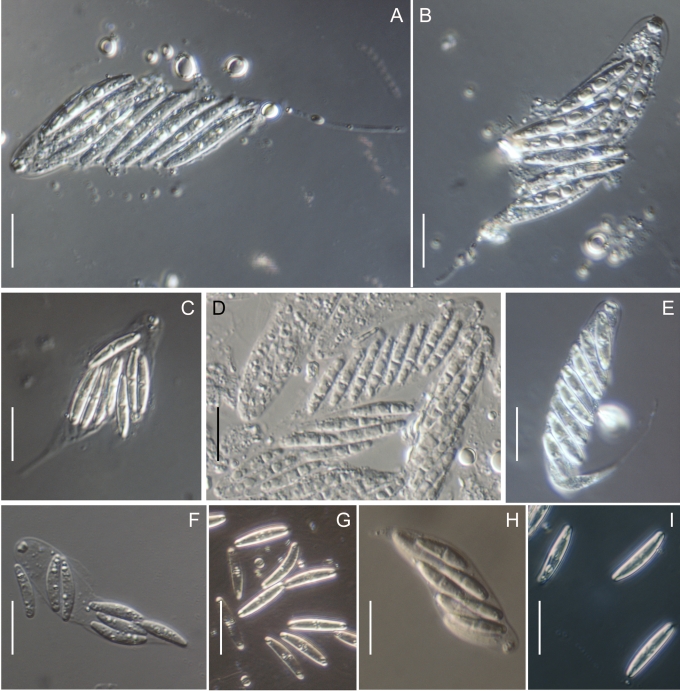
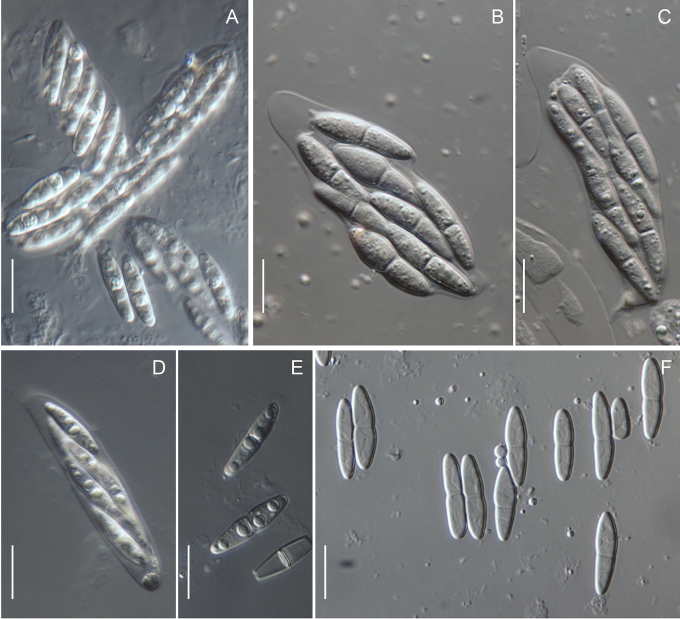
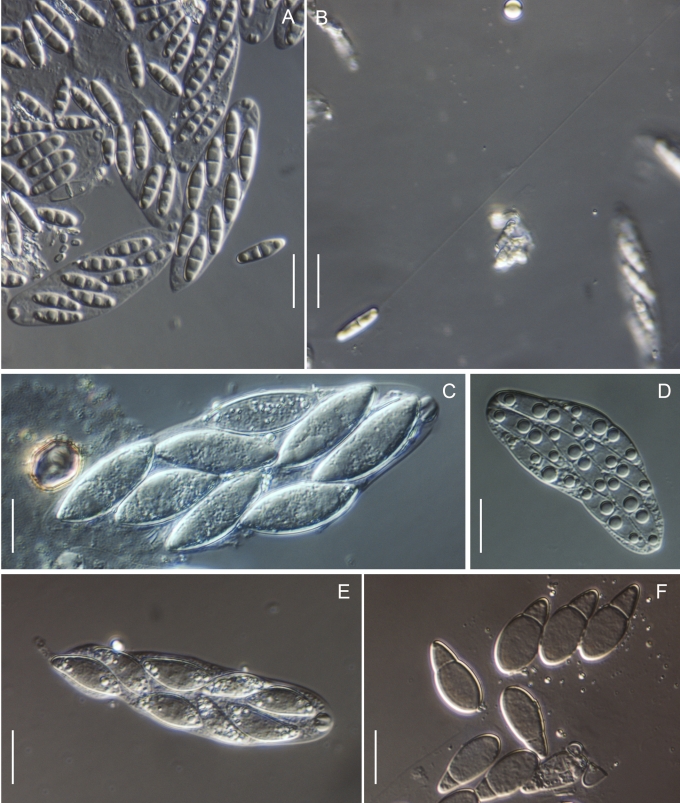
In the Gnomoniaceae the asci are generally broadly clavate to broadly cylindric with a conspicuous ascal ring always present. The width of the ascal ring may vary and, for Gnomonia orcispora, the ascal ring that is over 4.5 μm diam is diagnostic. The shape of the ascus base varies from being rounded to narrowing to the base with a distinct stalk. Asci may accumulate at the top of the often elongated necks. All species of Gnomoniaceae have eight-spored asci except Ditopella ditopa which has 32 ascospores in each ascus. The arrangement of the ascospores in the asci varies with the shape of the ascospores from obliquely distichous for shorter ascospores to irregularly parallel for elongated ascospores.
Within the Gnomoniaceae ascospores are hyaline but vary considerably in shape and septation including location of the septum, characters that traditionally been important for defining genera (Barr 1978, Monod 1983, Vasilyeva 1998). Ascospore shape and septation including the placement of the septum within the ascospore is no longer considered an important character for defining genera within the Gnomoniaceae. Ascospores range in shape from oval or short-fusiform to long cylindric and most species of Gnomoniaceae have non- or one-septate ascospores although Phragmoporthe conformis and three species of Pleuroceras have multi-septate ascospores. Species now known to be congeneric differ in ascospore shape and septation. Species with elongate, one-septate ascospores such as Ophiognomonia melanostyla and O. sassafras are congeneric with species having oval or fusiform ascospores such as O. intermedia and O. pseudoclavulata. Previously all species with non-septate ascospores were placed in the genus Gnomoniella; however, based on this study, species having non-septate ascospores are placed Ophiognomonia, O. nana, and Plagiostoma, P. euphorbiae-verrucosae and P. fraxini. Considerable variation in ascospore morphology occurs in the wood-inhabiting genus Cryptosporella that range from ellipsoid or fusiform to femoroid to elongated cylindric although most of the ascospores are non-septate (Mejia et al. 2008).
The location of the septum within the ascospore is variable in the Gnomoniaceae ranging from central i.e. in the middle of the ascospore, to below the septate, referred to as submedian or above the septum, referred to as supramedian. In this study the location of the septum was measured as the percent of the total length above the base of the ascospore, thus centrally located septa are generally 45–55 %. Within genera, location of the ascospore septum may vary from usually median in Ophiognomonia and Plagiostoma to median and supramedian in Gnomonia or median and submedian in Gnomoniopsis, and submedian, median or supremedian in Apiognomonia. At the species level, the location of the septum is consistent.
Many species of Gnomoniaceae bear appendages at both ends of the ascospore. These appendages vary in length from very short, stout to rather long, filiform but the appendages never envelop the entire ascospore. Within genera, the presence or absence of appendages is variable, although no species of Gnomoniopsis are known to have appendages. At the species level, the presence or absence of appendages is a useful diagnostic character although this may vary within species.
Anamorphs of members of the Gnomoniaceae have been placed in a number of genera including Cylindrosporella, Discula, Disculina, Gloeosporium and Neomarssonina but in general the anamorphs are similar within genera. All anamorphs in Cryptosporella for which anamorphs are known have been placed in Disculina (Sutton 1980). The conidia of species in the Gnomoniaceae are primarily non-septate, hyaline, and slimy. The asexual state appears in leaf spots often on the surface opposite the sexual state in late summer prior to leaf fall. Then, the sexual state develops on overwintered leaves in the spring as noted by Klebahn (1918). The nomenclature for the asexual states is complicated and is not dealt with here.
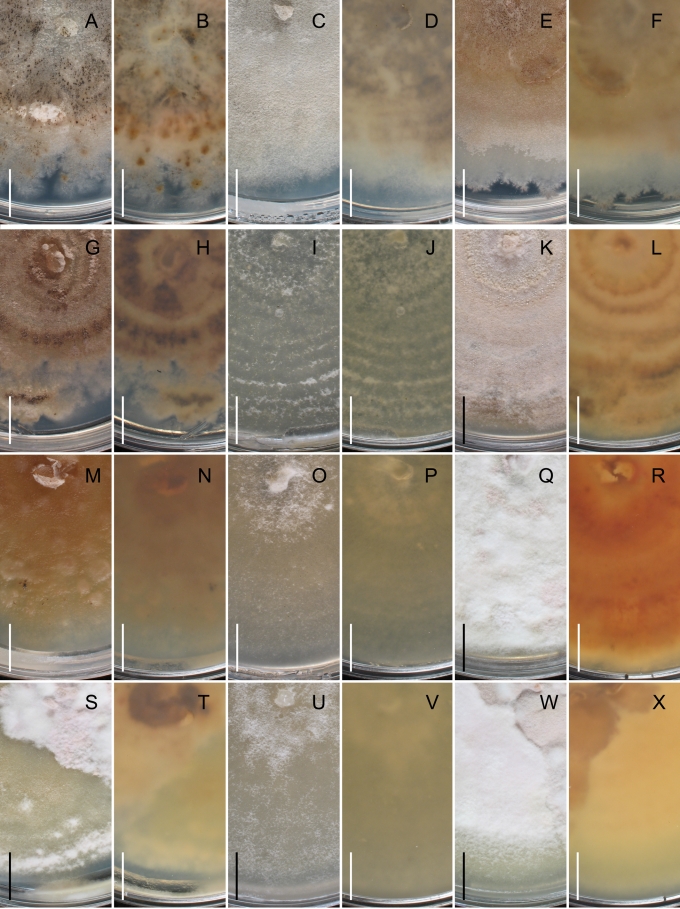
Cultures of species of Gnomoniaceae usually produce pale to dark grey, brown or black pigments, often with other colors such as yellow and orange that diffuse into the media. This pigmentation may vary considerably within a single species. Perithecia develop in isolates of some species relatively quickly within 2 wk while others will do so after several mo at the warm/cold light/dark regime described in the Materials and Methods. Some species never produced perithecia or conidia in culture.
TAXONOMY
Following is a key to the 59 species of Gnomoniaceae included in this study. These represent the commonly encountered species in the five genera treated here.
Key to the species of Gnomoniaceae in this study
1. Perithecia immersed in woody substrates, developing in groups, with perithecial necks oriented toward the centre, often on dead, still attached, one–two year old branches......................................................................................................................... 2
1'. Perithecia immersed or erumpent on overwintered, fallen or attached leaves or on dead herbaceous stems; not grouped with necks oriented toward the centre................................................................................................................................... 7
2. Ascospores non-septate, ellipsoid to cylindric including femoroid, with broadly rounded ends....... Cryptosporella (see Mejia et al. 2008)
2'. Ascospores one-septate, ellipsoid to fusiform........................................................................................................................................... 3
3. On overwintered twigs and branches of Acer spp..................................................................................................................................... 4
3'. On overwintered twigs and branches of woody hosts other than Acer, specifically Aesculus, Salix and Spiraea.................................... 5
4. Ascospores 14–20 × 2–2.5 μm fide Barr (1978). On Acer pseudoplatanus and various other hardwoods............. Apiognomonia hystrix
4'. Ascospores 7–12 × 1–2.5 μm fide Barr (1978). On Acer saccharum and A. spicatum................................... Plagiostroma petiolophilum
5. Ascospores 7.5–10 × 1.5–2.5 μm fide Barr (1978). On Spiraea.......................................................................... Gnomoniopsis macounii
5'. Ascospores greater than 10 μm long........................................................................................................................................................ 6
6. On Aesculus; ascospores 14–23 × 4.5–7 μm fide Barr (1978)................................................................................... Plagiostoma aesculi
6'. On Salix; ascospores 11–20 × 4.5–6 μm fide Barr (1978)..................................................................................... Plagiostoma salicellum
7. Individual necks surrounded with whitish powdery collars. Perithecia concave when dry, immersed in the substrate, on petioles or basal parts of major leaf veins........................................................................................................................... 8
7'. Lacking a collar around perithecial neck or neck lacking. Perithecia convex or concave when dry, in the latter case (partly) erumpent upon maturation........................................................................................................................................ 10
8. Ascospores (9–)11–12.5(–15) × 1.5–2 μm. On Liquidambar......................................................................... Ambarignomonia petiolorum
8'. Ascospores 2.5–3.2 μm wide. On Betulaceae.......................................................................................................................................... 9
9. Ascospores 17–23 × 2.5–3 μm fide Monod (1983). On Carpinus betulus in Europe and U.S.A. (TN)........................ Gnomonia amoena
9'. Ascospores 11–14.5 × 2.7–3.2 μm fide Monod (1983). On Corylus avellana in Europe and Canada............. Gnomonia pseudoamoena
10. Perithecia small, to 200 μm diam, with 2–3 necks opening on both sides of a leaf blade. Ascospores septum submedian, 12–15 × 2.7–4 μm fide Monod (1983). On Carpinus betulus in Europe .................................. Gnomonia carpinicola
10'. Perithecia with one ostiole....................................................................................................................................................................... 11
11. Perithecia concave when dry, (partly) erumpent upon maturation. Ascopores oval to fusiform with septum median or supramedian but ascospores always widest close to their middle. On Betulaceae............................................................... 12
11'. Perithecia convex when dry, remaining immersed in the substrate. If perithecia irregularly dented or concave when dry and erumpent with maturation, then ascospores filiform and upper cell wider than the lower, and septum supramedian ...... 23
12. Lacking elongated perithecial necks. On Alnus, Carpinus or Ostrya...................................................................................................... 13
12'. Elongated perithecial necks present, at least on fully mature perithecia; elongated necks may be absent in immature perithecia on Corylus. On Carpinus, Corylus or Ostrya....................................................................................................... 14
13. On Alnus. Ascospores (13.5–)16–17.5(–20.5) × (3.5–)4–4.5(–5) μm .............................................................................. Gnomonia alnea
13'. On Carpinus or Ostrya. Ascospores 17–23 × 3.5–4.5 μm fide Monod (1983) .................................................... Gnomonia arnstadtiensis
14. Ascospore septum median to slightly supramedian. Ascospores fusiform. Necks central, occasionally eccentric................................. 15
14'. Ascospore septum distinctly supramedian. Ascospores oval to fusiform. Necks eccentric to marginal except in G. incrassata and G. monodii.............................................................................................................................................................. 19
15. Ascospores (13.5–)15–16.5(–18.5) × 2–2.5 μm. On Carpinus caroliniana in Georgia, U.S.A.................................... Gnomonia rodmanii
15'. On Corylus.............................................................................................................................................................................................. 16
16. Ascospore septum slightly supramedian, located at 56 %. Ascospores (16.5–)17.5–19(–20.5) × 2–2.5(–3) μm. On Corylus californica in Washington, U.S.A. ...................................................................................................... Gnomonia skokomishica
16'. Ascospore septum median, located at 48–50 %, mean ascospore length exceeding 19 μm................................................................. 17
17. Ascospores (20–)21–22(–23) × (2.5–)3(–3.5) μm. On Corylus californica in North America................................. Gnomonia pendulorum
17'. Ascospores narrower than 2.5 μm.......................................................................................................................................................... 18
18. Ascospores (17–)19.5–21(–24.5) × 1.5–2(–2.5) μm. Perithecial necks central. On Corylus avellana in Europe ........ Gnomonia gnomon
18'. Ascospores (18.5–)20–22.5(–24.5) × 2–2.5 μm. Perithecial necks eccentric. On Corylus californica in North America....... Gnomonia neognomon
19. Ascospores (14–)15.5–17.5(–19.5) × (4.5–)5–5.5(–6) μm, oval, ends blunt, distinct hila on bases of evanescent appendages; two large guttules per cell. Apical ring exceeding 4.5 μm diam. Necks marginal. On Corylus californica in North America...................................................................................................................... Gnomonia orcispora
19'. Ascospores oval to fusiform or lanceolate, tapering toward ends or ends blunt, no hila present. Apical ring less than 4 μm diam. Necks marginal, eccentric to central. On Corylus avellana in Europe or Ostrya in Europe or North America..................................................................................................................................................................... 20
20. Ascospores mostly lanceolate or oval, broadened in upper part and distal ends broadly rounded or nearly truncated. Ascospores (12.5–)15–17(–20.5) × (3–)3.5–4.5(–6) μm. On Ostrya carpinifolia in Europe............. Gnomonia ostryae
20'. Ascospore fusiform, tapering to both ends. Mean ascospore width smaller than 3.5 μm. On Corylus avellana in Europe or Ostrya virginiana in North America.................................................................................................... 21
21. Necks marginal. Ascospores (12–)13–14(–14.5) × (2–)2.5–3 μm. On Ostrya virginiana in North America............... Gnomonia virginianae
21'. Necks eccentric or central. Mean ascospore length exceeding 14.5 μm. On Corylus avellana in Europe............................................. 22
22. Ascospores (13.5–)15.5–17(–18.5) × (2.5–)3–3.5(–4) μm, strongly constricted at septum...................................... Gnomonia incrassata
22'. Ascospores (14–)15–16(–18.5) × (2–)2.5(–3) μm, not or slightly constricted at septum.............................................. Gnomonia monodii
23. Ascospores one-celled............................................................................................................................................................................ 24
23'. Ascospores two-celled............................................................................................................................................................................ 26
24. Ascospores 20–22.5 × 5.3–6 μm fide Monod (1983), with pointed ends. On Euphorbia in Europe .... Plagiostoma euphorbiae-verrucosae
24'. Ascospores smaller, ends rounded......................................................................................................................................................... 25
25. Necks shorter than 200 μm. Ascospores (7.7–)8.6–12.7(–13.8) × (2.2–)2.8–5.9(–6.6) μm fide Redlin & Stack (1988). On Chionanthus and Fraxinus (Oleaceae) .............................................................................. Plagiostoma fraxini
25'. Necks longer than 400 μm. Ascospores 8–10 × 2.5–4 μm fide Monod (1983). On Betula nana in Europe ............. Ophiognomonia nana
26. Ascospores filiform, upper cell wider than lower cell. Necks long........................................................................................................... 27
26'. Ascospores oval, clavate or fusiform with variable septum position, or, if ascospores filiform, then septum median and cells of equal width. Necks short or long................................................................................................................ 28
27. Ascospores (30–)37–42.5(–44) × 1.5–2 μm. On Tilia spp., known from Europe and North America ................ Ophiognomonia melanostyla
27'. Ascospores 38–65 × 1 μm fide Barr (1978). On Sassafras............................................................................... Ophiognomonia sassafras
28. Ascospore septum supramedian, 15–17 × 3.7–4.5 μm fide Monod (1983). On Geranium spp. in Europe............ Apiognomonia borealis
28'. Ascospore septum median or submedian............................................................................................................................................... 29
29. Ascospore septum submedian, if septum nearly median, than upper cell wider than lower, ascospores clavate.................................. 30
29'. Ascospore septum median...................................................................................................................................................................... 39
30. Ascospores 13–16 × 4–5 μm fide Monod (1983), lower cell of ascospore conical, upper cell rounded. On overwintered but still attached pedicels and branches of Rhododendron spp. in Europe ............................. Plagiostoma rhododendri
30'. Both cells of ascospores of similar shape............................................................................................................................................... 31
31. Majority of asci clavate with base tapering to long, narrow stalk. Apical ring exceeding 1.8 μm diam, bluntly hexagonal in side view. Ascospores equally wide in upper and lower parts or wider in lower part.............................................. 32
31'. Majority of asci allantoid or obpyriform with bases rounded or shortly tapering. Apical ring less than 2.5 μm diam, more or less circular in side view. Ascospores usually slightly wider in upper part........................................................... 35
32. Ascospores straight or slightly curved, 10–13 × 2–2.5 μm fide Monod (1983). On Geum montanum in the Alps (Europe)...........................................................................................................................Ophiognomonia gei-montani
32'. Ascospores curved, wider than 3.5. On other hosts................................................................................................................................ 33
33. Perithecia exceeding 250 μm diam on average. Ascospores 15–24 × 4.5–5.5 μm fide Monod (1983). On Acer spp. in Europe .........Apiognomonia acerina
33'. Perithecia less than 250 μm diam on average. Ascospores shorter than 17 μm and narrower than 6 μm on average. Mostly on Fagus, Platanus, Quercus, and Tilia.......................................................................................................... 34
34. Ascospores (13–)15.5–17.5(–23) × (3.5–)5–5.5(–7.5) μm fide Sogonov et al. (2007). On Platanus spp. in temperate regions .................................................................................................................... Apiognomonia veneta
34'. Ascospores (10.5–)15–16.5(–19.5) × (3.5–)4.5–5.5(–6.5) μm fide Sogonov et al. (2007). On various hosts in temperate regions ............................................................................................................... Apiognomonia errabunda
35. Perithecia in groups of 3–9. Ascospores 7.5–11 × 2–3.5 μm fide Barr (1978). On Chamerion angustifolium in North America .................................................................................................... Gnomoniopsis racemula
35'. Perithecia scattered singly...................................................................................................................................................................... 36
36. Mean l:w of ascospores exceeding 3.5, upper ascospore cell slightly wider than lower. On Rosaceae................................................. 37
36'. Mean l:w of ascospores smaller than 3.5, upper ascospore cell distinctly wider than lower. On Fagaceae........................................... 38
37. Necks marginal. Ascospores 6.5–9 × 1.5–2 μm fide Monod (1983). On Potentilla spp. in Europe and North America.... Gnomoniopsis tormentillae
37'. Necks central. Ascospores (10–)10.5–11.5(–13) × (2–)2.5(–3) μm .................. Gnomoniopsis chamaemori, G. comari, and G. fructicola (These three species are morphologically similar. Additional work is required to clarify their morphological limits.)
38. Septum located above 37 % of ascospore length in the ascus. Ascospores (5–)8.5–9.5(–11) × (2–)3.5–4(–5.5) μm....... Gnomoniopsis clavulata
38'. Septum located below 37 % of ascospore length in the ascus. Ascospores (8–)9–10(–11) × (3–)3.5–4 μm Gnomoniopsis paraclavulata
39. Ascospores with l:w smaller than 3......................................................................................................................................................... 40
39'. Ascospores with l:w exceeding 3.5......................................................................................................................................................... 41
40. Necks 140–250 μm. Ascospores (6.5–)7.5–8(–9) × (2.5–)3–3.5 μm. On Carya in North America ........... Ophiognomonia pseudoclavulata
40'. Necks 300–500 μm long. Ascospores 8–11 × 2.2–3 μm fide Monod (1983). On Alnus viridis in Europe and Canada (BC) ................................................................................................... Ophiognomonia trientensis
41. Ascospores filiform, 39–51 × 1 μm fide Monod (1983). Necks central. On Prunus padus.................................. Ophiognomonia padicola
41'. Ascospores shorter or ascospore width exceeding 4.2 μm..................................................................................................................... 42
42. Ascospores 26–36 × 5.5–10 μm fide Barr (1978). Necks eccentric or lateral, stout. On Carya................... Ophiognomonia micromegala
42'. Ascospores shorter................................................................................................................................................................................. 43
43. On Rosaceae. Necks typically tapering to pointed ends. Asci with long, narrow stipe at the base. Ascospores 13–22 × 1–1.5 μm fide Monod (1983), with long, filamentous appendages............. Ophiognomonia rosae, Ophiognomonia rubi-idaei (These species cannot be reliably distinguished based on morphology. O. rubi-idaei occurs only on Rubus while O. rosae is found on various Rosaceae.)
43'. Not on Rosaceae. Necks cylindrical or slightly tapering, their width exceeding 25 μm below apex........................................................ 44
44. Necks marginal. Ascospores 8–10 × 2–3 μm fide Monod (1983) On Persicaria and Polygonum, rarely on Rumex and Vitis.......................................................................................................................................................................... Plagiostoma devexum
44'. Necks central or eccentric, few in a collection marginal. Ascospores generally longer than 10 μm. On other hosts.............................. 45
45. Necks shorter than 170 μm. Ascospores shorter than 18 μm................................................................................................................. 46
45'. Necks mostly longer or, if necks shorter than 170 μm, then ascospores longer than 18 μm on average............................................... 49
46. Ascospores (11.5–)14–15.5(–17.5) × (2.5–)3.5–4(–4.5) μm. On Acer in Washington, U.S.A.................................... Plagiostoma barriae
46'. On Euphorbia in Europe .......................................................................................................................................................................... 47
47. Necks shorter than 100 μm. Ascospores (12–)13–13.5(–15.5) × (3–)3.5(–4) μm............................................... Plagiostoma euphorbiae
47'. Necks longer than 100 μm...................................................................................................................................................................... 48
48. Ascospores 14–17.5 × 3.5–4.5 μm fide Monod (1983)................................................................................. Plagiostoma euphorbiaceum
48'. Ascospores 13–15.5 × 2.3–3 μm fide Monod (1983)......................................................................................... Plagiostoma amygdalinae
49. On dead stems of herbaceous plants, Geranium spp. in Europe. Ascospores 13–18 × 1.8–2.5 μm fide Monod (1983)... Plagiostoma geranii
49'. On overwintered leaves, one species also on twigs of trees and shrubs................................................................................................ 50
50. Necks shorter than 250 μm. Ascospores19–23 × 3.5 μm fide Monod (1983). On Juglans spp. in Europe and North America .................................................................................................................................................... Ophiognomonia leptostyla
50'. Necks typically longer than 250 μm. If necks shorter than 250 μm, then ascospores shorter than 19 μm on average.......................... 51
51. Necks 940–1150 μm. Ascospores (15–)18–19(–21) × 2.5–3(–3.5) μm. On Populus...................................Ophiognomonia balsamiferae
51'. Necks shorter; if longer than 900 μm, then ascospores shorter than 15 μm ........................................................................................... 52
52. Ascospores (17.5–)18.5–19.5(–21) × (2.5–)3(–3.5) μm. Ascal apical ring 2.7–3.2 μm diam. On Juglans.........Ophiognomonia vasiljevae
52'. Ascospores shorter than 17.5 μm on average........................................................................................................................................ 53
53. Ascal apical ring 3–3.5 μm diam. Ascospores 11–18 × 1.5–2.5 μm fide Monod (1983). On twigs of broad range of hosts, predominately on Acer........................................................................................................... Apiognomonia hystrix
53'. Ascal apical ring less than 2.7 μm diam. Ascospores mostly narrower than 2.6 μm. If ascospores wider than 2.6 μm, then ascospores shorter than 12 μm................................................................................................................................... 54
54. Ascospores (10–)11.5–13.5(–17) × (1.5–)2–2.5 μm with or without cuneiform appendages. On Castanea, Fagus and Quercus (Fagaceae)................................................................................................... Ophiognomonia setacea
54'. On Betulaceae, occasionally on other hosts........................................................................................................................................... 55
55. Ascospores (12.5–)13.5–15.5(–18.5) × (1.5–)2(–2.5) μm with cuneiform or long appendages. On Alnus, Betula, Corylus and Carpinus, possibly also Ostrya....................................................................... Ophiognomonia ischnostyla
55'. Ascospores generally smaller than 12 μm.............................................................................................................................................. 56
56. Ascospores (9.5–)10–11(–13.5) × (2.2–)2.5–3(–3.6) μm without appendages. On Alnus and Betula............ Ophiognomonia intermedia
56'. Ascospores (10–)11–11.5(–12.5) × 2–2.5 μm with long appendages. On Alnus and Betula, occasionally Myricaria and Vaccinium............................................................................................................... Ophiognomonia alni-viridis
DESCRIPTIONS OF GENERA AND SPECIES OF THE GNOMONIACEAE
Following are the descriptions of five genera and their generic synonyms representing the the most common leaf-inhabiting species in the Gnomoniaceae. The genus Cryptosporella is not included as it has been dealt with elsewhere (Mejia et al. 2008). A description for each of the five genera is included along with a description of the type species and the type species of any synonymous genera. Thirteen new species are described in full. In addition, many new combinations are made for taxa that should be placed in the phylogenetically defined genera.
GNOMONIA Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 231. 1863. Type: G. gnomon (Tode: Fr.) J. Schröt., designated by Höhnel (1917).
= Gnomonina Höhn., Ber. Deutsch. Bot. Ges. 35: 635. 1917. Type: Gnomonina alnea (Fr.) Höhn., herein recognised as Gnomonia alnea (Fr.) Sogonov
[≡ Laestadia Auersw., Hedwigia 8: 177. 1869 non Kunth ex Lessing, 1832.]
Perithecia solitary, without stroma, on overwintered, fallen or attached leaves of trees and shrubs, usually epiphyllous or on petioles, rarely hypophyllous. Perithecia black, immersed at first, later erumpent, rarely partly erumpent or with wide opening with white or pink powdery collar surrounding the neck, powdery substance not dissolving in water or 3 % KOH solution. Perithecia oblate-spheroidal to oblate when moist, concave when dry, circular in top view, rarely perithecia convex, nearly flat, with one neck, occasionaly with two or three necks emerging on both sides of a leaf blade. Necks central to marginal, never truly lateral, slightly curved to distinctly curved, their length mostly 1–2, sometimes to 5 times the perithecial diam, sometimes absent. Asci oval to fusiform, with an apical ring, with eight spores arranged unevenly parallel or irregularly multiseriate, occasionally obliquely uniseriate. Ascospores two-celled, fusiform to acerose, l:w 3–15, ends rounded; appendages cuneiform with diffuse ends or ovoid, subulate, or acicular, rarely absent.
Cultures: Colonies reaching (0.5–)1–4 cm diam after 2 wk at 23 °C dark/light on MEA/MYA and PDA. Colony surface velvety, pale grey, yellowish grey, greyish yellow, brownish grey, or brown. In some species fertile perithecia formed after 5–6 mo at 2/10 °C l/d on MEA, MYA, and PDA, rarely sterile perithecia noted within one month at 23 °C l/d. Conidiogenous structures not produced.
Hosts: Limited to family Betulaceae, mostly in the subfamily Coryloideae. Individual fungal species are host specific at plant species level or, less commonly, at genus level.
Gnomonia gnomon (Tode: Fr.) J. Schröt. in Cohn's Krypt. Fl. Schles. 3(2): 390. 1897. Figs 2A,B; 3A–C; 4A–M.
Fig. 3.
Morphology on natural substrates, asci and ascospores. A–C. Gnomonia gnomon. A. Epitype BPI 844273. B. BPI 871054A. C. BPI 844279. D–F. G. alnea, epitype BPI 877462A. G. G. incrassata, holotype BPI 611818A. H, I. G. monodii, holotype BPI 877499A. Scale 10 μm.
Fig. 4.
Gnomonia gnomon, cultures. A–F, M. Ex-type CBS 116383. G–L. CBS 121233. A–H. Colony habit, 40 d, 23 °C. A, C, E, G. Surface. B, D, F, H. Reverse. I, J. Perithecia, 4.5 mo, 2/10 °C. K–M. Ascospores and asci, 4.5 mo, 2/10 °C. A, B, G, H, J, M. PDA. C, D, K, L. MEA. E, F, I. MYA. Scale: A–H. 1 cm. I, J. 200 μm. K–M. 10 μm.
≡ Sphaeria gnomon Tode: Fr., Fungi Mecklenb. 2: 50. 1791: Syst. Mycol. 2: 517. 1823.
≡ Cryptosphaeria gnomon (Tode: Fr.) Grev., Fl. edin.: 360. 1824.
≡ Gnomonia vulgaris Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 232. 1863.
≡ Gnomoniella vulgaris (Ces. & De Not.) Sacc., Syll. Fung. 1: 416. 1882.
≡ Gnomoniella gnomon (Tode: Fr.) Magnus, Pilze von Tirol: 490. 1906.
Anamorph: Unknown.
Perithecia hypophyllous, scattered randomly over leaf blade, immersed at first, erumpent at maturity, black, oblate when moist, (58–)163–206(–239) μm high × (144–)206–262(–318) μm diam (mean = 179 × 230, SD 35, 40, n1=51, n2=96), concave when dry. Necks central, occasionally eccentric, straight or slightly sinuous, (179–)250–335(–561) μm long (mean = 296, SD 70, n=71), (18–) 27–37.5(–53) μm wide at base, (14.5–)23.5–33(–42.5) μm wide at apex. Asci fusiform with narrow tapering stipe, (28.5–)35.5–43(–56.5) × (5.5–)7–9.5(–11.5) μm (mean = 39.5 × 8, SD 6, 1.5, n=70), apical ring 1.5–2.5 μm diam, with eight ascospores more or less parallel. Ascospores fusiform, straight to slightly curved, (17–) 19.5–21(–24.5) × 1.5–2(–2.5) μm (mean = 20.5 × 1.5, SD 1.5, 0.2, n=276), l:w (8.5–)11–13(–16.5), two-celled, slightly constricted at septum; septum located at (36–)47–51(–61) % (mean = 49, SD 3, n=266) of ascospore length; cells tapering to blunt, rounded ends, 4–8 guttules per cell, usually one large guttule close to septum; appendages 2.5–4 μm long, cuneiform, sometimes whip-shaped, up to 27 μm long, sometimes absent.
Cultures: Colony diam after 14 d at 23 °C 25–30 mm diam on PDA, 5–25 mm on MEA, 10–20 mm on MYA. Colonies flat, radially or irregularly furrowed, velvety or with few loose felty tufts, pale grey to orange-grey, yellow-brown or dark brown; margin even or lobate; reverse yellow-brown to dark brown. On MEA colonies flat, orange-brown or greyish orange, aerial mycelium nearly lacking or whitish dendroid tufts formed; margin wavy; reverse greyish orange. On MYA colonies greyish orange to brownish orange, aerial mycelium scant; margin irregular; reverse brownish orange, pale brown or dark brown. Perithecia typically produced on PDA, MEA and MYA in cultures incubated at 2/10 °C dark/light regime, fertile or sterile depending on the strain, best sporulation observed on PDA. Asci and ascospores in culture not significantly different from those produced on natural substrates, although ascospores sometimes shorter, swollen, and with more guttules.
Habitat: On overwintered leaves of Corylus spp., most common on C. avellana L. (Betulaceae), one collection on Populus nigra L. (Salicaeae).
Distribution: Europe (Austria, Bulgaria, Czech Republic, Finland, Germany, Russia, Slovakia, Sweden, Switzerland, Ukraine, United Kingdom).
Lectotype: Germany, Mecklenburg, Corylus avellana, 1791, H.I. Tode, illustration in `Fungi mecklenb.', Tab. 16, Figs 125a–125f designated by Sogonov et al. (2005).
Epitype: Finland, Helsinki, Helsinki University Botanical Garden, overwintered fallen leaves of C. avellana, 19 Apr 2004, D.S. Shchigel MS0036 (BPI 844273; ex-type culture AR 4062 = CBS 116383) designated by Sogonov et al. (2005).
Additional specimens examined: All on dead leaves of Corylus avellana except where noted. Austria, Lower Austria, Krems, Apr. 1871, Thümen (BPI 611815); Vienna, 1 May 2004, W. Jaklitsch WJ2501 (BPI 844279, culture CBS 116384); Czech Republic, Bohemia, Žlutice (Luditz), Krašov (Krasch), May 1913, R. Steppan (BPI 611818); Bohemia, Turnov, 02 May 1907, J. M. Kabat (BPI 611820); Hranice, 1913, F. Petrak (BPI 611817); France, Haute-Savoie, Petit-Saleve, near Geneve, Feb. 1852, collector unknown (BPI 596635). Germany, Brandenburg, Prignitz, Triglitz, 14 Apr. 1906, O. Jaap, Fungi selecti exsiccati 220 (BPI 596638); Brandenburg, Prignitz, Triglitz, Willd., 06 Apr. 1911, O. Jaap (Jaap, Fungi selecti exsiccati 519, BPI 596287, BPI bound); Dillkreis, 02 Apr. 1934, collector unknown (BPI 596633); same details (BPI 596634); Dillkreis, Langenaubach, Apr. 1923, collector unknown (BPI 611435); Hessen, Oestrich, near Dillkreis, Langenaubach, 30 Apr. 1933, A. Ludwig (BPI 611808); Nossen, 24 Apr. 1889, W. Krieger (BPI 611809); Leipzig, May 1871, G. Winter (BPI 611810); same location, May 1874, G. Winter (BPI 611811); Schleussig, near Leipzig, May 1871, G. Winter (BPI 611812); Thüringen, Steiger near Erfurt, 13 May 1905, collector unknown (BPI 596632); locality unknown, date unknown, J.C. Schmidt & G. Kunze, Deutschlands Schwämme 57, BPI (611814); Russia, Nizhniy Novgorod oblast, Pil'na, birch park close to the river P'yana, 20 May 2004, G.M. Sogonova MS0103 (BPI 863598); Novgorod oblast, Kholm, Dendropark, Jun. 2005, M.V. Sogonov MS0274b (BPI 877514C); Novgorod oblast, Kholm, ulitsa Naberezhnaya Reki Lovat', 07 Jun. 2005, D.N. Borisov MS0275b (BPI 877517C,); Slovakia, Prenčov, 28 Mar. 1887, A. Kmet (BPI 611821); Sweden, E.M. Fries, Scleromyceti Sueciae 285, BPI (bound); Switzerland, Bischofszell, date unknown, H. Wegelin (ZT); Oberbuchsiten, 01 Mar. 1946, coll. J.A. von Arx (ZT); Changins, 1 March 1976, M. Monod, No. 2 (LAU); Vaud, Bex, Le Bévieux, 13 May 1976, M. Monod, No. 47 (LAU); Valais, Populus nigra, 13 May 1977, A. Bolay, No. 267 (LAU, culture CBS 829.79); Misox, Grono, 17 May 1988 E. Müller (ZT); Domleschg, Rodel, 04 May 1988, E. Müller (ZT); Albulatal, Filisur, Solis, 20 May 1988, E. Müller (ZT); Schanfigg, Lüen, 24 May 1989, E. Müller (ZT); Vorderrheintal, Panix, 13 June 1989, E. Müller (ZT); Valais, vicinity of Martigny, overwintered but still hanging leaves, 21 May 2005, M. Monod MS0335a (BPI 877499B); Ticino, Monte San Salvatore, 28 May 2005, M.V. Sogonov MS0205 (BPI 871054A, culture AR4189 = CBS 121233) GenBank EU254779; Vaud, St-Cergue, 20 May 2005, M.V. Sogonov MS0336 (BPI 877492); same data, MS0392 (BPI 877490A) GenBank EU254780; Ukraine, Ivano-Frankivsk oblast, Kalkhingel, near Podluze, 12 May 1918, F. Petrak (BPI 611816); United Kingdom, England, 1873?, Plowright (BPI 611813).
Notes: Gnomonia gnomon, the type species of the genus Gnomonia, was described and illustrated by Sogonov et al. (2005). This species is generally restricted to Corylus spp. in Europe although the one specimen on Populus exists for which the host identification has been verified. Species previously reported as G. gnomon from North America have been determined to be G. neognomon or G. pendulorum.
Gnomonia alnea (Fr.) Sogonov, comb. nov. MycoBank MB 512161. Figs 2C–E; 3D–F. Basionym: Sphaeria alnea Fr., Syst. Mycol. 2: 520. 1823.
≡ Sphaeriella alnea (Fr.) Auersw. in Gonn. & Rabenh., Mycol. Europ. 5/6: Tab. 2, Fig. 15. 1869.
≡ Laestadia alnea (Fr.) Auersw., Hedwigia 8: 177. 1869.
≡ Guignardia alnea (Fr.) Schröt., Pilze Schlesiens 2: 330. 1894.
≡ Gnomonina alnea (Fr.) Höhn., Ber. Deutsch. Bot. Ges. 35: 628. 1917.
≡ Plagiostoma alneum (as alnea) (Fr.) Arx, Antonie van Leeuwenhoek 17: 264. 1951.
= Gnomonia perversa Rehm, Hedwigia 24: 70. 1885.
Fig. 15.
Morphology on natural substrate, perithecia. Ambarignomonia petiolorum. A, B. Epitype BPI 844274. C, D. BPI 877511. A, C. Intact air-dry perithecia on petioles. B. Air-dry perithecium on fragment of petiole with removed outer tissue. D. Extracted and rehydrated perithecia. Scale 200 μm.
Anamorph: Unknown.
Perithecia solitary, without stroma, hypophyllous, scattered randomly over leaf blade, immersed at first, erumpent or partly erumpent at maturity, black, oblate when moist, 100–140 μm high × 140–240 μm diam, collapsed concave or convex, occasionally irregularly wrinkled or flat when dry. Neck absent, ostiole marginal. Asci fusiform, (47.5–)58–67.5(–78) × (9.5–)12–13(–16) μm (mean = 63 × 12.5, SD 7, 1.5, n=19), apical ring 4–4.5 μm diam, with eight ascospores arranged obliquely uniseriate or irregularly multiseriate. Ascospores fusiform (13.5–)16–17.5(–20.5) × (3.5–)4–4.5(–5) μm (mean = 17 × 4.5, SD 1.5, 0.5, n=89), l:w (3.1–)3.7–4.3(–5), two-celled with septum located at (44–)48–51(–55) % (mean = 49, SD 3, n=60) of ascospore length, ends blunt, rounded, each cell with two large guttules; appendages ovoid to cuneiform, ca. 2 μm long, or absent.
Habitat: On fallen overwintered leaves of Alnus glutinosa (L.) Gaertn., A. incana (L.) Moench and A. viridis (Chaix) DC. (Betulaceae).
Distribution: Europe (Bulgaria, Czech Republic, Germany, Sweden, Ukraine).
Lectotype (designated here): Sweden, date unknown, E.M. Fries, Scleromyceti Sueciae 59, specimen bound in Shear's Types and Rarities (BPI 799019).
Additional specimens examined: Bulgaria, Sredna Gora Mt (western), Lozenska Planina, along the track to Barbeka Lake locality, near river, 21 May 2005, D. Stoykov MS0310 (BPI 877462A) GenBank EU 254767. Czech Republic, Bohemia, 1913, J.A. Stevenson (BPI 611541); Bohemia, Žlutice (Luditz), Krašov (Krasch), Jun. 1913, R. Steppan (BPI 611543); Germany, Bielatal near Königstein, Apr. 1884, W. Krieger (BPI 611540, lectotype of Gnomonia perversa designated here); Ukraine, Ivano-Frankivsk oblast, Czarny Las, near Rybno, 08 Jun. 1918, F. Petrak (BPI 611542).
Notes: Gnomonia alnea is restricted to species of Alnus in Europe. The synonymy of G. perversa is based on an examination of its type specimen as listed above. The taxonomic synonymy of Gnomonia vleugelii Kleb. listed by Monod (1983) is rejected because Monod's description disagrees with the original one. Klebahn (1918) described the species as having necks up to 1 mm long while necks are absent in G. alnea. Gnomonia alnea is unlike the other species of Gnomoniaceae on Alnus in the lack of an elongated neck. Ophiognomonia alni-viridis, O. ischnostyla and O. trientensis all have elongated necks on the perithecium.
New and revised species of Gnomonia
Gnomonia incrassata Sogonov, sp. nov. MycoBank MB 512162, Figs 2F,G; 3G.
Perithecia 130–310 μm alta × 190–380 μm diam. Rostrum 220–420 μm longum, basi 40–65 μm diam, apice 30–50 μm diam. Ascosporae fusiformes, leviter curvatae, (13.5–)15.5–17(–18.5) × (2.5–)3–3.5(–4) μm, L:l (4–)4.5–5(–6). Differt a speciebus aliis Gnomoniae ascosporis insigniter constrictis et septatis supra medio, et rostris crassis. Holotypus: BPI 611818A.
Anamorph: Unknown.
Etymology: Refers to thickened ascospore cells separated by the septum and thick necks.
Perithecia solitary, without stroma, hypophyllous, in irregular groups, immersed at first, erumpent at maturity, black, oblate to spheroidal when moist, 130–310 μm high × 190–380 μm wide, concave when dry. Necks eccentric or central, slightly sinuous, 220–420 μm long, 40–65 μm wide at base, 30–50 μm wide at apex, compressed when dry. Asci fusiform, (46–)49–51(–61.5) × (9.5–)12.5–13.5(–15) μm (mean = 51.5 × 12.5, SD 5, 1.5, n=10), apical ring 2.5–3.5 μm diam, with eight ascospores arranged irregularly multiseriate. Ascospores fusiform, slightly curved (13.5–) 15.5–17(–18.5) × (2.5–)3–3.5(–4) μm (mean = 16 × 3.5, SD 1, 0.5, n=72), l:w (3.9–)4.5–5.1(–6.2), two-celled, strongly constricted at septum, septum located at (55–)60–65(–75) % (mean = 63, SD 4, n=72) of ascospore length, ends blunt, rounded, each cell with 2 (–3), large and sometimes several smaller guttules; appendages absent or ovoid to subulate to 7 μm long.
Cultures: Not observed.
Habitat: On overwintered fallen leaves of Corylus avellana (Betulaceae).
Distribution: Europe (Czech Republic, France, Germany, Switzerland).
Holotype: Czech Republic, Bohemia, Žlutice, Krašov, May 1913, R. Steppan (BPI 611818A).
Additional specimen examined: France, Petit Saleve, Geneve, 05 Apr. 1851, J. Müller (BPI 596639); Germany, Thüringen, Steiger near Erfurt, 13 May 1905, H. Diedicke Mycotheca Germanica 482 (BPI bound); Switzerland, Calancatal, ob Buseno, 17 Jun. 1989, E. Müller (ZT).
Notes: Some of the specimens here regarded as Gnomonia incrassata were identified as Apiognomonia ostryae by Monod (1983).
Gnomonia monodii Sogonov, sp. nov. MycoBank MB 512163, Figs 2H, I; 3H, I.
Perithecia 180–220 μm high × 270–310 μm diam. Rostrum 320–420 μm longum, basi 40–45 μm diam, apice 35–40 μm diam. Ascosporae fusiformes, rectae vel inaequilaterae, (14–)15–16(–18.5) × (2–)2.5(–3) μm, L:l (5–)5.5–6.5(–7.5). Ad aliis cum rostro eccentrico Gnomoniae speciebus ascosporae magnitudine differt. Holotypus: BPI 877499A.
Anamorph: Unknown.
Etymology: Named after Michel Monod, Lausanne, Switzerland, in recognition of his contribution to the taxonomy of the Gnomoniaceae.
Perithecia solitary, without stroma, hypophyllous, on leaf blade or veins, in loose irregular groups, immersed at first, erumpent, black, oblate or suboblate when moist, concave when dry, 180–220 μm high × 270–310 μm diam. Necks eccentric, straight or slightly curved, 320–420 μm long, 40–45 μm wide at base, 35–40 μm wide at apex. Asci fusiform, (42.5–)45.5–46.5(–51) × 9–11(–13) μm (mean = 46 × 10.5, SD 3, 1.5, n=6), apical ring 2–2.5 μm diam, with eight ascospores arranged irregularly multiseriate. Ascospores fusiform, straight or inequilateral (14–)15–16(–18.5) × (2–)2.5(–3) μm (mean = 15.5 × 2.5, SD 1, 0.2, n=54), l:w (4.9–)5.7–6.4(–7.3) (mean = 6, SD 0.6, n=54), two-celled, septum located at (53–)60–64(–70) % (mean = 62, SD 4, n=54) of ascospore length, not or slightly constricted at septum, ends blunt, rounded, each cell with 2(–3) large, some smaller, guttules; appendages absent or ovoid, cuneiform or subulate to whip-shaped, to 20 μm long.
Habitat: On overwintered leaves of Corylus avellana (Betulaceae).
Distribution: Europe (Denmark, Switzerland).
Holotype: Switzerland, Valais, vicinity of Martigny, on overwintered but still attached leaves, 21 May 2005, M. Monod & M.V. Sogonov MS0335 (BPI 877499A).
Additional specimen examined: Denmark, Jylland, Krabbesholm Skov, on leaves of Corylus avellana, 07 Apr. 1901, J. Lind (BPI 611819); Switzerland, Valais, La Tallaz, vallon de Gueuroz, 05 Jun. 1979, M. Monod, No. 741 (LAU).
Notes: Two of the specimens here regarded as Gnomonia monodii were identified as Apiognomonia ostryae by Monod (1983).
Gnomonia neognomon Sogonov, sp. nov. MycoBank MB 512254, Figs 5A,B; 6A–C; 7A–O.
Fig. 6.
Morphology on natural substrates, asci and ascospores. A–C. Gnomonia neognomon, holotype BPI 877465A. D–F. G. orcispora. D. BPI 877466B. E, F. Holotype BPI 877465C. G, H. G. ostryae. G. BPI 611536. H. BPI 871051. Scale 10 μm.
Fig. 7.
Culture morphology. A–O. Gnomonia neognomon. A–F. CBS 121244. G–L. CBS 121265. M–O. Ex-type CBS 121246. P–X. G. orcispora. P–S. AR 4286. T–X. CBS 121247. A–L, P–S, U–X. Colony habit, 40 d, 23 °C. A, C, E, G, I, K, P, R, U, W. Surface. B, D, F, H, J, L, Q, S, U, X. Reverse. M–O, T. Perithecia, 2/10 °C. M, N, T. 4.5 mo. O. 8 mo. A, B, G, H, P, Q, U, V. PDA. C, D, I, J, N, R, S, W, X. MEA. E, F, K–M, O, T. MYA. Scale: A–L, P–S, U–X. 1 cm. M–O, T. 200 μm.
Perithecia 170–250 μm alta × 220–370 μm diam. Rostrum 400–1075 μm longum, basi 35–52 μm diam, apice 31–40 μm diam. Ascosporae fusiformes, leviter curvatae, (18.5–)20–22.5(–24.5)×(2–)2(–2.5) μm, L:l (8–)9.5–11(–12.5). Ad alteris Gnomoniae speciebus ascosporae longitudine latitudineque differt. Ascosporae longitudo latitudoque similares G. gnomon, sed perithecii rostris longioribus et semper eccentricis differt. Holotypus: BPI 877465A.
Anamorph: Unknown.
Etymology: Refers to the morphological similarity with G. gnomon.
Perithecia solitary, without stroma, hypophyllous, loosely scattered on blades and veins, immersed at first, erumpent at maturity, black, suboblate when moist, 170–250 μm high × 220–370 μm diam, concave when dry. Necks eccentric, straight or slightly sinuous, 400–1075 μm long, 35–52 μm wide at base, 31–40 μm wide at apex. Asci fusiform, (39.5–)41–44.5(–48.5) × (8–)9–11(–12.5) μm (mean = 43.5 × 10, SD 3, 1.5, n=13), apical ring 1.5–3 μm diam, with eight parallel ascospores. Ascospores fusiform, slightly curved, (18.5–)20–22.5(–24.5) × 2–2.5 μm (mean = 21.5 × 2, SD 1.5, 0.2, n=29), l:w (7.9–)9.7–10.8(–12.7) (mean = 10.2, SD 1, n=29), two-celled, slightly constricted at septum, septum located at (45–)49–52(–57) % (mean = 50, SD 3, n=29) of ascospore length, cells tapering, at ends blunt, rounded, each cell with 4–7, guttules, usually one large guttule close to septum; appendages usually 2.5–4 μm long, cuneiform, sometimes absent, sometimes whip-shaped, to 16 μm long.
Cultures: Colonies on PDA attaining 35 mm diam after 40 d at 23 °C, radially furrowed, velvety, greyish orange to brownish orange, with droplets of clear exudate; margin well-defined, wavy; reverse dark brown. Colonies on MEA attaining 60 mm diam after 40 d at 23 °C, flat, pale brown to dark brown, smooth with scant aerial mycelium; margin diffuse, wavy; reverse pale brown to dark brown. Colonies on MYA attaining 40 mm after 40 d at 23 °C, flat or radially furrowed, greyish brown or pale brown to dark brown, velvety, with scant droplets of clear exudate; margin well-defined, even or broadly wavy; reverse dark brown. Cultures incubated at 2/10 °C dark/light regime produce sterile perithecia after 4.5 mo on MEA and MYA but not on PDA. Perithecia remain sterile.
Habitat: On overwintered fallen or hanging leaves of Corylus californica (A. DC.) Rose (Betulaceae).
Distribution: Canada (British Columbia) and U.S.A. (WA).
Holotype: U.S.A., Washington, Mason Co., Potlatch State Park, next to U.S. route 101, on overwintered fallen leaves, 16 May 2006, M.V. Sogonov MS0364 (BPI 877465A, ex-type culture AR 4287 = CBS 121246) GenBank EU254786.
Additional specimens examined: Canada, British Columbia, Vancouver Island, Goldstream Provincial Park, on veins of overwintered but still hanging leaves, 11 May 2006, M.V. Sogonov MS0408b (BPI 877526C, culture AR 4336 = CBS 121265); U.S.A., Washington, Mason Co., Potlatch State Park, on overwintered leaves, 16 May 2006, M.V. Sogonov MS0363 (BPI 877466A, culture AR 4285 = CBS 121244).
Notes: Specimens previously identified as Gnomonia gnomon on Corylus in North America may actually be G. neognomon or G. pendulorum.
Gnomonia orcispora Sogonov, sp. nov. MycoBank MB 512164. Figs 5C–H; 6D–F; 7P–X.
Perithecia 150–280 μm alta × 160–310 μm diam. Rostrum usque ad 350 μm longum, basi 32–42 μm diam, apice 24–29 μm diam. Ascosporae ovales, inaequilaterae, (14–)15.5–17.5(–19.5) × (4.5–) 5–5.5(–6) μm. Ad plerumque Gnomoniae speciebus ascosporae longitudine latitudineque differt. Ascosporae longitudo latitudoque similares G. ostryae, sed ascosporis in extremitatibus hilo terminatis et guttulis majoribus in ascosporae cellulis differt. Holotypus: BPI 877465C.
Anamorph: Unknown.
Etymology: Refers to the appearance of spores from Latin orca (barrel-shaped) and spora (spore).
Perithecia solitary, without stroma, hypophyllous, loosely scattered on blades and veins, immersed at first, erumpent at maturity, black, spherical when moist, 150–280 μm high × 160–310 μm diam, concave when dry. Necks marginal, straight or slightly sinuous, absent at ascospore formation, growing at maturity of ascospores, reaching 350 μm long, 32–42 μm wide at base, 24–29 μm wide at apex. Asci oval to fusiform, (53–)58.5–62.5(–69.5) × (19.5–) 20–20.5(–22) μm (mean = 61 × 20.5, SD 5.9, 0.9, n=5), apical ring 5–5.5 μm diam, with eight ascospores with eight ascospores evenly or slightly unevenly parallel. Ascospores oval, inequilateral, (14–)15.5–17.5(–19.5) × (4.5–)5–5.5(–6) μm (mean = 16.5 × 5, SD 1.1, 0.4, n=45), l:w (2.8–)3–3.3(–3.7) (mean = 3.2, SD 0.2, n=45), two-celled, constricted at septum, septum located at (54–)59–63(–66) % (mean = 61, SD 3, n=45) of ascospore length, ends blunt, rounded or somewhat truncated, each cell with two large guttules; appendages navicular, large to 15 μm long, 8 μm wide but very thin and often indistinct, with base surrounded by a pronounced hilum on ascospore wall.
Cultures: Two cultures (CBS 121247 and AR 4286) differ significantly in their morphology. CBS 121247: Colonies on PDA attaining 18 mm diam after 40 d at 23 °C, irregularly furrowed, shortly velvety, dark brown and dark grey to almost black, with whitish submerged mycelium at margin; margin diffuse, irregular; reverse dark brown, almost black with orange-brown to whitish margins. Colonies on MEA attaining 17 mm diam after 40 d at 23 °C, flat, glabrous with scant aerial mycelium, black with dark brown margins; margin clear, slightly wavy; black with dark brown margins. AR 4286: Colonies on PDA attaining 33 mm diam after 40 d at 23 °C, radially furrowed, velvety, whitish to greyish orange, with droplets of clear exudate; margin well-defined, slightly wavy; reverse reddish orange to dark brown; agar stained with orange pigment. Colonies on MEA attaining 15 mm diam after 40 d at 23 °C, slightly radially furrowed, greyish orange overlaid by white short felty aerial mycelium; margin well-defined, slightly wavy; reverse reddish orange. Sterile perithecia observed in CBS 121247 incubated at 2/10 °C dark/light regime for 4.5 mo on MYA.
Habitat: On overwintered fallen or still hanging leaves of Corylus californica (Betulaceae).
Distribution: Canada (British Columbia) and U.S.A. (WA).
Holotype: U.S.A., Washington, Mason Co., Potlatch State Park, next to U.S. route 101, on overwintered fallen leaves, 16 May 2006, M.V. Sogonov MS0364b (BPI 877465C, ex-type culture CBS 121247).
Additional specimen examined: Canada, British Columbia, Vancouver Island, Goldstream Provincial Park, on overwintered but still hanging leaves, 11 May 2006, M.V.Sogonov MS0408 (BPI 877526A) GenBank EU254789; U.S.A., Washington, Mason Co., Potlatch State Park, on overwintered fallen leaves, 16 May 2006, M.V. Sogonov MS0363a culture AR 4286 (BPI 877466B).
Notes: Perithecia of Gnomonia orcispora have marginal necks and are thus distinct from other species of Gnomonia on Corylus in North America.
Gnomonia ostryae De Not., Sfer. Ital. cent. 1, fasc. 1: 42. 1863. 5. 8I–L; 6G–H; 8A–H.
≡ Apiognomonia ostryae (De Not.) M. Monod, Beih. Sydowia 9: 50 1983.
= Gnomonia veneta Speg., Michelia 1: 457. 1879.
Perithecia solitary, without stroma, hypophyllous, on leaf blade or veins, in loose irregular groups, immersed at first, erumpent later, black, oblate or suboblate when moist, 130–200 μm high × 170–260 μm diam, concave when dry. Necks eccentric to marginal, slightly curved, 175–290 μm long, 25–45 μm wide at base, 25–45 μm wide at apex. Asci fusiform, 49–57 × 12–16 μm, apical ring 2.9–3.8 μm diam, with eight ascospores arranged irregularly multiseriate to obliquely uniseriate. Ascospores varying from fusiform with both ends gradually tapering although rounded at tips to oblanceolate or obovoid, broadened in upper part, with distal end extensively rounded or smoothly truncated, inequilateral, (12.5–)15–17(–20.5) × (3–)3.5–4.5(–6) μm (mean = 16 × 4, SD 1.5, 0.5, n=90), l:w (3–)3.6–4.1(–4.9) (mean = 3.9, SD 0.4), two-celled, constricted at septum, septum located at (55–)62–65(–71) % (mean = 63, SD 3) of ascospore length, ascospore cells usually with two large or/and several small guttules; appendages subulate 2–5 μm long.
Cultures: Colonies on PDA attaining 30 mm diam after 40 d at 23 °C, radially furrowed, felty orange-white to greyish orange in central part, velvety, dark brownish grey at margin; droplets of clear exudate present all over the colony but more abundant at margin; margin well-defined, crenate; reverse dark brown with pale brown patterns. Colonies on MEA attaining 50 mm diam after 40 d at 23 °C, flat, superficial, with almost no aerial mycelium, greyish orange, with tufts of orange-white aerial mycelium in central part; margins submerged; margin irregular; reverse greyish orange. Colonies on MYA attaining 30 mm diam after 40 d at 23 °C, radially furrowed, short felty orange-grey in central part, velvety, dark brownish grey at margin; droplets of clear exudate present all over the colony but more abundant at margin; margin well-defined, crenate; reverse dark brown with pale brown patterns. No perithecia observed in cultures at 2/10 °C dark/light regime. Cultures on PDA after 8 mo at 2/10°C regime produce amorphous slimy conidiomata. Conidia oval or allantoid, 4.9–7.1 × 1.9–2.7 μm.
Habitat: On overwintered leaves of Ostrya carpinifolia Scop. (Betulaceae).
Distribution: Europe (Bulgaria, Italy, Switzerland).
Specimens examined: Italy, Varone near Riva (Valsesia), Garda-See, May 1900, H. Rehm (BPI 611536). Switzerland, Ticino, Monte San Salvatore, 28 May 2005, M.V. Sogonov MS0204, culture CBS 121242 (BPI 871051) GenBank EU254790.
Notes: The asexual state of Gnomonia ostryae has been placed in Cylindrosporella (Monod, 1983). The varieties of G. ostryae as Apiognomonia ostryae defined by Monod (1983) are herein recognised as distinct species.
Gnomonia pendulorum Sogonov, sp. nov. MycoBank MB 512165, Figs 8I,J; 9A,B; 10A,B.
Fig. 8.
Culture morphology. A–H. Gnomonia ostryae CBS 121242. I, J. G. pendulorum ex-type CBS 121264. K–P. G. rodmanii ex-type CBS 121909. A–F, I–P. Colony habit, 40 d, 23 °C. A, C, E, I, K, M, O. Surface. B, D, F, J, L, N, P. Reverse. G. Conidiomata, 4 mo, 2/10 °C. H. Conidia, 4.5 mo, 2/10 °C. A, B, G, H, K, L. PDA. C, D, M, N. MEA. E, F, I, J, O, P. MYA. Scale: A–F, I–P. 1 cm. G. 200 μm. H. 10 μm.
Fig. 10.
Morphology on natural substrate, asci and ascospores. A, B. Gnomonia pendulorum, holotype BPI 877526B. C, D. G. rodmanii, holotype BPI 878211A. E, F. G. skokomishica, holotype BPI 877465B. Scale 10 μm.
Perithecia 230–280 μm alta × 470–580 μm diam. Rostrum 770–970 μm longum, basi 65–70 μm diam, apice 40–55 μm diam. Ascosporae fusiformes, leviter curvatae, (20–)21–22(–23)×(2.5–)3(–3.5) μm, L:l (6–)7–7.5(–8). Similares G. gnomon, sed ascosporae latioribus et perithecii rostris longioribus differt. Holotypus: BPI 877526B.
Anamorph: Unknown.
Etymology: Refers to the fact that perithecia of this species were found on hanging, overwintered leaves.
Perithecia solitary, without stroma, hypophyllous, mostly on major veins, immersed at first, later erumpent, black, suboblate when moist, 230–280 μm high × 470–580 μm diam, concave when dry. Necks central, straight or slightly sinuous, 770–970 μm long, 65–70 μm wide at base, 40–55 μm wide at apex. Asci fusiform, 55–60 × 10–11 μm, apical ring 2.5 μm diam, with eight ascospores arranged unevenly parallel or irregularly multiseriate. Ascospores fusiform, slightly curved (20–)21–22(–23) × (2.5–)3(–3.5) μm (mean = 21.5 × 3, SD 0.5, 0.3, n=16), l:w (6–)6.8–7.4(–8.2) (mean = 7.1, SD 0.6), two-celled, constricted at septum, septum located at (47–)49–51 % (mean = 49, SD 1.5) of ascospore length, ends blunt, rounded, each cell with 2–10 guttules, usually one large guttule close to septum; appendages whip-shaped, 7–32 μm long.
Cultures: Not observed on PDA and MEA. Colonies on MYA attaining 30 mm diam after 40 d at 23 °C, thick, radially furrowed, short felty, orange-white; margin well-defined, even; reverse orange to brownish orange. Neither perithecia nor conidiomata observed in cultures at 2/10 °C after 4.5 mo.
Habitat: On overwintered dead but still attached leaves of Corylus californica (Betulaceae).
Distribution: Canada (British Columbia).
Holotype: Canada, British Columbia, Vancouver Island, Goldstream Provincial Park, 11 May 2006, M.V. Sogonov MS0408a (BPI 877526B; ex-type culture CBS 121264) GenBank EU254791.
Notes: Among species of Gnomonia on Corylus, G. pendulorum in North America is similar to Gnomonia gnomon in Europe in having a central neck on the perithecia.
Gnomonia rodmanii Sogonov, sp. nov. MycoBank MB 512166, Figs 8K–P; 9C,D; 10C,D.
Perithecia 180–260 μm alta × 220–330 μm diam. Rostrum 300–530 μm longum, basi 41–48 μm diam, apice 22–28 μm diam. Ascosporae fusiformes, rectae, interdum leviter curvatae, (13.5–)15–16.5(–18.5) × 2–2.5 μm, L:l (6–)6.5–7.5(–8.5). Abd aliis cum rostro eccentrico Gnomoniae speciebus ascosporae longitudine latitudineque differt. Ascosporae longitudo latitudoque similares G. monodii et G. virginianae, sed septi positione et hospitis genere differt. Holotypus: BPI 878211A.
Anamorph: Unknown.
Etymology: Named after Dr. James Rodman in recognition of his promotion of taxonomic research. The holotype specimen of this species was collected during the 6th meeting of National Science Foundation Partnership for Enhancing Expertise in Taxonomy program that was initiated by Dr. Rodman.
Perithecia solitary, without stroma, hypophyllous, mostly next to midrib but some scattered over leaf blade, immersed at first, erumpent at maturity, black, suboblate when moist, 180–260 μm high × 220–330 μm diam, concave when dry. Necks eccentric, slightly sinuous, 300–530 μm long, 41–48 μm wide at base, 22–28 μm wide at apex. Asci fusiform with narrow tapering stipe, (40.5–)43.5–46(–50.5) × (11–)13–14(–14.5) μm (mean = 45 × 13, SD 2.4, 1.1, n=11), apical ring 2–2.5 μm diam, with eight ascospores arranged irregularly multiseriate to obliquely uniseriate. Ascospores fusiform, straight, occasionally slightly curved, (13.5–)15–16.5(–18.5) × 2–2.5 μm (mean = 16 × 2.5, SD 1.1, 0.2, n=64), l:w (5.8–)6.6–7.3(–8.3) (mean = 6.9, SD 0.5), two-celled, not constricted at septum, septum located at (41–)48–50(–55) % (mean = 49, SD 2) of ascospore length, cells tapering, at ends blunt, rounded, each cell with 2–4 guttules, usually one large guttule close to septum; appendages whip-shaped, to 30 μm long.
Cultures: Colonies on PDA attaining 85 mm diam after 40 d at 23 °C, flat, loosely velvety, granular from young perithecia in central part, dark brown; margin diffuse; reverse black. Colonies on MEA attaining 90 mm after 40 d at 23 °C, flat, dark brown to black, smooth with scant appressed aerial mycelium, with black dots of submerged young perithecia; margin diffuse; reverse black. Colonies on MYA attaining 85 mm diam after 40 d at 23 °C, shallowly and irregularly furrowed in centre, flat at marginal parts, velvety, greyish dark brown to black; margin diffuse; reverse dark brown to black.
Habitat: On overwintered fallen leaves of Carpinus caroliniana Walter (Betulaceae).
Distribution: U.S.A. (GA).
Holotype: U.S.A., Georgia, Clarke Co., Athens, Botanical Garden, Orange Trail, 28 Mar. 2007, M.V. Sogonov MS0535 (BPI 878211A, ex-type culture CBS 121909).
Notes: Among species of Gnomonia on Carpinus, G. rodmanii has an elongated, eccentric neck on each perithecium unlike G. arnstaeditensis in which an elongated neck is lacking. In addition, the necks of G. rodmanii lack a collar unlike G. amoena that has a central neck surrounded by a collar and is unlike G. carpinicola, a species having two or three necks on each perithecium.
Gnomonia skokomishica Sogonov, sp. nov. MycoBank MB512167, Figs 9E–G; 10E,F; 11A–L.
Fig. 11.
Gnomonia skokomishica, culture morphology. A–L. Ex-type CBS 121245. M–Q. CBS 121398. A–F. Colony habit, 14 d, 23 °C. G–L. Colony habit, 40 d, 23 °C. A, C, E, G, I, K. Surface. B, D, F, J, L. Reverse. M–P. Perithecia, 2/10 °C. M. 40 d. N. 40 d. O. 4.5 mo. P. 8 mo, ×1/2. Q. Asci and ascospores, 8 mo, 2/10 °C. A, B, G, H, M. PDA. C, D, I, J. MEA. E, F, K, L, N–Q. MYA. Scale: A–L. 1 cm. M–O. 200 μm. P. 500 μm. Q. 10 μm.
Perithecia 190–340 μm alta × 260–480 μm diam. Rostrum 610–930 μm longum, basi (34.5–)36.5–42(–44) μm diam, apice (22.5–)29.5–34.5(–37) μm diam. Ascosporae fusiformes, rectae, (16.5–)17.5–19(–20.5) × 2–2.5(–3) μm, L:l (7–)7.5–8.5(–9.5). Similares G. gnomon, sed ascosporae latioribus et ascosporarum septis leviter sed semper supra medio differt. Holotypus: BPI 877465B.
Anamorph: Unknown.
Etymology: Refers to the Native American tribe near whose reservation the holotype was collected.
Perithecia solitary, without stroma, hypophyllous, scattered loosely on midribs or in dense groups on petioles, immersed at first, erumpent, partly erumpent or immersed at maturity, black, oblate when moist, 190–340 μm high × 260–480 μm diam, concave when dry. Necks central, curved, 610–930 μm long (mean = 722, SD 134, n=6), (34.5–)36.5–42(–44) μm wide at base, (22.5–)29.5–34.5(–37) μm wide at apex. Asci fusiform with tapering stipe, (38.5–)40–46.5(–51) × (8.5–)10–10.5(–11.5) μm (mean = 43.5 × 10, SD 4, 1, n=12), apical ring 2–2.5 μm diam, with eight ascospores arranged evenly or unevenly parallel. Ascospores fusiform, straight (16.5–)17.5–19(–20.5) × 2–2.5(–3) μm (mean = 18.5 × 2.5, SD 1, 0.1, n=37), l:w (6.8–)7.5–8.5(–9.6) (mean = 8, SD 0.7, n=37), two-celled, constricted at septum, septum located at (51)54–58(–62) % (mean = 56, SD 3) of ascospore length, each cell with 2–3 large or one large and 3–5, smaller guttules, largest guttule close to septum; appendages whip-shaped to 27 μm long.
Cultures: Colonies on PDA and MYA at 23 °C initially forming velvety pale brown or brown surface, later overgrown by orange-grey to whitish (PDA) or snow-white (MYA) short felty mycelium expanding from the centre of colonies, attaining 80 mm diam after 40 d; margin well-defined, low, wavy or irregularly serrate; reverse greyish brown to dark brown. Colonies on MEA attaining 90 mm diam after 40 d at 23 °C, flat, thin, greyish orange for the most part, with dark brown areas or almost entirely dark brown, thin, smooth with scant appressed aerial mycelium, with black dots of submerged young perithecia; margin diffuse; reverse of same colours as front side. Cultures at 2/10 °C produce sterile perithecia after 4.5 mo on PDA and MYA, asci and ascospores were observed only on MYA in CBS 121245 after 8 mo at 2/10 C. Perithecia produced in culture often with 2–3, rarely 4 necks; necks often hairy. Ascospores similar to those on natural substrates.
Habitat: On overwintered fallen leaves of Corylus californica (Betulaceae).
Distribution: U.S.A. (WA).
Holotype: U.S.A., Washington, Mason Co., Potlatch State Park, next to U.S. route 101, on overwintered fallen leaves, 16 May 2006, M.V. Sogonov MS0364a (BPI 877465B, ex-type culture CBS 121245).
Additional specimen examined: U.S.A., Washington, King Co., Tiger Mountain State Forest, near U.S. route 18, 16 May 2006, M.V. Sogonov MS0393 (BPI 877535, culture CBS 121398) GenBank EU254798.
Notes: This new taxon is similar to Gnomonia californica described by Monod (1983) as compared with the description and illustrations only. According to Monod the type specimen of G. californica is deposited in TRTC from where it was requested but was not found. Gnomonia skokomishica lacks a dark red collar as described for G. californica by Monod (1983) as “collum rubrum rostrum cingens ex substrato”, “le substrat forme une couronne rougeâtre, non pulvérulente à la sortie du bec”. In addition G. skokomishica has a submedian ascospore septum while G. californica is described as having a median septum (“ascosporae... septatae dimidio longitudinis”, “ascospores... cloisonnées à mi-longueur”) (Monod 1983).
Gnomonia virginianae Sogonov, sp. nov. MycoBank MB 512168. Figs 12A–C; 13A–C; 14A–F.
Fig. 13.
Morphology on natural substrate, asci and ascospores. A–C. Gnomonia viriginianae. A. BPI 877565A. B. Holotype BPI 844264. C. BPI 878209. D, E. G. amoena, BPI 877469. F, G. G. pseudoamoena. F. BPI 877518. G. Stirpes Cryptogamae Vogeso-Rhenanae 1251, BPI bound. Scale 10 μm.
Fig. 14.
Culture morphology. A–F. Gnomonia virginianae ex-type CBS 121913. G–L. G. amoena CBS 121262. M–T. G. pseudoamoena CBS 121261. A–R. Colony habit. A–N, Q, R. 40 d, 23 °C. O, P. 14 d, 23 °C. S. A, C, E, G, I, K, M, O, Q. Surface. B, D, F, H, J, L, N, P, R. Reverse. Perithecium, 4.5 mo, 2/10 °C. T. Asci, 4.5 mo, 2/10 °C. A, B, G, H, M, N. PDA. C, D, I, J, O, P, S, T. MEA. E, F, K, L, Q, R. MYA. Scale: A–R. 1 cm. S. 200 μm. T. 10 μm.
Perithecia 115–160 μm high × 150–260 μm diam. Rostrum 200–430 μm longum, basi 21–33 μm diam, apice 18–28 μm diam. Ascosporae fusiformes, leviter curvatae, (12–)13–14(–14.5) × (2–) 2.5–3 μm, L:l (4–)4.5–5(–6). Ad plerumque cum rostro eccentrico Gnomoniae speciebus ascosporae longitudine latitudineque differt. Ascosporae longitudo latitudoque similares G. romanii, sed septi positione et hospitis genere differt. Holotypus: BPI 844264.
Anamorph: Unknown.
Etymology: Refers to species epithet of the host plant.
Perithecia hypophyllous, on leaf blade or veins, in loose irregular groups, immersed at first, erumpent later, black, suboblate to oblate spheroidal when moist, 115–160 μm high × 150–260 μm diam, concave when dry. Necks marginal, straight or slightly sinuous, 200–430 μm long, 21–33 μm wide at base, 18–28 μm wide at apex. Asci fusiform with narrow tapering stipe, (33–)34.5–42(–47.5) × (8–)9.5–11(–14) μm (mean = 38.5 × 10.5, SD 5, 1.5, n=14), apical ring 2–2.5 μm diam, with eight ascospores arranged unevenly parallel, less commonly in evenly parallel or irregularly multiseriate. Ascospores fusiform, slightly curved (12–)13–14(–14.5) × (2–)2.5–3 μm (mean = 13.5 × 2.5, SD 0.5, 0.2, n=47), l:w (4.2–)4.7–5.3(–5.8) (mean = 5, SD 0.4), two-celled, slightly constricted at septum, septum located at (55–)59–62(–66) % (mean = 61.5, SD 3) of ascospore length, cells tapering, at ends blunt, rounded, distal cell usually with two, basal cell with two or three large guttules, several smaller guttules may be present in each cell; appendages whip-shaped, 7–45 μm long.
Cultures: Colonies on PDA attaining 30 mm diam after 40 d at 23 °C, with knobbed surface, velvety, greyish orange, brownish orange, pale brown or brownish grey, with droplets of clear exudate; margin well-defined, irregular; reverse pale brown to dark brown. Colonies on MEA attaining 30 mm diam after 40 d at 23 °C, radially wrinkled and furrowed, velvety, orange-grey to brownish orange; margins with two brims differing from the rest of colony, 1–2 mm wide each, a dark brown velvety inner brim and greyish orange waxy outer brim; margin well-defined, even; reverse greyish orange and orange-grey to brownish orange and brown. Colonies on MYA attaining 22 mm diam after 40 d at 23 °C, densely radially wrinkled, velvety to felty, orange-white with pale brown 3 mm brim; margin well-defined, even; reverse greyish brown to pale brown. Neither perithecia nor conidiomata observed in cultures on PDA, MEA and MYA after 8 mo at 2/10 C. Scarce fertile perithecia were observed in cultures on CMA after 5 mo at 15 °C in darkness.
Habitat: On overwintered fallen leaves of Ostrya virginiana (Betulaceae).
Distribution: U.S.A. (AR, GA, MD, NC).
Holotype: U.S.A., Maryland, Montgomery Co., Chesapeake & Ohio Canal National Historic Park, 10 April 2004, M.V. Sogonov MS0016 (BPI 844264, ex type culture CBS 121913).
Additional specimens examined: U.S.A., Arkansas, Ozark Natural Science Center, 21 June 2006, L.N. Vasilyeva (BPI 877565A) GenBank EU254804; Georgia, Clarke Co., Athens, Oconee Forest Park, 30 March 2007, M.V. Sogonov MS0532 (BPI 878209) GenBank EU254805; Georgia, Clarke Co., Athens, Botanical Garden, Orange Trail, 28 March 2007, M.V. Sogonov MS0534 (BPI 878210); Maryland, Mongomery Co., Chesapeake & Ohio Canal National Historic Park, 10 April 2004, M.V. Sogonov MS0023 (BPI 877564) GenBank EU254802; North Carolina, Wake Co., Raleigh, William B. Umstead State Park, hardwood forest, 03 April 2005, M.V. Sogonov MS0169 (BPI 877566) GenBank EU254803; same collecting data MS0170 (BPI 877422).
Notes: Gnomonia virginianae is the only species of Gnomonia on Ostrya in North America and is distinct from the two European species on Ostrya, G. arnestadtiensis and G. ostryae. Gnomonia arnstadtiensis has perithecia lacking an elongated neck while ascospores of G. ostryae are longer and wider than those of G. virginianae. Many specimens of Gnomonia virginianae were identified as Apiognomonia ostryae variété 2 by Monod (1983).
Additional species accepted in Gnomonia
Gnomonia amoena (Nees: Fr.) Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 232. 1863. Figs 12D–F; 13D,E; 14G–L.
≡ Sphaeria amoena Nees: Fr., Nova Acta Acad. Caes. Leop.-Carol. Nat. Cur. 9: 257. 1818: Syst. Mycol. 2: 517. 1823.
≡ Gnomoniella amoena (Nees: Fr.) Sacc., Syll. Fung. 1: 414. 1882.
Habitat: On dead leaves and petioles of Carpinus betulus and C. caroliniana (Betulaceae).
Distribution: Europe (Germany, Switzerland) and U.S.A. (TN).
Specimens examined: Switzerland, Lausanne, on overwintered leaves of Carpinus betulus, 25 May 2005, coll. M.V. Sogonov (BPI 877468) GenBank EU254770; Vaud, near hospital St-Loup, on overwintered leaves of Carpinus betulus, 24 May 2005, EU254769.
Notes: Gnomonia amoena is unique among species of Gnomonia on Carpinus in having a distinct collar around the central neck. Monod (1983) provides a detailed description of this species. Barr (1978) erred in reporting this species on Corylus based on specimens later identified by Monod (1983) as G. californica M. Monod. Specimens of G. amoena on Liquidambar styraciflua L. are reidentified as Ambarignomonia petiolorum.
Gnomonia arnstadtiensis Auersw. in Gonn. & Rabenh., Mycol. Europ. 5/6: 22. 1869. Fig. 12G.
≡ Plagiostoma arnstadtiense (Auersw.) M. Monod, Beih. Sydowia 9: 143. 1983.
= Hypospila rehmii Sacc., Syll. Fung. 2: 189. 1883 fide Monod 1983.
≡ Gnomonina rehmii (Sacc.) Höhn., Ann. Mycol. 16: 52. 1918.
≡ Plagiostoma rehmii (Sacc.) Arx, Antonie van Leeuwenhoek 17: 264. 1951.
Habitat: On fallen leaves of Carpinus betulus and Ostrya carpinifolia (Betulaceae).
Distribution: Europe (Bulgaria, Germany, Switzerland, “Yugoslavia”)
Specimens examined: Bulgaria, Mt Belasitsa, nearby Belasitsa challet, alt. ca. 750 m, on overwintered leaves of Ostrya carpinifolia, 30 Apr 2005, Stoykov, D. (BPI 877470) GenBank EU254772; Balkan foot-hill region, Golyama Zhelyazna village, Promkombinat locality, on overwintered leaves of Ostrya carpinifolia, 4 Apr 2005, Stoykov, D. (BPI 877472B) GenBank EU254773.
Notes: Gnomonia arnstadtiensis is unique among species of Gnomonia on Carpinus and Ostrya in lacking an elongated neck on the perithecium.
Gnomonia carpinicola (Höhn.) Sogonov, comb. nov. MycoBank MB 512169. Basionym: Plagiostomella carpinicola Höhn., Ann. Mycol. 16: 52. 1918.
≡ Apioplagiostoma carpinicola (Höhn.) M.E. Barr, Mycol. Mem. 7: 103. 1978.
= Gnomonia stahlii Kleb., Haupt- und Nebenfruchtformen der Ascomyzeten: 279. 1918 fide Monod 1983.
= Apiospora carpinea Rehm, Ber. naturalist. Ver. Augburg 26: 119. 1881 non Gnomonia carpinea (Fr.) Kleb. 1918 fide Monod 1983.
Habitat: On fallen leaves of Carpinus betulus L. (Betulaceae).
Distribution: Europe (Bulgaria, Germany, Switzerland).
Notes: The perithecia of Gnomonia carpinicola are unusual in often having two or three necks emerging on both sides of the leaf blades. In addition the ascospores have a submedian septum.
Gnomonia pseudoamoena M. Monod, Beih. Sydowia 9: 86. 1983. Figs 12H; 13F,G; 14M–T.
Habitat: On fallen leaves of Corylus avellana and C. californica (Betulaceae).
Distribution: Canada (British Columbia) and Europe (Bulgaria, Germany, Sweden, Switzerland)
Specimens examined: Switzerland, Vaud, near hospital St-Loup, on overwintered leaves of Corylus avellana, 24 May 2005, coll. M.V. Sognov (BPI 877512) GenBank EU254794; Flühli, on overwintered leaves of Corylus avellana, 27 May 2005, coll. M.V. Sogonov (BPI 877513) GenBank EU254793; same as above (BPI 877516) GenBank EU254792.
Notes: Gnomonia pseudoamoena is unique among species of Gnomonia on Corylus in having a distinct collar around the central neck. Stoykov & Denchev (2006) reported this species from Bulgaria as G. amoena.
AMBARIGNOMONIA Sogonov gen. nov. MycoBank MB 512170.
Perithecia solitaria, sine stromate, immerse, in foliis caducis, in sicco concava. Rostri centralia, recti, apice contracti, basi circumcincti collo albo pulveraceo. Ascosporae fusiformes, bicellulares. Holotypus: Ambarignomonia petiolorum (Schwein.: Fr.) Sogonov, comb. nov.
Perithecia solitary, without stroma, on fallen leaves, on petioles and basal parts of major veins of fallen leaves, immersed, black, suboblate when moist, concave when dry, round in top view. Necks central, straight, tapering to their ends, at base surrounded by white powdery collar not soluble in water or 3 % KOH, length 3–4 times greater than perithecial diam. Asci fusiform, with apical ring, with eight spores arranged irregularly fasciculate. Ascospores fusiform, l:w ca. 7, two-celled; appendages ovoid to subulate. Colonies usually slowly to moderately growing, reaching 0.5 cm diam or less in 2 wk at 23 °C dark/light. Colony surface glabrous, sometimes velvety in central part, yellowish brown. Conidiogenous structures or perithecia never formed in culture. Known only from Liquidambar styraciflua (Hamamelidaceae).
Ambarignomonia petiolorum (Schwein.: Fr.) Sogonov, comb. nov. MycoBank MB 512171. Figs 15A–D; 16A–C; 17A–F. Basionym: Sphaeria petiolorum Schwein.: Fr., Schr. Naturf. Ges. Leipzig 1: 41. 1822: Syst. Mycol. 2: 517. 1823.
Fig. 16.
Morphology on natural substrate, asci and ascospores. Ambarignomonia petiolorum. A. Epitype BPI 844274. B, C. BPI 877509. Scale 10 μm.
Fig. 17.
Ambarignomonia petiolorum ex-type CBS 121227, culture morphology, colony habit. A, C, E. Surface. B, D, F. Reverse. A, B. PDA. C, D. MEA. E, F. MYA. Scale 1 cm.
≡ Gnomonia petiolorum (Schwein.: Fr.) Cooke, Grevillea 7: 54. 1878.
≡ Gnomoniella amoena var. petiolorum (Schwein.: Fr.) Sacc., Syll. Fung. 1: 414. 1882.
Perithecia solitary, without stroma, evenly and densely distributed over petioles of fallen leaves, sometimes also on basal parts of major veins, immersed, black, suboblate when moist, 180–220 μm high × 300–420 μm diam, concave when dry, round in top view. Necks central, straight, 200–700 μm long, 65–80 μm wide at base, 35–45 μm wide at apex. Asci fusiform with narrow tapering stipe, (24–)27.5–29.5(–33.5) × (6.5–)8–9.5(–11) μm (mean = 29 × 9, SD 2.5, 1.2, n=25), apical ring 1.3–2 μm diam, with eight ascospores arranged irregularly multiseriate or unevenly parallel. Ascospores fusiform, slightly curved (9–)11–12.5(–15) × 1.5–2 μm (mean = 11.5 × 2, SD 1.5, 0.2, n=44), l:w (5–)6.1–6.9(–8) (mean = 6.5, SD 0.6), two-celled, not constricted at septum located at (36–)45–50(–54) % (mean = 47, SD 4) of ascospore length, each cell with several (usually 3–5) guttules, usually one large guttule close to septum; appendages subulate 1–4 μm long, rarely whip-like to 10 μm long.
Cultures: Colonies on PDA attaining 40 mm diam after 40 d at 23 °C, branched, at margin with patches of aerial mycelium connected with each other, in centre surface knobbed, aerial mycelium velvety to felty, orange-white, orange-grey or pale orange; margin well-defined, irregular, in some parts submerged; reverse dark brown; agar stained with orange-brown pigment. Colonies on MEA extremely slow growing, forming a brim of only 1–2 mm around original inoculum after 40 d at 23 °C, velvety, orange-grey; margin clear; reverse dark brown. Colonies on MYA attaining 10 mm diam after 40 d at 23 °C, mostly submerged, radially stringy, pale brown, with an entire spot of orange-grey felty aerial mycelium, orange-white with pale brown 3 mm brim; margin diffuse; reverse pale brown to dark brown. Neither perithecia nor conidiomata observed in cultures on PDA, MEA and MYA after 4.5 mo at 2/10 C.
Habitat: On petioles and basal vein parts of fallen leaves of Liquidambar styraciflua (Hamamelidaceae).
Distribution: U.S.A. (AL, DE, GA, LA, MD, MS, NC, NJ, SC, TN, TX, VA).
Lectotype designated here: U.S.A., North Carolina, L.D. Schweinitz (Shear Study Collection Types & Rarities Series I, BPI 800519). Epitype designated here: U.S.A., Virginia, Accomack Co., southern part of Assateague Island, 09 May 2004, M.V. Sogonov MS0037 (BPI 844274, ex-type culture CBS 121227).
Additional specimens examined: U.S.A., Alabama, Septent, 2 June 1854, T.M. Peters, D.A. Watt Herb. 569, Missouri Bot. Gard. Herb. 60514 (BPI 611281); Delaware, Newark, 25 May 1908, H.S. Jackson 2159 (BPI 611282); Georgia, Darien, date unknown, H.W. Ravenel, Fungi Americani Exsiccati 374 (BPI 611547); Louisiana, 04 March 1896, collector unknown, Herbarium of Rev.A.B. Langlois (BPI 596288); Louisiana, 22 Dec. 1888, and others, collector unknown, Herbarium of Rev. A.B. Langlois (BPI 596290); Louisiana, 23 March 1893, collector unknown, Herbarium of Rev. A.B. Langlois (BPI 596291); Louisiana, St. Martinsville, March 1890, Rev. A.B. Langlois, Ellis & Everhart 2543 (BPI 596292); same location, 11 Nov. 1890, Rev. A.B. Langlois, Herb. S.M. Tracy (BPI 596295); Louisiana, near St. Martinsville, 05 Nov. 1899, Rev. A.B. Langlois, Flora Ludoviciana (BPI 596294); Maryland, St. Mary's, 29 May 1921, C.L. Shear (BPI 611546); Maryland, Suitland, date unknown, H.H. Whetzel, R.W. Davidson et al. (BPI 611280); Maryland, Prince George Co., Greenbelt, Greenbelt Park, 21 Apr. 2004, M.V. Sogonov MS0028 (BPI 877511); Maryland, Prince George's Co., Beltsville, BARC, forest near B011A, 06 Apr. 2005, M.V. Sogonov MS0178 (BPI 877510); Mississippi, Pike Co., Percy Quinn State Park, 27 Feb. 2006, M.V. Sogonov MS0331 (BPI 877509); New Jersey, Monmouth Co., Turkey Swamp Wildlife Management Area, 08 Jan. 1995, G. Bills (BPI 802807); South Carolina, date unknown, collector unknown, Michener Collection, Shear Study Collection Types & Rarities Series I (BPI 800520); ibid. (BPI 800521); Tennessee, Great Smoky Mountains National Park, Cosby Cabin, 13 May 2002, L.N. Vasilyeva (BPI 843530, culture CBS 116866); Tennessee, Great Smoky Mountains National Park, Tremont, 04 June 2002, L.N. Vasilyeva (BPI 863545); Texas, Houston, year 1869, H.W. Ravenel (BPI 596289).
Additional sequence from GenBank: U.S.A., North Carolina, Durham, Duke Forest, litter, date unknown, H.E. O'Brien, J.L. Parrent, J.A. Jackson, J.-M. Moncalvo, R. Vilgalys, nrDNA ITS1–5.8S–ITS2 (AY969703).
Notes: Ambarignomonia petiolorum is an extremely common species on petioles of overwintered leaves of sweetgum (Liquidambar styraciflua) in eastern North America. This species is easily identified by the whitish powdery collar surrounding the central neck.
APIOGNOMONIA Höhn., Ber. Deutsch. Bot. Ges. 35: 635. 1917.
Type species: Apiognomonia veneta (Sacc. & Speg.) Höhn.
Perithecia solitary, on fallen leaves, epiphyllous or on petioles, or on dead but still attached pedicels of trees and shrubs, or on dead parts of herbaceous plants, otherwise in groups of 5–15 perithecia with or without weakly developed stroma on twigs of trees and shrubs. Perithecia black, remaining immersed in substrate, oblate to spherical when moist, convex, sometimes with some irregular dents when dry, round in top view, with one neck. Necks central to marginal, never truly lateral, mostly 0.5–2 perithecial diam long but varying from almost lacking to length 3–4 times perithecial diam. Asci fusiform, with an apical ring, with eight spores arranged irregularly multiseriate or obliquely uniseriate. Ascospores mostly two-celled, rarely one-celled, oval to fusiform, l:w 2.5–6; ends mostly rounded, rarely pointed; appendages mostly absent or less commonly present, subulate, navicular or whip-shaped, to 30 μm long.
Cultures: Colonies fast growing, often reaching edges of 90 mm Petri plates after 2 wk at 23 °C l/d or at least 60–70 mm diam. Colonies floccose or lanose all over surface or in lobes or concentric rings intermingled with glabrous or velvety areas. Colonies whitish, grey, orange-grey, brownish orange, dark brown, olive. Some species produce fertile perithecia in culture after 5–6 mo at 2/10 °C l/d. Conidiomata often produced after 2–4 wk at 23 °C l/d.
Hosts: In diverse taxonomic groups (Aceraceae, Ericaceae, Euphorbiaceae, Fagaceae, Geraniaceae, Hippocastanaceae, Oleaceae, Platanaceae, Polygonaceae, Salicaceae). Most species are specific to one host species or genus, however, a few species are on a diverse range of plants.
Apiognomonia veneta (Sacc. & Speg.) Höhn., Ann. Mycol. 16: 51. 1918.
≡ Laestadia veneta Sacc. & Speg., Michelia 1: 351. 1878.
≡ Apiospora veneta (Sacc. & Speg.) Kleb., Z. Pflanzenkrankh. 7: 258. 1902.
[≡ Gnomonia veneta (Sacc. & Speg.) Kleb., Jahrb. Wiss. Bot. 41: 533. 1905 non Speg. 1879.]
≡ Gnomonia platani Kleb., Vortr. Ges. Bot. 1: 28. 1914.
Habitat: On overwintered leaves of Platanus occidentalis and P. orientalis (Platanaceae), rarely, Tilia sp. (Tiliaceae).
Distribution: Widespread in temperate regions including Canada (British Columbia), Europe (Bulgaria, France, Germany, Switzerland), New Zealand, and U.S.A. (MD, TN).
Notes: A detailed description of Apiognomonia veneta and its distinction from the closely related A. errabunda is provided by Sogonov et. al. (2007).
Additional species of Apiognomonia
The following taxa are accepted species of Apiognomonia based on their inclusion in multigene and ITS phylogeny.
Apiognomonia acerina (Starbäck) M. Monod, Beih. Sydowia 9: 63. 1983. Figs 18A–C; 19A,B.
Fig. 18.
Morphology on natural substrate, perithecia. A–C. Apiognomonia acerina. A, C. BPI 877677. B. BPI 877678. D, E. A. hystrix. D. Monod 464, LAU bound. E. BPI 877692. A, B, D, E. Intact air-dry perithecia on stems, twigs, leaves and petioles. C. Extracted and rehydrated perithecia. Scale 200 μm.
Fig. 19.
Morphology on natural substrate, asci and ascospores. A, B. Apiognomonia acerina, BPI 877677. C–E. A. hystrix. C. BPI 877697. D. BPI 877698. E. BPI 877692. Scale 10 μm.
≡ Gnomonia acerina Starbäck, Bih. K. Svenska Vetensk Akad. Handl. 14, Afd. 3, n. 5: 17. 1889.
Habitat: On fallen leaves of Acer opalus Mill., A. platanoides L., and A. pseudoplatanus L. (Aceraceae).
Distribution: Europe (Bulgaria, Germany, Switzerland)
Specimen examined: Switzerland, Valais, Salvan/Les Marécottes, Pont du Triège, 1300 m a.s.l., on overwintered leaves of Acer pseudoplatanus, May 2005, coll. M. Monod (BPI 877677) GenBank EU254990.
Notes: Among species of Gnomoniaceae on Acer, Apiognomonia acerina is unique in having ascospores that are wider than 3.5 μm and having a central neck. Barr (1978) considered the basionym Gnomonia acerina to be synonym of Apioplagiostoma aceriferum (Cooke) M.E. Barr but Monod (1983) recognised this name as a distinct species and provides detailed descriptions of both species. ITS sequences of Apiognomonia acerina and Apioplagiostoma aceriferum show these species to be distinct. Fig. 1 shows Apioplagiostoma acerifum in Pleuroceras, a genus not detailed in this study.
Apiognomonia borealis (J. Schröt.) M. Monod, Beih. Sydowia 9: 61. 1983. Figs 20A–F.
Fig. 20.
Apiognomonia borealis CBS 799.79, culture morphology, colony habit, 40 d, 23 °C. A, C, E. Surface. B, D, F. Reverse. A, B. PDA. C, D. MEA. E, F. MYA. Scale 1 cm.
≡ Gnomonia borealis J. Schröt., Jahresber. Schles. Ges. Vaterl. Cult. 65: 275. 1888.
= Gnomonia pratensis Svrček, Česká Mykol. 28: 219. 1974 fide Monod 1983.
Habitat: On overwintered leaves and stems of Geranium pratense L., G. sanguineum L., and G. sylvaticum L. (Geraniaceae).
Distribution: Europe (Czech Republic, Finland, Norway, Sweden, Switzerland).
Specimen examined: Switzerland, Vaud, col du Mollendruz, on Geranium sylvaticum, Monod 274, CBS 796.79, GenBank EU254999.
Notes: Apiognomonia borealis is distinguished from other species of Gnomoniaceae on Geranium by the ascospores having a supramedium septum. Monod (1983) provides a detailed description of this species.
Apiognomonia errabunda (Roberge) Höhn., Ann. Mycol. 16: 51. 1918.
≡ Sphaeria errabunda Roberge in Desm., Ann. Sci. nat. Bot., ser. 3 10: 355. 1848.
≡ Gnomonia errabunda (Roberge) Auersw. in Gonn. & Rabenh., Mycol. Eur. 5/6, p. 25. 1869.
More synonyms are listed in Sogonov et al. (2007).
Habitat: On overwintered leaves primarily of hardwoods trees in the Fagaceae, Salicaceae, and Tiliaceae as well as other woody and herbaceous plants including Chamerion angustifolium (L.) Holub, Rhus glabra L. and Sorbus aria (L.) Crantz as listed in Sogonov et al. (2007). Distribution: Widespread in northern temperate regions as listed in Sogonov et al. (2007).
Notes: Apiognomonia errabunda is the cause of oak anthracnose (Sinclair & Lyon 2005 as A. veneta). A detailed description including the differences between A. errabunda and the closely related A. veneta are provided by Sogonov et. al. (2007). Like many species in the Gnomoniaceae, A. errabunda is frequently isolated as an endophyte in woody plants.
Apiognomonia hystrix (Tode: Fr.) Sogonov, comb. nov. MycoBank MB512172. Figs 18D,E; 19C–E. Basionym: Sphaeria hystrix Tode, Fungi Meckl. 2: 53. 1791.
≡ Diatrype hystrix Tode: Fr., Sum. Veg. Scand.: 383. 1846.
≡ Mamiana hystrix (Tode: Fr.) De Not., Comment. Soc. Crittog. Ital. 1: 43. 1863.
≡ Cryptospora hystrix (Tode: Fr.) Fuckel, Jb. Nassau Ver. Naturk. 23–24: 194. 1870.
≡ Diaporthe hystrix (Tode: Fr.) Sacc., Fung. Ven. 4: 6. 1873.
≡ Chorostate hystrix (Tode: Fr.) Traverso, Fl. Ital. Crypt. 2: 212. 1906.
≡ Cryptodiaporthe hystrix (Tode: Fr.) Petr., Ann. Mycol. 19: 119. 1921.
= Valsa longirostris Tul. & C. Tul., Sel. Fung. Carp. 2: 200. 1863 fide Wehmeyer 1933.
≡ Diaporthe longirostris (Tul. & C. Tul.) Sacc., Syll. Fung. 1: 609. 1882.
= Diaporthe mamiania Sacc., Syll. Fung. 1: 609. 1882 fide Wehmeyer 1933.
≡ Chorostate mamiania (Sacc.) Traverso, Fl. Ital. Crypt. 2: 201. 1906.
= Sphaeria cerastis Riess, Hedwigia 1: 24. 1853.
≡ Gnomonia cerastis (Riess) Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 233. 1863.
= Sphaeria petioli Fuckel, Jahrb. Ver. Naturkunde Herzogthume Nassau 15: 68. 1860 fide Monod 1983.
≡ Gnomonia petioli (Fuckel) Cooke in Rabenh., Fungi Europaei exsiccati 927. 1866.
= Gnomoniella brunaudiana Pass. in Brunaud, Champ. Saint. 5, 1, 1891 fide Monod 1983.
= Gnomoniella hippocastani Brunaud, Bull. Soc. Bot. Fr. 36: 336. 1889 fide Monod 1983.
= Gnomonia aesculi Oudem., Beih. bot. Centralbl. 11: 527. 1902 fide Monod 1983.
= Gnomonia cerastis Riess f. nedundinis Karakulin, Morbi plant. Script. Lect. Phyto. Hort. Bot. U.S.S.R. 14: 81. 1925 fide Monod 1983.
Habitat: On overwintered leaves, twigs and branches of Acer pseudoplatanus (Aceraceae) and various other hardwoods.
Distribution: Europe (Austria, Bulgaria, Czech Republic, Germany, Switzerland).
Specimens examined: Canada, Ontario, Etobicoke, Dean West Park, on overwintered leaves of Acer saccharum, 1 Apr 2005, coll. M.V. Sogonov (BPI 877696) GenBank EU255019. Russia, Novgorod province, Kholm, Arboretum (Dendropark), on overwintered leaves of Fraxinus excelsior, 7 Jun 2005, coll. M.V. Sogonov (BPI 877698) GenBank255022. The Netherlands, Baarn, Garen Eemnersserweg 90, on leaf spot of seedling of Acer negundo, Oct 1997, coll. H.A. van der Aa 12406 (CBS 100566) GenBank EU255032.
Notes: Apiognomonia hystrix as Cryptodiaporthe hystrix with its synonym Gnomonia cerastis and its relationship to members of the Gnomoniaceae was recognised by Monod (1983) who provides a detailed description of this species as C. hystrix.
GNOMONIOPSIS Berl., Icon. Fung. 1: 93. 1894.
Type species: Gnomoniopsis chamaemori (Fr.) Berl.
Perithecia solitary or groups up to 5, without stroma, on fallen, overwintered leaves and twigs of trees and shrubs, usually epiphyllous or on petioles, on dead parts of herbaceous plants.
Perithecia black, remaining immersed, spheroidal to suboblate when moist, convex or irregularly dented when dry, round in top view, with one neck. Necks central to lateral, slightly curved to curved, shorter or slightly longer than perithecial diam, sometimes almost absent. Asci oval to fusiform, with an apical ring, with eight spores arranged mostly biseriate or obliquely uniseriate, less commonly irregularly multiseriate. Ascospores two-celled, oval to fusiform, l:w 1.5–5, usually somewhat ovoid or pyriform; ends rounded; appendages absent.
Cultures: Colonies fast growing, often reaching egdes of 90 mm Petri plates after 2 wk at 23 °C l/d, or moderately growing 40–60 mm diam. Colony surface usually glabrous, velvety or lanose. Colonies whitish, grey, dark brown, olive. Some species produce fertile perithecia in culture after 5–6 mo at 2/10 Cl/d; rarely fertile perithecia produced after 2–4 wk at 23 °C l/d. Conidiomata produced by most species after 2–4 wk at 23 °C l/d.
Hosts: In diverse taxonomic groups (Ericaceae, Fagaceae, Pinaceae, Rosaceae). Most species are specific at the host species or genus level; however, a few species occur on a diverse range of plant hosts.
Type species of Gnomoniopsis
Gnomoniopsis chamaemori (Fr.) Berl., Icon. Fung. 1: 93. 1894. Figs 21A–C; 22A–C; 23A–F.
Fig. 21.
Morphology on natural substrate, perithecia. A–C. Gnomoniopsis chamaemori, BPI 877438. D–G. G. clavulata. D–F. Epitype BPI 877443. G. Lectotype BPI 611339. H–K. G. paraclavulata, holotype BPI 877448. L, M. G. fructicola. L. BPI 877446. M. BPI 877454. N, O. G. racemula, BPI 871003. P. Q. G. cf. chamaemori. P. BPI 877452A. Q. BPI 877456. A, B, D, H, I, L–N, P, Q. Intact air-dry perithecia on leaves and stems. C, E–G, J, K, O. Extracted and rehydrated perithecia. Scale 200 μm.
Fig. 22.
Morphology on natural substrate, asci and ascospores. A–C. Gnomoniopsis chamaemori, neotype BPI 877438. D–F. G. clavulata. D. BPI 877440. E. BPI 877442. F. BPI 877441. G, H. G. paraclavulata. G. Holotype BPI 877448. H. BPI 877449. I. G. fructicola, BPI 877446. J. G. racemula, BPI 871003. K, L. G. cf. chamaemori. K. BPI 877455. L. BPI 877456. Scale 10 μm.
Fig. 23.
Gnomoniopsis chamaemori CBS 803.79, culture morphology, colony habit. A, C, E. Surface. B, D, F. Reverse. A, B. PDA. C, D. MEA. E, F. MYA. Scale 1 cm.
≡ Sphaeria chamaemori Fr., Syst. Mycol. 2: 519. 1823.
Perithecia hypophyllous or less commonly epiphyllous, immersed, subepidermal, mostly on veins, black, oblate spheroidal when moist, 150–220 μm high × 210–320 μm diam, convex when dry. Necks central, straight, 60–85 μm long, diam 45–65 μm. Asci fusiform or obclavate, 28–40 × 7–9 μm, apical ring 1.5–2 μm diam, with eight ascospores arranged irregularly multiseriate or obliquely uniseriate. Ascospores fusiform, straight to slightly curved, (10–)10.5–11.5(–13) × (2–)2.5(–3) μm (mean = 11 × 2.5, SD 0.5, 0.3, n=28), l:w (3.3–)4–4.8(–5.5) (mean = 4.4, SD 0.6), two-celled, not constricted or slightly constricted at septum, septum located at (27–)33–38(–46) % (mean = 36, SD 5) of ascospore length, ends blunt, rounded, each cell with two large guttules, or with one big guttule and several small ones, or several indistinct guttules; appendages absent.
Cultures: Colonies on PDA usually attaining 90 mm after 40 d at 23 °C, flat, velutinous to shortly woolly, dark brown in centre, gradually lightening to pale reddish grey at margin; margin diffuse; reverse of almost same colours as surface. Colonies on MEA attaining 90 mm after 40 d at 23 °C, flat, almost glabrous, overlaid by loose and short woolly-like mycelium, pale reddish grey with indistinct pale orange-brown patterns in centre; margin diffuse; reverse of almost same colours as surface. Colonies on MYA attaining 90 mm after 40 d at 23 °C, flat, dark brown in centre, brown with some shades of red, becoming pale reddish grey at margin, overlaid by whitish woolly, aerial mycelium; margin diffuse; reverse of same colours as surface.
Habitat: On overwintered leaves of Rubus chamaemorus L. (Rosaceae).
Distribution: Europe (Finland, Russia).
Specimen examined: Russia, Novgorod oblast, Kholm raion, Rdeyskiy Zapovednik, vicinity of Fryunino, on overwintered leaves of Rubus chamaemorus, 11 Jun. 2005, M.V. Sogonov & D.A. Maykov MS0273 (BPI 877438) GenBank EU254809. Additional culture examined: Finland, Oulanka, on overwintered leaves of Rubus chamaemorus, 10 Jul. 1977, M. Monod, No. 345 (culture CBS 803.79). Specimens examined of G. aff. chamaemori: Bulgaria, Sredna Gora Mt (western), Lozenska Planina, above Pancharevo lake, near the track from `Stenata' locality to VEC Kokaljane, on overwintered stems of Agrimonia eupatoria, 21 May 2005, coll. D. Stoykov (BPI 877452A) GenBank EU254812. Russia, Novgorod province, Kholm, on dead petioles of Potentilla anserina, 7 Jun 2005, coll. M.V. Sogonov (BPI 877455), GenBank EU254811; Tver' province, Toropets district, v. Bubonitsy, on overwintered stems of Potentilla canescens, 14 Jun 2005, coll. M.V. Sogonov (BPI 877456) GenBank254810.
Notes: Monod (1983) provided a detailed description of this species as Gnomonia chamaemori.
New and revised species of Gnomoniopsis
Gnomoniopsis clavulata (Ellis) Sogonov, comb. nov. MycoBank MB 512173. Figs 21D–G; 22D–F; 24A–W; 25A–I. Basionym: Gnomonia clavulata Ellis, Amer. Nat. 17: 318. 1883.
Fig. 24.
Gnomoniopsis clavulata, culture morphology. A–F. CBS 121231. G–L. CBS 121257. M–R. Ex-type CBS 121259. S–W. G. cf. clavulata, CBS 119028. A–T, V, W. Colony habit, 40 d, 23 °C. A, C, E, G, I, K, M, O, Q, S, V. Surface. B, D, F, H, J, L, N, P, R, T, W. Reverse. U. Conidiomata, 17 d, 23 °C. A, B, G, H, M, N, S, T. PDA. C, D, I, J, O, P, U. MEA. E, F, K, L, Q, R, V, W. MYA. Scale: A–T, V, W. 1 cm. U. 1 mm.
Fig. 25.
Gnomoniopsis clavulata, culture morphology, 40 d, 23 °C. A–F. Ex-type CBS 121259. G–I. CBS 121257. A–C. Conidiomata. D–I. Conidia. A, D, G. PDA. B, E, H. MEA. C, F, I. MYA. Scale: A–C. 1 mm. D–I. 10 μm.
≡ Didymiella clavulata (Ellis) Sacc., Syll. Fung. 9: 666. 1891.
≡ Cercidospora clavulata (Ellis) Kuntze, Rev. Gen. Pl. 3 (2): 453. 1898.
Perithecia solitary, without stroma, hypophyllous, scarce, mostly in upper and marginal parts of leaf blades, spheroidal when moist, 110–150 μm high × 120–140 μm wide, convex when dry. Necks central, slightly flexuous, (158–)160–166(–169) μm long (mean = 163, SD 8, n=2), (37–)37.5–39(–39.5) μm wide at base, (34.5–)36–39(–40.5) μm wide at apex. Asci fusiform to cylindrical, (28–)33.5–41.5(–47) × (6.5–)7–10(–11) μm (mean = 38 × 8.5, SD 5.5, 1.5, n=34), apical ring 1.5–2.5 μm diam, with eight ascospores biseriate or obliquely uniseriate. Ascospores pyriform, inequilateral, (5–)8.5–9.5(–11) × (2–)3.5–4(–5.5) μm (mean = 9 × 4, SD 1, 0.5, n=149), l:w (1.8–)2.2–2.4(–3) (mean = 2.3, SD 0.2), two-celled, constricted at septum; septum located at (29–)37–43(–49) % (mean = 40, SD 4) of ascospore length; ends broadly rounded, distal cell with 2–3 and basal cell with 1–2 small guttules, sometimes both cells without guttules; appendages absent.
Cultures: Colonies on PDA usually attaining 90 mm after 40 d at 23 °C, in CBS 121257 slower growing, attaining 50 mm, flat, velutinous to woolly, pale brown to brown, overlaid by scant or abundant orange-grey, greyish orange or brownish orange slimy conidial mass drops, ex-epitype culture CBS 121259 with pale grey woolly mycelium; margin clear, even to lobate; reverse pale brown or orange-brown to dark brown; agar stained by yellow soluble pigment in some strains; conidia oval to oblong, sometimes slightly obovoid, straight or curved, allantoid or sigmoid, (5–)6–6.5(–8) × (2–)2.5–3(–4) μm (mean = 6.5 × 3, SD 0.5, 0.3, n=285), l:w (1.4–)2.1–2.6(–3.7) (mean = 2.4, SD 0.4, n=285). Colonies on MEA attaining 90 mm after 40 d at 23 °C, flat, thin, semitransparent, loosely woolly, colourless or whitish, with areas of pale orange, brown or dark brown, with numerous orange-grey or greyish orange slimy conidial mass drops; margin diffuse; reverse of same colours as surface; conidia oval to oblong, sometimes slightly obovoid, straight or slightly curved (4.5–)6–7(–8) × (2–)2.5–3(–3.5) μm (mean = 6.5 × 2.5, SD 0.5, 0.3, n=315), l:w (1.4–)2.2–2.7(–3.8) (mean = 2.5, SD 0.4). Colonies on MYA attaining 90 mm after 40 d at 23 °C, flat, with areas of whitish to greyish orange felty aerial mycelium and areas of brownish yellow, pale brown, brown or brownish grey woolly, partly fasciculate mycelium; orange-grey or greyish orange slimy conidial mass drops; margin clear, even or irregular; reverse greyish orange, orange-brown, greyish brown or dark brown; conidia oval to oblong, sometimes slightly obovoid, straight or slightly curved (4.5–)6–6.5(–8) × (2–)2.5–3(–3.5) μm (mean = 6 × 3, SD 0.6, 0.4, n=151), l:w (1.4–)2–2.5(–3.5) (mean = 2.2, SD 0.4). No perithecia observed in cultures at 2/10 °C after 8 mo.
Habitat: On overwintered leaves of Quercus spp. (Fagaceae).
Distribution: U.S.A. (MD, NC, NJ, TN, VA).
Lectotype designated here: U.S.A., New Jersey, Newfield, on Quercus nigra, May 1884, J.B. Ellis, North American Fungi 1685 (lectotype BPI 611339; isotype BPI bound).
Epitype designated here: U.S.A., Maryland, Prince George's Co., Riverdale, Anacostia River Park, on Quercus marilandica, 12 Jun. 2006, M.V. Sogonov MS0401 (BPI 877443, ex-epitype culture CBS 121259) GenBank EU254820.
Additional specimens examined: U.S.A., Maryland, Prince George's Co., Beltsville, Beltsville Agricultural Research Center, near building 011A, on Q. falcata, 06 Apr. 2005, M.V. Sogonov MS0181 (BPI 877441); same location, Q. rubra, 29 Jun. 2005, M.V. Sogonov MS0206 (BPI 877444); same location, Q. prinus, 19 May 2006, M.V. Sogonov MS0371 (BPI 877477); same location, Q. rubra, 19 May 2006, M.V. Sogonov MS0434 (BPI 877522); same location, Q. falcata, 08 Jun. 2006, M.V. Sogonov MS0397 (BPI 877439, culture CBS 121255) GenBank EU254818; North Carolina, Wake Co., Raleigh, Carl Alwin Schenk memorial forest, on Q. ilicifolia, 03 Apr. 2005, M.V. Sogonov MS0161 (BPI 877442, culture CBS 121239) GenBank EU254816; Tennessee, Sevier Co., Greenbrier, University of Tennessee field station, Conley Huskey Way, on Q. falcata, 25 May 2004, M.V. Sogonov MS0399 (BPI 877440, culture CBS 121257) GenBank EU254819; Virginia, Albermarle Co., Charlottesville, University of Virginia campus, between Edgement Road and U.S. route 29 BYP, on Q. prinus, 02 Mar. 2005, M.V. Sogonov MS0139 (BPI 871056, culture CBS 121231) GenBank EU254815.
Additional cultures examined: U.S.A., Maryland, Prince George's Co., Patuxent Wildlife Research Center, on Quercus rubra, S. Cohen (R153 = AR 4123 = CBS 121911); same data, (R154 = AR 4124) GenBank EU254814.
Additional culture G. cf. clavulata: Switzerland, isol. from Fagus sylvatica, AR 4183 = CBS 119028 (BPI 871052) GenBank EU254817.
Notes: Gnomoniopsis clavulata is common on overwintered leaves of oak (Quercus spp.) in eastern North America and was frequently isolated as an endophyte from Quercus rubra (Cohen, 1999; 2004) mistakenly identified as Discula umbrinella. Gnomoniopsis clavulata and G. paraclavulata are distinct from most species of Gnomoniaceae on Quercus in having ascospores with a submedian septum. The ascospores of G. clavulata are larger than those of G. paraclavulata.
Gnomoniopsis paraclavulata Sogonov, sp. nov. MycoBank MB 512174. Figs 21H–K; 22G,H; 26A–M.
Fig. 26.
Gnomoniopsis paraclavulata, culture morphology. A–M. Ex-type CBS 121263. N–S. G. cf. paraclavulata CBS 121269. A–F, N–S. Colony habit, 40 d, 23 °C. A, C, E, N, P, R. Surface. B, D, F, O, Q, S. Reverse. G–J. Conidiomata, 40 d, 23 °C. K–M. Conidia, 40 d, 23 °C. A, B, G, K, N, O. PDA. C, D, H, I, L, P, Q. MEA. E, F, J, M, R, S. MYA. Scale: A–F, N–S. 1 cm. G–J. 1 mm. K–M. 10 μm.
Perithecia (139–)149–170(–180) μm alta × (156–)188–231(–241) μm diam. Rostrum (157–)180–21)180–210(–216) μm longum, basi (37.3–)38.9–41(–41.5)–41.5) μm diam, apice (40.8–)41.1–42.9(–44.3) μm diam. Ascosporae pyriformes, inaequilaterae (8–)9–10(–11) × (3–)3.5–4 μm, L:l (2.1–)2.4–2.8(–3.6). Similis to G. clavulatae, sed ascosporarum Longitudo/latitudo ratione leviter majore et septi positione inferiore differt. Holotypus: BPI 877448.
Etymology: Refers to similarity and affinity with G. clavulata.
Perithecia solitary, without stroma, hypophyllous, scarce, mostly in upper and marginal parts of leaf blades, spheroidal when moist, (139–)149–170(–180) μm high × (156–)188–231(–241) μm diam (mean = 159 × 206, SD 20, 44, n=3), convex when dry. Necks central, slightly flexuous, (157–)180–210(–216) μm long (mean = 192, SD 31, n=3), (37–)39–41(–42) μm wide at base, 41–43(–45) μm wide at apex. Asci fusiform to cylindrical, (38–)45–48(–51.5) × 7–8.5(–10.5) μm (mean = 46 × 8, SD 4, 1.5, n=7), apical ring 2–2.5 μm diam, with eight ascospores biseriate or obliquely uniseriate. Ascospores pyriform, inequilateral, (8)9–10(–11) × (3–)3.5–4 μm (mean = 9.5 × 3.5, SD 0.5, 0.3, n=24), l:w (2.1–)2.4–2.8(–3.6) (mean = 2.6, SD 0.3), two-celled, constricted at septum; septum located at (25–)31–37(–44) % (mean = 34, SD 4) of ascospore length; distal and basal cells usually with correspondingly 1–5 and 0–2 small guttules; appendages absent.
Cultures: Colonies on PDA usually attaining 90 mm after 40 d at 23 °C, flat, with pale red to greyish orange, smooth to shortly woolly and whitish to reddish white woolly areas, overlaid by abundant pale orange to orange-grey slimy conidial masses; margin submerged, irregular; reverse with areas of yellow-grey, pale orange and brownish orange; conidia oval to oblong, sometimes slightly obovoid, straight or slightly curved (6–)7.5–8(–9.5) × (2–)3–3(–3.5) μm (mean = 7.5 × 3, SD 0.5, 0.3, n=108), l:w (1.6–)2.4–2.9(–4.2) (mean = 2.6, SD 0.4). Colonies on MEA attaining 90 mm after 40 d at 23 °C, flat, thin, semitransparent, colourless with brown centre and scattered flocks of white aerial mycelium, with black conidiomata which, at maturity, produce orange-grey slimy conidial mass drops; margin diffuse; reverse greyish orange to orange-grey; conidia oval to oblong or obovoid, straight or slightly curved (6.5–)7.5–8.5(–9.5) × (3–)3–3.5 μm (mean = 8 × 3.5, SD 0.5, 0.2, n=75), l:w (2–)2.3–2.6(–3.2) (mean = 2.5, SD 0.2). Colonies on MYA attaining 90 mm after 40 d at 23 °C, flat, whitish to orange-white felty, with small areas of shorter brownish grey woolly, partly fasciculate mycelium; moderate number of orange-grey or greyish orange slimy conidial mass; margin irregular, partly submerged; reverse greyish orange, orange-brown, greyish brown or brown, conidia obovoid to oblong, straight or slightly curved (6–)7–7.5(–8.5) × (2.5–)3(–3.5) μm (mean = 7 × 3, SD 0.5, 0.2, n=60), l:w (1.6–)2.3–2.6(–3.4) (mean = 2.4, SD 0.3). No perithecia observed in cultures at 2/10 °C after 8 mo.
Habitat: On overwintered leaves of Quercus alba L. (Fagaceae).
Distribution: U.S.A. (MD, TN).
Holotype: U.S.A., Tennessee, Sevier Co., Greenbrier, University of Tennessee field station, Conley Huskey Way, 22 May 2004, M.V. Sogonov MS0406 (BPI 877448, ex-type culture CBS121263)
Additional specimens examined: U.S.A., Maryland, Prince George's Co., Beltsville, Beltsville Agricultural Research Center, near building 011A, 14 Feb. 2005, M.V. Sogonov MS0127 (BPI 877450) GenBank EU 254837; same location, 20 Mar. 2005, M.V. Sogonov MS0152 (BPI 877449) GenBank EU 254838.
Additional cultures examined: U.S.A., Maryland, Prince George's Co., Patuxent Wild Life Research Center, S. Cohen W623 = AR 4125; same data W633 = AR 4126, GenBank EU254835; same data (W645 = AR 4127 = CBS 123202).
Notes: Gnomoniopsis clavulata and G. paraclavulata are distinct from most species of Gnomoniaceae on Quercus in having ascospores with submedian septum. The ascospores of G. clavulata are larger than those of G. paraclavulata.
Additional species accepted in Gnomoniopsis
Gnomoniopsis comari (Karst.) Sogonov, comb. nov. MycoBank MB 512175. Basionym: Gnomonia comari Karst., Mycol. Fenn. 2: 122. 1873.
≡ Gnomoniella comari (Karst.) Sacc., Syll. Fung. 1: 415. 1882.
Habitat: On overwintered leaves of Comarum palustre L. (Rosaceae).
Distribution: Europe (Finland, Germany, Switzerland)
Specimen examined: Finland, on Comarum palustre, Monod 366, CBS 806.79, GenBank EU254821; Monod 353, CBS 807.79, GenBank EU 254822.
Notes: The concept of Gnomoniopsis comari is conceived here in a much narrower sense than by Monod (1983), thus the numerous taxonomic synonyms listed by Monod (1983) are not included. The multigene phylogeny presented here suggests that G. comari is distinct from G. fructicola (Fig. 1).
Gnomoniopsis fructicola (Arnaud) Sogonov, comb. nov. MycoBank MB 512176. Figs 21L,M; 22I; 27A–N. Basionym: Gnomonia fragariae f. fructicola Arnaud, Traité de Pathol. Veg. p. 1558. 1931.
Fig. 27.
Culture morphology. A–N. Gnomoniopsis fructicola CBS 121226. O–S. G. macounii CBS 121468. T–Y. G. racemula CBS 121469. A–F, O, P, R–Y. Colony habit, 40 d, 23 °C. A, C, E, O, R, T, V, X. Surface. B, D, F, P, Q, U, W, Y. Reverse. G. Perithecia and coniodiomata, 40 d, 23 °C. I. Perithecia immersed in agar, reverse view, 40 d, 23 °C. J. Coniodiomata, 40 d, 23 °C. K, L. Asci and ascospores, 40 d, 23 °C. M. Conidia, 40 d, 23 °C. N. Conidia, 40 d, 2/10 °C. Q. Conidia, 4.5 mo, 2/10 °C. A, B, G, K, M–P, T, U. PDA. C, D, I, L,Q, V, W. MEA. E, F, J, R, S, X, Y. MYA. Scale: A–F, O, P, R–Y. 1 cm. G–J. 1 mm. K–N, Q. 10 μm.
≡ Gnomonia fructicola (Arnaud) Fall., Can. J. Bot. 29: 309. 1951.
Habitat: On overwintered leaves and fruits of Fragaria spp. (Rosaceae), occasionally pathogenic on fruits causing strawberry stem-end rot. The causal organism has often been referred to as Gnomonia comari, now considered Gnomoniopsis comari.
Distribution: Canada (British Columbia), Europe (Belgium, France) and U.S.A. (MD, NY).
Specimens examined: Belgium, on Fragaria sp., CBS 255.61, GenBank EU254828. Canada, Ontario, on Fragaria sp., CBS 275.51, GenBank EU254829. France, on Fragaria sp. coll. G. Arnaud, CBS 208.34, GenBank EU254826. U.S.A., New York, Sullivan Co., Roscoe, area around Campbell Inn, on dead petioles of Fragaria sp., Jul 2005, coll. M.V. Sogonov (BPI 877446) GenBank EU254830.
Specimens examined G. cf. fructicola: Russia, Novgorod province, Kholm, valley of Lovat' river, on dead petioles of Geum rivale, 10 Jun 2005, coll. M.V. Sogonov (BPI 877454) GenBank EU254832.
Notes: Considerable confusion has existed among the species of Gnomoniopsis on Fragaria. Gnomonipsis fructicola is herein recognised to be distinct from G. comari. “Gnomonia” fragariae Kleb. causes another disease of strawberry in Europe called leaf blotch, root rot and petiole blight (Maas 1998, Moročko et al. 2006). Moročko & Fatehi (2007) determined that “Gnomonia” fragariae belongs outside of the Gnomoniaceae in the Sydowiellaceae.
Gnomoniopsis macounii (Dearn.) Sogonov, comb. nov. MycoBank MB 512177. Figs 27O–S. Basionym: Diaporthe macounii Dearn., Mycologia 8:100. 1916.
≡ Cryptodiaporthe macounii (Dearn.) Wehm., The Genus Diaporthe: 191. 1933.
Habitat: On overwintered branches of Spiraea douglasii Hook. var. menziesii (Hook.) C. Presl and Spiraea sp. (Rosaceae).
Distribution: Canada (British Columbia) and U.S.A. (NH, NY).
Notes: Barr (1978) and Wehmeyer (1933) provide a detailed description of G. macounii as C. macounii.
Gnomoniopsis racemula (Cooke & Peck) Sogonov, comb. nov. MycoBank MB 512178. Figs 21N,O; 22J; 27T–Y. Basionym: Sphaeria racemula Cooke & Peck in Peck, Ann. Rep. New York State Museum 26: 87. 1874
≡ Diaporthe racemula (Cooke & Peck) Sacc., Syll. Fung. 1: 691. 1882.
≡ Ditopellopsis racemula (Cooke & Peck) M.E. Barr, Mycol. Mem. 7: 91. 1978.
Habitat: On overwintered stalks of Chamerion angustifolium (Onagraceae).
Distribution: Canada (British Columbia) and U.S.A. (ME, MN, NY, OR).
Notes: Gnomoniopsis racemula is unusual in this genus in having perithecia in groups of 3–9 that occur on fibrous overwintered stalks. Barr (1978) provided a detailed description of G. racemula as Ditopellopsis racemula.
Gnomoniopsis tormentillae (Lind) Sogonov, comb. nov. MycoBank MB 512179. Basionym: Gnomoniella tormentillae Lind, Bot. Tidsskr. 41: 217. 1931.
≡ Plagiostoma tormentillae (Lind) Bolay, Ber. Schweiz. Bot. Ges. 81: 436. 1971.
Habitat: On overwintered petioles, veins and stalks of Potentilla canadensis L. and P. erecta (L.) Raeusch (Rosaceae).
Distribution: Europe (Switzerland) and U.S.A. (MA).
Notes: Both Barr (1978) and Monod (1983) provide a detailed description of Gnomoniopsis tormentillae as P. tormentillae. The perithecial neck of this species is marginal.
OPHIOGNOMONIA (Sacc.) Sacc., Syll. Fung. 14: 613. 1899. Lectotype species designated by Höhnel (1919): Ophiognomonia melanostyla (DC.: Fr.) Berl.
≡ Gnomoniella subgenus Ophiognomonia Sacc., Syll. Fung. 1: 419. 1882.
Perithecia solitary, without stroma, on underside of leaf blade, petioles or rachises, occasionally on upper side of blade of overwintered fallen leaves, rarely on dead but attached pedicels, and on dead stems of herbaceous plants. Perithecia dark brown to black, remaining immersed or becoming partly erumpent at maturity, oblate when moist, convex or irregularly shrunk when dry, in some species, part of perithecia may be concave, round in top view, with one neck. Neck central to eccentric, rarely marginal, never truly lateral, mostly length 2.5–5 perithecial diam, in some species shorter, down to length of one perithecial diam. Asci oval to almost filiform, with an apical ring, with eight spores per ascus arranged mostly unevenly parallel, also irregularly multiseriate or obliquely uniseriate, occasionally evenly parallel. Ascospores mostly two-celled, rarely one-celled, oval to filiform, l:w 2.5–25; ends rounded, with or without appendages, may vary within species.
Cultures: Colonies growing at a moderate rate, reaching 1–6 cm diam in 2 wk at 23 °C l/d, in some strains reach edges of 90 mm Petri plates in 2 wk on PDA. Colony surface leathery to coarsely farinose or velvety, in some species with floccose areas. Colonies mostly whitish, yellow, greyish yellow, pale orange, olive-brown. Some species produce fertile perithecia in culture after 5–6 mo at 2/10 °C l/d, rarely sterile perithecia formed within one month at 23 °C l/d. Conidiomata in cultures formed by a few species but then not requiring long-term cultivation at low temperatures.
Hosts: Mostly on Fagales (Betulaceae, Fagaceae, Juglandaceae), a few species on Lauraceae, Rosaceae, Salicaceae, and Tiliaceae. Individual fungal species are host-specific at genus or, less commonly, at family level.
Type species of Ophiognomonia
Ophiognomonia melanostyla (DC.: Fr.) Berl., Icon. Fung. 2: 146. 1899. Figs 28A–C; 29A–C.
Fig. 28.
Morphology on natural substrates, perithecia. A–C. Ophiognomonia melanostyla. A. Lectotype G 00053951. B. Epitype BPI 877610. C. BPI 877611. D–F. O. balsamiferae, holotype BPI 877606. G, H. O. pseudoclavulata. G. BPI 877615B. H. BPI 877631. A, D, F, G. Intact air-dry perithecia on leaves and petioles. B, C, E, H. Extracted and rehydrated perithecia. Scale 200 μm.
Fig. 29.
Morphology on natural substrates, asci and ascospores. A–C. Ophiognomonia melanostyla. A, B. Epitype BPI 877610. C. BPI 877607. D, E. O. balsamiferae, holotype BPI 877606. F–H. O. pseudoclavulata. F. Holotype BPI 844280. G. BPI 877632. H. BPI 877633A. Scale 10 μm.
≡ Sphaeria melanostyla DC.: Fr., Fl. Franç., 5/6: 129. 1815: Syst. Mycol. 2: 517. 1823.
≡ Gnomonia melanostyla (DC.: Fr.) Auersw. in Gonn. & Rabenh., Mycol. Europ. 5/6: 28. 1869.
≡ Gnomoniella melanostyla (DC.: Fr.) Sacc., Syll. Fung. 1: 419. 1882.
≡ Cryptoderis melanostyla (DC.: Fr.) G. Winter, Rabenhorst's Kryptogamen-Flora I, Abt. 2: 592. 1887.
Perithecia solitary, without stroma, hypophyllous, evenly distributed over large areas of leaf blades, sometimes on upper part of petioles, immersed at first, partly erumpent at maturity, oblate to suboblate when moist, 180–220 μm high × 220350 μm diam, convex, occasionally irregularly dented or concave when dry. Necks central or eccentric, usually sinuous, 550–1100 μm long, 30–45 μm wide at base, 25–33 μm wide at apex. Asci narrowly fusiform, 55–65 × 4.5–5 μm, apical ring 1–1.5 μm diam, with eight ascospores evenly or slightly unevenly parallel. Ascospores clavately filiform, slightly sinuous (30–)37–42.5(–44) × 1.5–2 μm (mean = 39 × 1.5, SD 4, 1, n=16), l:w (20.3–)22.4–26.4(–29.3) (mean = 24.6, SD 2.6), two-celled, slightly constricted at septum, septum located at (55–)65–68(–71) % (mean = 66, SD 4) of ascospore length, ends blunt, rounded, basal cell is narrower than distal cell, ca. 1.2 μm wide, each cell with a few small guttules; appendages subulate to whip-shaped, 5–25 μm long.
Habitat: On fallen overwintered leaves of Tilia spp. (Tiliaceae).
Distribution: Europe (Austria, Bulgaria, Czech Republic, Germany, Switzerland, Ukraine), Canada (Ontario) and U.S.A. (NY, PA) Lectotype: Switzerland, vicinity of Geneva, Tilia sp., March, year unknown, M. Chaillet, (G 00053951).
Additional specimens examined: Austria, Sonntagberg, near Rosenau, on Tilia sp., Apr., year unknown, P.P. Strasser (BPI 596571); Czech Republic, Moravia, Hranice na Moravě, Teplice, on Tilia platyphyllos, Apr. 1914, F. Petrak (BPI 596581); same location, on Tilia sp., May 1924, F. Petrak (BPI 596572); Germany, Frankensteinerkopf near Oestrich (Nassau), on Tilia parvifolia, Spr. 1894, L. Fuckel (BPI 596576, BPI 596577); Oestrich (Nassau), on Tilia parvifolia, 1894, L. Fuckel (BPI 596575); Switzerland, Vaud, Lausanne, Parc Bourge, on Tilia cordata, 28 May 2005, M.V. Sogonov MS0333 (BPI 877611) GenBank EU254913; Vaud, St. Cergue, on Tilia cordata, 20 May 2005, M.V. Sogonov MS0197 (BPI 877610) GenBank EU254911; Ukraine, Lviv oblast, Stryi raion, Pidhirtsi, on Tilia platyphyllos, 27 Mar. 1918, F. Petrak (BPI 596579); U.S.A., New York, Heldenburg Mts., on Tilia americana, May, year unknown, C.H. Peck (BPI 596574); New York, Ithaca vicinity, Arnot forest, on Tilia americana, 10 Jul. 2002, L.N. Vasilyeva MS0353 (BPI 877609); New York, Sullivan Co., Roscoe vicinity, area around Campbell Inn, on Tilia americana, Jul. 2005, M.V. Sogonov MS0299 (BPI 877608) GenBank EU254912; Pennsylvania, Franklin Co., Cove Gap, Buchanan Birthplace State Park, on Tilia americana, 05 May 2006, M.V. Sogonov MS0358 (BPI 877607).
Notes: Ophiognomonia melanostyla is relatively common on overwintered leaves of Tilia spp. The very long ascospores over 35 μm long distinguish this species from other species of the Gnomoniaceae on Tilia.
New species of Ophiognomonia
Ophiognomonia balsamiferae Sogonov, sp. nov. MycoBank MB 512180. Figs 28D–F; 29D,E; 30A–N.
Fig. 30.
Culture morphology. A–N. Ophiognomonia balsamiferae ex-type CBS 121266. O–Z. O. pseudoclavulata. O–T. Ex-type CBS 121236. U–Z. CBS 121232. A–L, O–Z. Colony habit. A, C, E, G, I, K, O, Q, S, U, W, Y. Surface. B, D, F, H, J, L, P, R, T, V, X, Z. Reverse. M. Conidiomata. N. Conidia. A–F. 14 d, 23 °C. G–Z. 40 d, 23 °C. A, B, G, H, O, P, U, V. PDA. C, D, I, J, M, N, Q, R, W, X. MEA. E, F, K, L, S, T, Y, Z. MYA. Scale: A–L, O–Z. 1 cm. M. 200 μm. N. 10 μm.
Perithecia 320–390 μm alta × 370–425 μm diam. Rostrum 940–1150 μm longum, basi 73–90 μm diam, apice 42–55 μm diam. Ascosporae fusiformes, leviter curvatae, (15–)18–19(–21) × 2.5–3(–3.5) μm, L:l (4.9–)6.2–7.2(–8.1). Ad aliis Ophiognomoniae speciebus morphologiae characteribus combinatis differt. Singularis Ophiognomoniae species lecta in Salicaceis. Holotypus: BPI 877606.
Etymology: Named after the epithet of the plant host.
Perithecia solitary, without stroma, evenly and densely distributed over petioles, immersed or partly emerging, dark brown, oblate spheroidal when moist, 320–390 μm high × 370–425 μm diam, convex when dry, round from top. Necks central, straight, curved or flexuous when dry, straight when moist, 940–1150 μm long, 73–90 μm wide at base, 42–55 μm wide at apex. Asci fusiform with tapering stipe, 36–70 × 9–17 μm, apical ring 2.5–3 μm diam, with eight ascospores arranged obliquely uniseriate, irregularly multiseriate or unevenly parallel. Ascospores fusiform, slightly curved, (15–)18–19(–21) × 2.5–3(–3.5) μm (mean = 18.5 × 3, SD 1.5, 0.3, n=29), l:w (4.9–)6.2–7.2(–8.1) (mean = 6.7, SD 0.7), two-celled, slightly constricted at septum; septum located at (44–)47–51(–52) % (mean = 48, SD 2) of ascospore length, cells tapering, at ends blunt, rounded or indistinctly truncated, each cell with 3–5, guttules, often one large guttule close to septum; appendages subulate to navicular, 10–15 μm long.
Cultures: Colonies on PDA attaining 90 mm after 40 d at 23 °C, flat, short woolly in centre, velvety with loose tufts at margin, pale brownish grey to brown; margin very irregular; reverse dark brown. Colonies on MEA attaining 90 mm after 40 d at 23 °C, flat, woolly, whitish, with scarce dark brown amorphous conidiomata attaining 500 μm diam; margin diffuse; reverse orange-white to orange and brownish orange; conidia oval, cylindrical, oblanceolate or allantoid, (6–)8.5–10(–13.5) × (1.5–)2–2.5(–3) μm (mean = 9.5 × 2.5, SD 1.5, 0.5, n=61); l:w (1.8–)3.6–4.5(–7.2) (mean = 4.1, SD 0.9). Colonies on MYA almost attaining 90 mm after 40 d at 23 °C, flat, felty to woolly, pale brownish grey to dark brown, with droplets of clear exudate; margin irregular; reverse dark brown. Cultures at 2/10 °C after 4.5 mo produce dark thick-walled conidiomata with conidia on MYA, sterile conidioma-like structures on PDA with sparse conidia after 8 mo, on MEA, sterile after 8 mo.
Distibution: Canada (British Columbia).
Habitat: On overwintered petioles of Populus balsamifera L. (Salicaceae).
Holotype: Canada, British Columbia, Manning Provincial Park, rest area at West Gate, beginning of Engineers Loop Trail, 13 May 2006, M.V. Sogonov, MS0409 (BPI 877606, ex-type culture AR 4320 = CBS 121266).
Notes: Ophiognomonia balsamiferae has a central neck on the perithecium unlike other species of Gnomoniaceae on Populus, specifically Apioplagiostoma populi and Plagiostoma salicella in which the necks are lateral. Gnomonia gnomon is known to occur rarely on Populus but has ascospores that are considerably narrower, 1.5–2 μm wide, than those of O. balsamiferae.
Ophiognomonia pseudoclavulata Sogonov, sp. nov. MycoBank MB 512181. Figs 28G–H; 29F–H; 30O–Z.
Perithecia 170–190 μm alta × 210–280 μm diam. Rostrum 140–250 μm longum, basi 37–54 μm diam, apice 34–44 μm diam. Ascosporae late ellipsoidal vel ellipsoidal, rectae vel leviter inaequilateralae, (6.5–)7.5–8(–9) × (2.5–)3–3.5(–3.6) μm (mean = 7.7 × 3.1, SD 0.6, 0.3, n=112), L:l (2.12–)2.3–2.7(–3.4). Ad aliis Ophiognomoniae speciebus parvis peritheciis et brevibus ascosporis differt. Similis Gnomoniopsis clavulata et G. paraclavulata, sed ascosporis raro clavatis et septis ascosporarum fere semper in medio differt. Holotypus: BPI 844280.
Etymology: Refers to the confusion with Gnomoniopsis clavulata. The oldest specimen of O. pseudoclavulata observed in this study was originally identified as G. clavulata.
Perithecia solitary, without stroma, hypophyllous, mostly on and next to midrib, or scattered randomly over leaf blade, immersed, dark brown, oblate when moist, 170–190 μm high × 210–280 μm diam, convex when dry. Necks curved when dry, slightly curved when moist, 140–250 μm long, 37–54 μm wide at base, 34–44 μm wide at apex. Asci ellipsoidal to fusiform, with tapering stipe, (30–)33.5–41.5(–46) × (6.5–)7–9.5(–11.5) μm (mean = 37.5 × 8.5, SD 4.5, 1.5, n=24), apical ring 2–3 μm diam, with eight ascospores arranged biseriate or irregularly multiseriate. Ascospores broadly ellipsoidal to ellipsoidal, often broader in upper part, straight or slightly inequilateral, (6.5–)7.5–8(–9) × (2.5–)3–3.5 μm (mean = 7.5 × 3, SD 0.5, 0.3, n=112), l:w (2.1–)2.3–2.7(–3.4) (mean = 2.5, SD 0.3), two-celled, not constricted at septum; septum located at (40–)47–51(–58) % (mean = 49, SD 3) of ascospore length; cells broadly rounded at ends, without guttules or with 2–5 small guttules; appendages absent or of different irregular shapes or filiform to 20 μm long.
Cultures: Two cultures (CBS 121232 and CBS 121236) differ significantly in their morphology, especially on MEA and MYA. CBS 121232: Colonies on PDA attaining 60 mm diam after 40 d at 23 °C, with lobate convex concentric zones, felty to woolly, whitish; margin well-defined, lobate; reverse dark brown in central part, then greyish orange, at margin whitish. Colonies on MEA attaining 22 mm diam after 40 d at 23 °C, slightly radially furrowed, velvety to woolly, orange-white; margin well-defined, slightly wavy; reverse dark brown in central part, then greyish orange, at margin orange-white. Colonies on MYA attaining 30 mm diam after 40 d at 23 °C, slightly furrowed, in centre glabrous greyish orange with tufts of white aerial mycelium, then woolly, whitish, at margin felty, whitish; margin well-defined, lobate; reverse dark brown in central part, then greyish orange, at margin orange-white. CBS 121236: Colonies on PDA attaining 65 mm diam after 40 d at 23 °C, flat, felty to woolly, whitish; margin well-defined, serrately lobate; reverse dark brown in central part, then greyish orange, at margin whitish. Colonies on MEA attaining 70 mm diam after 40 d at 23 °C, flat, felty to woolly, white; margin diffuse, broadly indistinctly lobate; reverse orange-white with small greyish orange area in centre. Colonies on MYA attaining 65 mm diam after 40 d at 23 °C, flat, felty, whitish; margin well-defined, even; reverse dark brown in central part, then orange. Neither perithecia nor conidiomata observed in cultures on PDA, MEA and MYA after 8 mo at 2/10 C.
Habitat: On overwintered leaves of Carya spp., primarily C. tomentosa (Lam.) Nutt. (mockernut hickory) (Juglandaceae).
Distribution: U.S.A. (DC, IL, IN, MD, NC, NJ, PA, TN, VA).
Holotype: U.S.A., Pennsylvania, Kennett Square Co., vicinity of Philadelphia, near Phillips mushroom farm, Carya tomentosa, 17 Apr. 2004, M.V. Sogonov MS0025 (BPI 844280, ex-holotype culture AR 4059 = CBS 121236).
Additional specimens examined: U.S.A., District of Columbia, National Arboretum, Carya tomentosa, 03 May 2005, M.V. Sogonov MS0355a (BPI 877615B); Illinois, Hancock Co., St. Mary's Township, Section 27, Apr. 2006, L.C. Castlebury MS0527 (BPI 877520); Indiana, Clark State Forest, Carya sp., 16 Jan 2005, M.V. Sogonov MS113 (BPI 877630) GenBank EU254925; Maryland, Prince Georges Co., Beltsville, B.A.R.C., Entomology Rd., Carya tomentosa, 04 Apr 2004, M.V. Sogonov MS0012a (BPI 877613B) GenBank EU254922; Maryland, Prince George's Co., Beltsville, B.A.R.C., forest near Building 011A, Carya sp., 13 Jan 2005, M.V. Sogonov MS0112 (BPI 877631) GenBank EU254924; North Carolina, Wake Co., Raleigh, Carl Alwin Schenk memorial forest, Carya tomentosa, 03 Apr 2005, M.V. Sogonov MS0165 (BPI 877633A) GenBank EU254927; New Jersey, Newfield, Carya sp., Apr 1891, J.B. Ellis, North American Fungi 3429 (BPI 611620); Tennessee, Blount Co., Great Smoky Mountains National Park, Cades Cove, Anthony Creek Trail, Carya tomentosa, 24 May 2006, M.V. Sogonov MS0488 (BPI 877521A); Tennessee, Blount Co., Great Smoky Mountains National Park, Cades Cove, Carya tomentosa, 24 May 2006, A.Y. Rossman, MS0470 (BPI 877632); Tennessee, Sevier Co., Univ. of Tennessee field station, Conley Huskey Way, Carya tomentosa, 23 May 2006, M.V. Sogonov MS0469 (BPI 877519); same data MS0471a (BPI 877667B); Virginia, Albermarle Co., Charlottesville, University of Virginia Campus, between Edgement Road and highway US 29 BYP, Carya sp., 02 Mar. 2005, M.V. Sogonov MS0140 (BPI 871057, culture CBS 121232) GenBank EU254926.
Notes: Ophiognomonia pseudoclavulata is distinguished from similar species on Carya in the Gnomoniaceae by the relatively short ascospores compared to those of O. micromegala (Ellis & Everh.) Sogonov that are 26–36 × 5.5–10 μm and Gnomonia caryae Wolf that are (16–)22–30(–37) × (2–)3–5.5 μm fide Barr (1978).
Ophiognomonia vasiljevae Sogonov, sp. nov. MycoBank MB 512182. Figs 31A,B; 32A–B; 33A–I.
Fig. 31.
Morphology on natural substrates, perithecia. A, B. Ophiognomonia vasiljevae, holotype BPI 877671. C. O. alni-viridis, BPI 877585A. D. O. gei-montani, BPI 877589. E–G. O. intermedia. E. BPI 877498. F. BPI 877598. G. BPI 877496. A, C–E, G. Intact air-dry perithecia on leaves and stems. B, F. Extracted and rehydrated perithecia. Scale 200 μm.
Fig. 32.
Morphology on natural substrates, asci and ascospores. A, B. Ophiognomonia vasiljevae, holotype BPI 877671. C. O. alni-viridis, BPI 877600. D. O. gei-montani, BPI 877589. E–I. O. intermedia. E. BPI 877498. F. BPI 877598. G. BPI 877602. H. BPI 877488B. I. BPI 877496. Scale 10 μm.
Fig. 33.
Culture morphology. A–I. Ophiognomonia vasiljevae ex-type CBS 121253. J–S. O. cf. alni-viridis CBS 121250. A–F, J–O. Colony habit, 40 d, 23 °C. A, C, E, J, L, N. Surface. B, D, F, K, M, O. Reverse. G–I. Sterile perithecia or perithecium-like bodies, 2/10 °C. P. Young perithecia, 2/10 °C. Q, R. Perithecia, 2/10 °C. G, H, Q, R. 4.5 mo. I. 8 mo. P. 40 d. A, B, G, H, Q¬–S. PDA. C, D, I, P. MEA. E, F. MYA. G, H, Q–S. Scale: A–F, J–O. 1 cm. G–I, P–R. 200 μm. S. 10 μm.
Perithecia 310–390 μm alta × 590–690 μm diam. Rostrum 520–640 μm logum, basi 75–85 μm diam, apice 32–42 μm diam. Ascosporae fusiformes, leviter curvatae (17.5–)18.5–19.5(–21) × (2.5–)3(–3.5) μm (mean = 19 × 3, SD 1, 0.2, n=31), L:l (5.4–)5.9–6.5(–7.4) (mean = 6.3, SD 0.5, n=31). Ad aliis Ophiognomoniae speciebus morphologiae characteribus combinatis differt. Holotypus: BPI 877671.
Etymology: Named after the Russian mycologist Larissa Vasilyeva in recognition of her contribution to the taxonomy of the Gnomoniaceae.
Perithecia solitary, without stroma, in small loose groups on compound leaf rachises, immersed, from upper side not easily detachable from plant tissue, peroblate when moist, 310–390 μm high × 590–690 μm diam, convex when dry. Necks eccentric to lateral, slightly flexuous, 520–640 μm long, 75–85 μm wide at base, 32–42 μm wide at apex. Asci fusiform with narrow stipe, (52.5–) 58.5–64.5(–74) × (10–)11.5–13.5(–17) μm (mean = 62 × 12.5, SD 5.5, 1.7, n=13), apical ring 2.5–3 μm diam, with eight ascospores arranged obliquely uniseriate or irregularly multiseriate. Ascospores fusiform, slightly curved, (17.5–)18.5–19.5(–21) × (2.5–)3(–3.5) μm (mean = 19 × 3, SD 1, 0.2, n=31), l:w (5.4–)5.9–6.5(–7.4) (mean = 6.3, SD 0.5), two-celled, not constricted at septum; septum located at (42–)48–50(–54) % (mean = 49, SD 3) of ascospore length; cells strongly tapering, at ends blunt, rounded, each cell with 2–3 large guttules with largest guttule close to septum or with numerous small guttules; appendages absent.
Cultures: Colonies on PDA attaining 90 mm after 40 d at 23 °C, flat, short, loose, woolly, orange, in some areas overlaid with whitish aerial mycelium; margin diffuse; reverse orange to brownish orange. Colonies on MEA attaining 90 mm after 40 d at 23 °C, flat, short, loose, woolly, brownish orange, in some areas overlaid with whitish aerial mycelium; margin diffuse; reverse brownish orange. Colonies on MYA attaining 80 mm diam after 40 d at 23 °C, flat, woolly, whitish; margins submerged, orange; margin irregular; reverse orange. Cultures at 2/10 °C produce sterile perithecia and dark amorphous bodies on PDA after 4.5 mo, on MEA after 8 mo. No such structures were observed after 8 mo on MYA.
Habitat: On overwintered leaf rachises of Juglans nigra L. (Juglandaceae).
Holotype: U.S.A., Tennessee, Blount Co., Great Smoky Mountains National Park, along loop near the Methodist Church, 24 May 2006, M.V. Sogonov MS0388 (BPI 877671, ex-holotype culture CBS 121253).
Notes: In distinguishing species of Ophiognomonia on Juglans, O. vasiljevae is similar to O. ischnostyla in having a neck longer than 250 μm but, unlike O. leptostyla, which has a neck less than 250 μm long. Ascospores of O. vasiljevae are generally greater than 17.5 μm long while those of O. ischnostyla are less than 17.5 μm.
Additional species accepted in Ophiognomonia
Ophiognomonia alni-viridis (Podlahova & Svrček) Sogonov, comb. nov. MycoBank MB 512215. Figs 31C; 32C; 33J–S. Basionym: Gnomonia alni-viridis Podlahova & Svrček, Česká Mykol. 24: 129. 1970.
= Gnomonia intermedia var. alni M.E. Barr, Mycol. Mem. 7: 55. 1978 fide Monod 1983.
Habitat: On overwintered leaves of Alnus viridis (Chaix) DC. (Betulaceae).
Distribution: Canada (British Columbia), Europe (Bulgaria, Czech Republic, Switzerland) and U.S.A. (WA).
Specimens examined: Switzerland, Valais, vicinity of Martigny, on overwintered leaves of Alnus viridis, 21 May 2005, coll. M. Monod (BPI 877585A) GenBank EU254866. Canada, British Columbia, 15 km S from Princeton, near Indian reserve #3, on overwintered leaves of?Betula papyrifera, 13 May 2006, coll. M.V. Sogonov (BPI 877600) GenBank EU254869. U.S.A., Washington, King Co., Mount Baker-Snoqualmie National Forest, Snoqualmie Ranger District, near exit 42 on the highway US 90, road to mines, on overwintered leaves of Alnus viridis, 16 May 2006 (BPI 877595) GenBank EU254867.
Notes: Ophiognomonia alni-viridis can be distinquished from the other species of Gnomoniaceae on Alnus. Gnomonia alnea lacks an elongated neck unlike O. alni-viridis, O. ischnostyla and O. trientensis. Ophiognomonia trientense has an ascospore l:w less than 3 while O. alni-viridis and O. ischnostyla both have an ascospore l:w greater than 3. The ascospores of O. alni-viridis are 10–12.5 × 2–2.5 μm fide Podlahova & Svrček (1970) while those of O. ischnostyla fide Monod (1983) are longer, 12.5–18.5 × 1.5–2.5 μm. For a more detailed description of O. alni-viridis, see Monod (1983) and Podlahova & Svrček (1970).
Ophiognomonia gei-montani (Ranoj.) Sogonov, comb. nov. MycoBank MB 512183. Figs 31D; 32D. Basionym: Gnomonia gei-montani Ranoj., Ann. Mycol. 8: 362. 1910.
Habitat: On overwintered leaves of Geum bulgaricum Panc., G. coccineum Sm., G. montanum L., and G. rhodopeum Stoj. & Stef. (Rosaceae).
Distribution: Europe (Bulgaria, Switzerland)
Specimen examined: Switzerland, Salvan, La Tendraz, 1600 m a.s.l., on dead leaves of Geum montanum, 28 May 2005, coll. M. Monod (BPI 877589) GenBank EU254872.
Notes: See Monod (1983) for a detailed description.
Ophiognomonia intermedia (Rehm) Sogonov, comb. nov. MycoBank MB 512185. Figs 31E–G; 32E–I; 33A–F. Basionym: Gnomonia intermedia Rehm, Ann. Mycol. 6: 489. 1908.
Habitat: On overwintered leaves of Betula nana L., B. papyrifera Marshall, B. pendula Roth, and B. pubescens Ehrh. (Betulaceae).
Distribution: Canada (British Columbia), Europe (Germany, Russia, Scotland, Switzerland, United Kingdom) and U.S.A (MD).
Specimens examined: Canada, British Columbia, 15 km NE from Agassiz, route #7, on overwintered leaves of Betula papyrifera, 13 May 2005, coll. M.V. Sogonov (BPI 877599) GenBank EU 254884; Burnaby Lake Regional Park, on overwintered leaves of Betula papyrifera, 12 May 2006, coll. M.V. Sogonov (BPI 877602) GenBank EU254886.. Russia, Tver' province, Toropets district, v. Kosilovo, on overwintered leaves of Betula pendula, 5 Jun 2005, coll. M.V. Sogonov (BPI 877488B) GenBank EU254887; Novgorod province, Kholm district, Rdeysky Natural Reserve, vicinity of the village Fryunino, on overwintered leaves of Betula nana, 11 Jun 2005, coll. M.V. Sogonov (BPI 877496) GenBank EU254881; Naberezhnaya reki Lovat' str., 9, on overwintered leaves of Betula pendula, 23 Aug 2004, coll. M.V. Sogonov (BPI 877498) GenBank EU254878. U.S.A., Maryland, Prince George's Co., Beltsville, Little Paint Branch Park, on overwintered leaves of Betula nigra, 17 Mar 2005, coll. M.V. Sogonov (BPI 877597) GenBank EU254879; 11 Apr 2005, coll. M.V. Sogonov (BPI 877598) GenBank EU254880.
Notes: Among other species of Ophiognomonia on Betula, Ophiognomonia intermedia with two-celled ascospores is distinct from O. nana with one-celled ascospores. Ophiognomonia intermedia is similar to O. alni-viridis and O. ischnostyla in ascospore size except that the ascospores of O. intermedia lack appendages and tend to have a length wide ratio of less than 5. Ophiognomonia intermedia causes a foliar disease of birch that also causes dieback of young shoots (Green 2004). Green & Castlebury (2007) proved the connection between O. intermedia as G. intermedia with the asexual state Discula betulae (Westend.) Pennycook (Pennycook, 2007).
Ophiognomonia ischnostyla (Desm.) Sogonov, comb. nov. MycoBank MB 512186. Figs 34G–L; 35A–E; 36A–H. Basionym: Sphaeria ischnostyla Desm., Annals Sci. nat., Bot., sér. 3. 11: 357. 1849.
Fig. 34.
Culture morphology, colony habit. A–F. Ophiognomonia cf. intermedia CBS 121229. G–L. O. cf. ischnostyla CBS 121234. M–R. O. cf. ischnostyla BPI 877467B. S–X. O. cf. ischnostyla CBS 121252. A, C, E, G, I, K, M, O, Q, S, U, W. Surface. B, D, F, H, J, L, N, P, R, T, V, X. Reverse. A, B, G, H, M, N, S, T. PDA. C, D, I, J, O, P, U, V. MEA. E, F, K, L, Q, R, W, X. MYA. Scale 1 cm.
Fig. 35.
Morphology on natural substrates, perithecia. A, B. Ophiognomonia ischnostyla. A. BPI 877514B. B. BPI 877620. C–E. O. cf. ischnostyla. C, D. BPI 877605A. E. BPI 877616. A, C, E. Intact air-dry perithecia on leaves and petioles. B, D. Extracted and rehydrated perithecia. Scale 200 μm.
Fig. 36.
Morphology on natural substrates, asci and ascospores. A, B. Ophiognomonia ischnostyla. A. BPI 877514B. B. BPI 877619. C–H. O. cf. ischnostyla. C, D. BPI 877605A. E. BPI 877605B. F. BPI 877467B. G. BPI 877622. H. BPI 877616. Scale 10 μm.
≡ Gnomonia ischnostyla (Desm.) Auersw. in Gonn. & Rabenh., Mycol. Europ. 5/6: 2. 1869.
= Gnomonia setacea f. alni Kleb., Haupt- und Nebenfruchtformen der Ascomyzeten: 244. 1918 fide Monod 1983.
= Sphaeronema amenticolum Ces., Bot. Z. 15: 173. 1857 fide Monod 1983.
≡ Gnomonia amenticola (Ces.) Prihoda, Česká Mykol. 10:122. 1956 fide Monod 1983.
Habitat: On overwintered leaves of Alnus, Betula, Carpinus (Betulaceae), Juglans (Juglandaceae), and other hardwood trees.
Distribution: Europe (Bulgaria, France, Russia, Switzerland) and U.S.A. (TN).
Specimens examined: Russia, Tver' province, Toropets district, v. Kosilovo, on overwintered leaves of Alnus glutinosa, 5 Jun 2005, coll. M.V. Sogonov (BPI 877617) GenGank EU254907; Tver' province, Toropets district, vicinity of v. Bubonitsy, biological research station “Chisty Les”, on fallen leaves of Betula? pubescens, 31 Aug 2004, coll. M.V. Sogonov (BPI 877616) EU254919; on overwintered leaves of Alnus glutinosa, 14 Jun 2005, coll. M.V. Sogonov (BPI 877618) GenBank EU254908; Novgorod province, Kholm district, Rdeysky Natural Reserve, vicinity of the village Fryunino, on overwintered leaves of Alnus glutinosa, 11 Jun 2005, coll. M.V. Sogonov (BPI 877619) GenBank EU294900; Arboretum (Dendropark), near the tree #560, on overwintered leaves of Corylus avellana, Jun 2005, coll. M.V. Sogonov (BPI 877514B) GenBank EU254899. Switzerland, Wallis, Mörel, on overwintered leaves of Alnus incana, 28 May 2005, coll. M.V. Sogonov (BPI 877620) GenBank EU254898.
Specimen examined O. cf. ischnostyla: Russia, Tver' province, Toropets district, vicinity, the beginning of the Ecotrail, on fallen leaves of Betula pubescens, 31 Aug 2004, coll. M.V. Sogonov (BPI 877605A) GenBank EU254895.
Notes: Ophiognomonia ischnostyla occurs on a variety of hardwood trees especially in the Betulaceae. Opiognomonia ischnostyla has an elongated neck unlike Gnomonia alnea on Alnus. The ascospores of O. alni-viridis and O. ischnostyla have a l:w greater than 3 while O. trientense has an ascospore l:w less than 3. The ascospores of O. alni-viridis are 10–12.5 × 2–2.5 μm while those of O. ischnostyla are (12.5–)13.5–15.5(–18.5) × (1.5–)2(–2.5) μm fide Monod (1983). For a more detailed description of O. ischnostyla, see Monod (1983).
The name Gnomonia nervisequa (Wallr.) Fuckel based on Sphaeria nervisequa Wallr., was proposed by Monod (1983) as the correct name for O. ischnostyla but this is rejected. Wallroth's (1833) original description of the basionym is poor, does not indicate ascospore size, and lacks reference to any type specimen. The host plant mentioned in the description, Salix caprea, has been never reported in any later study. Nevertheless, Fuckel (1870) used this epithet creating the new combination Gnomonia nervisequa (Wallr.) Fuckel in reference to his own collection on Carpinus betulus. None of the species of Ophiognomonia are known to be associated with hosts in the Salicales. The next available basionym for G. nervisequa sensu Monod (1983) is Sphaeria ischnostyla Desm. The type specimen of Sphaeria ischnostyla on Carpinus betulus in France was examined at BPI (Desmazieres, Pl. crypt. France 2084-bound).
Ophiognomonia leptostyla (Fr.) Sogonov, comb. nov. MycoBank MB 512187. Basionym: Sphaeria leptostyla Fr., Syst. Mycol. 2: 517. 1823.
≡ Gnomonia leptostyla (Fr.) Ces. & De Not., Comment. Soc. Crittog. Ital. 1(4): 232. 1863.
Habitat: On overwintered leaves of Juglans spp. (Juglandaceae).
Distribution: Canada (Ontario), Europe (Austria, Bulgaria, Germany, Poland, Russia, Switzerland) and U.S.A. (AL, DE, IA, IL, MA, MD, NY, PA, VA, WV).
Specimen examined: Switzerland, on Juglans regia, coll. M. Monod 439, GenBank EU254910.
Notes: Ophiognomonia leptostyla is the cause of walnut anthracnose or walnut leaf blotch, a disease that is particularly virulent in the midwestern and eastern United States (Neely & Black 1976, Berry 1981, Juhasova et al. 2006). The anamorph of O. leptostyla has been called Marssoniella juglandis (Lib.) Hohn. but that anamorphic genus is a later homonoym of an alga, thus Braun (1991) established the genus Neomarssoniella U. Braun. For more detailed descriptions see Barr (1978) and Monod (1983).
Ophiognomonia micromegala (Ellis & Everh.) Sogonov, comb. nov. MycoBank MB 512188. Figs 37A,B; 38A,B. Basionym: Diaporthe micromegala Ellis & Everh., Proc. Acad. nat. Sci. Philad. for 1893: 449. 1894.
Fig. 37.
Morphology on natural substrates, perithecia. A, B. Ophiognomonia micromegala. A. BPI 877613A. B. BPI 877634B. C. O. rosae, BPI 877586. D. O. rubi-idaei, BPI 877637. A, C, D. Intact air-dry perithecia on leaves. B. Extracted and rehydrated perithecium. Scale 200 μm.
Fig. 38.
Morphology on natural substrates, asci and ascospores. A, B. Ophiognomonia micromegala, BPI 877615A. C, D. O. rosae. C. BPI 877636. D. BPI 877588. E, F. O. rubi-idaei, BPI 877637. Scale 10 μm.
≡ Plagiostoma micromegalum (Ellis & Everh.) M.E. Barr, Mycol. Memoir, 7: 112. 1978.
Habitat: On overwintered leaflets and rachises of Carya spp. (Juglandaceae).
Distribution: U.S.A. (DC, DE, GA, MD)
Specimens examined: U.S.A., Maryland, Montogomery Co., Chesapeake & Ohio Canal National Historic Park, on overwintered leaves of Carya tomentosa, 10 Apr 2004, coll. M.V. Sogonov (BPI 877614) GenBank EU254916; Wheaton Regional Park, on overwintered leaves of Carya sp., 6 Mar 2005, coll. M.V. Sogonov (BPI 877612) GenBank EU254917; Prince George's Co., Beltsville, end of Entomology Road, on overwintered leaves of Carya tomentosa, 30 Mar 2004, coll. M.V. Sogonov (BPI 877634B) GenBank EU254914; 4 Apr 2004 (BPI 877613A) GenBank EU254915.
Notes: Ophiognomonia micromegala with ascospores 32–45 × 5.5–8 μm fide Barr (1978 as Plagiostoma micomegalum) is similar to Gnomonia caryae with ascospores (16–)22–30(–37) × (2–)3–5.5 μm fide Barr (1978), however, the latter species has thinner ascospores and central necks on the perithecia. Ophiognomonia pseudoclavulata has shorter ascospores than those of O. micromegala. Barr (1978 as Plagistoma micromegalum) provides a detailed description of this species as does Wehmeyer (1933 as Diaporthe micromegala).
Ophiognomonia nana (Rehm) Sogonov, comb. nov. MycoBank MB 512189. Basionym: Gnomoniella nana Rehm, Hedwigia 42: 349. 1903.
Habitat: On dead leaves of Betula nana L. (Betulaceae).
Distribution: Europe (Finland, Germany, Switzerland).
Notes: Ophiognomonia nana is distinguished from other species of Gnomoniaceae on Betula by the non-septate ascospores and beaks longer than 400 μm, and is unlike species of Gnomonia in having perithecia that do not become concave upon drying. For a more detailed description, see Monod (1983 as Gnomoniella nana).
Ophiognomonia padicola (Lib.) M. Monod, Beih. Sydowia 9: 158. 1983.
≡ Sphaeria padicola Lib., Plant. Cryptog. Arduenn. Cent. 2, 149. 1832.
≡ Gnomonia padicola (Lib.) Kleb., Z. Pflkrankh. 18:137. 1908.
= Ophiognomonia padi Jaap, Verh. bot. Ver. Prov. Brandenburg 47: 87. 1905 fide Monod 1983.
Habitat: On overwintered leaves of Prunus padus L. (Rosaceae).
Distribution: Europe (Germany, Switzerland)
Notes: Ophiognomonia padicola is descried in detailed by Monod (1983) who placed the asexual state in Cylindrosporella. Gnomonia cerastis, previously reported on this host, is now considered a synonym of Apiognomonia hystrix. Ophiognomonia padicola has filiform ascospores more than 30 μm much longer than the ascospores of A. hystrix.
Ophiognomonia rosae (Fuckel) Kirschst., Ann. Mycol. 37: 129. 1939. Figs 37C; 38C,D; 39A–H.
Fig. 39.
Culture morphology. A–H. Ophiognomonia rosae CBS 121263. I–Q. O. sassafras CBS 121243. A–F, I–N. Colony habit, 40 d, 23 °C. A, C, E, I, K, M. Surface. B, D, F, J, L, N. Reverse. G, O, P. Perithecia. H, Q. Asci. G, H. 40 d, 23 °C. O–Q. 4.5 mo, 2/10 °C. A, B, I, J, P. PDA. C, D, G, H, K, L, Q. MEA. E, F, M, O. MYA. Scale: A–F, I–N. 1 cm. G, O. 500 μm. P. 200 μm. H, Q. 10 μm.
≡ Gnomonia rosae Fuckel, Jb. Nassau Ver. Naturk. 23–24: 122. 1870.
≡ Gnomoniella rosae (Fuckel) Sacc., Syll. Fung. 1: 416. 1882.
Habitat: On Rosa spp. and possibly other genera in the Rosaceae.
Distribution: Europe (Germany, Russia) and U.S.A. (ME).
Specimen examined: Russia, Tver' province, Toropets district, v. Bubonitsy, near Bologovs' house, on dead but attached twigs of Rosa sp., 14 Jun 2005, coll. M.V. Sogonov (BPI 877635) GenBank EU 254933.
Notes: Ophiognomonia rosae having filiform ascospores has been reported on a number of rosaceous hosts; however, many of these specimens were not examined, thus a narrow concept of this species is retained. Monod (1983) provides a detailed description of this species as Gnomonia rosae.
Ophiognomonia rubi-idaei (M. Monod) Sogonov, comb. nov. MycoBank 512190. Figs 37D; 38E,F. Basionym: Gnomonia rubi-idaei M. Monod, Beih. Sydowia 9: 106. 1983.
Habitat: On overwintered leaves of Rubus idaeus L. (Rosaceae).
Distribution: Canada (British Columbia) and Europe (Switzerland).
Specimens examined: Canada, British Columbia, Manning Provincial Park, on overwintered leaves of Rubus sp., 13 May 2006, coll. M.V. Sogonov (BPI 877559B) GenBank EU254939; Victoria Island, Route 14, on overwintered leaves of Rubus spectabilis, 10 May 2006, coll. M.V. Sogonov (BPI 877638) GenBank EU 254938. Switzerland, on overwintered leaves of Rubus idaeus, 21 May 2005, coll. M.V. Sogonov (BPI 877637) GenBank EU254937).
Notes: Ophiognomonia rubi-idaei is distinguished from other species in the Gnomoniaceae on Rubus by the filiform ascospores. Monod (1983) provides a detailed description as G. rubi-idaei.
Ophiognomonia sassafras (Ellis & Everh.) M. Monod, Beih. Sydowia 9: 86. 1983. Figs 39I–Q; 40A–C; 41A.
Fig. 40.
Morphology on natural substrates, perithecia. A–C. Ophiognomonia sassafras. A, B. BPI 877639. C. BPI 611592. D. O. cf. trientensis, BPI 877672. A, D. Intact air-dry perithecia on leaves. B, C. Extracted and rehydrated perithecia. Scale 200 μm.
Fig. 41.
Morphology on natural substrates, asci and ascospores. A. Ophiognomonia sassafras, BPI 877639. B. O. cf. trientensis, BPI 877672. Scale 10 μm.
≡ Gnomonia sassafras Ellis & Everh., Bull. Torrey Bot. Club 10: 98. 1883.
≡ Pleuroceras sassafras (Ellis & Everh.) M.E. Barr, Mycol. Mem. 7: 122. 1978.
Habitat: On overwintered leaves of Sassafras officinale Nees. & Eberhm. (Lauraceae).
Distribution: U.S.A. (MD, NJ, OH, PA).
Specimen examined: U.S.A., Maryland, Howard Co., Columbia, Centennial Park, on overwintered leaves of Sassafras albidum, 9 Apr 2005, coll. M.V. Sogonov (BPI 877642) GenBank EU254940.
Notes: Ophiognomonia sassafras is the only species of Gnomoniaceae known on this plant host. For a detailed description, consult Barr (1978).
Ophiognomonia setacea (Pers.: Fr.) Sogonov, comb. nov. MycoBank MB 512191. Figs 42A–X. Basionym: Sphaeria setacea Pers.: Fr., Syn. Method. Fung. p. 62. 1801: Syst. Mycol. 2: 517. 1823.
Fig. 42.
Ophiognomonia setacea, culture morphology, colony habit, 40 d, 23 °C. A–F. CBS 116406. G–L. CBS CBS116408. M–P. CBS 121235. Q, R. CBS 121237. S–X. O. cf. setacea CBS 121256. A, C, E, G, I, K, M, O, Q, S, U, W. Surface. B, D, F, H, J, L, N, P, R, T, V, X. Reverse. Scale 1 cm.
≡ Gnomonia setacea (Pers.: Fr.) Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 232. 1863.
Habitat: On overwintered leaves of Castanea dentata L., Castanea sp. and Quercus alba L., Q. bicolor Willd., Q. cerris L., Q. macrocarpa Michx., Q. montana Willd., Q. palustris Münchh., Q. phellos L., Q. pubescens Willd., Q. robur L., and Quercus sp. (Fagaceae).
Distribution: Canada (Ontario), Europe (Austria, Bulgaria, Germany, Italy, Montenegro, Sweden, Switzerland) and U.S.A. (LA, MD, NJ, NY, OH, PA, TN, VA, WV).
Notes: Ophiognomonia setacea was originally retained in Gnomonia by Sogonov et al. (2005) based on an analysis of the LSU of relatively few species in the Gnomoniaceae. However, the multigene phylogeny presented in this paper reveals that this species is allied with species of Ophiognomonia. Ophiognomonia setacea is conspicuous on overwintered leaves of chestnut and oak due to the very long, often over 500 μm, thin, black necks emerging from the leaf surface. Sogonov et al. (2005) provides an epitype and a detailed description of this species.
Ophiognomonia trientensis (M. Monod) Sogonov, comb. nov. MycoBank MB 512192. Figs 40D; 41B. Basionym: Gnomonia trientensis M. Monod, Beih. Sydowia 9: 90. 1983.
Habitat: On overwintered leaves of Alnus viridis (Betulaceae).
Distribution: Canada (British Columbia), Europe (Switzerland) and U.S.A. (WA).
Specimens examined: Canada, British Columbia, Hope, on overwintered leaves of Alnus tenuifolia, 13 May 2006, coll. M.V. Sogonov (BPI 877672) GenBank EU254986; Manning Provincial Park, Engineers Trail, on overwintered leaves of Alnus viridis, 13 May 2006 (BPI 877673) GenBank EU254987. U.S.A., Washington, King Co., Mount Baker-Snoqualmie National Forest, Snoqualmie Ranger District, near exit 42 on the highway US 90, road to mines, on overwintered but still hanging leaves of Alnus viridis, 16 May 2006 (BPI 877674) GenBank EU254985.
Notes: Ophiognomonia trientensis can be distinquished from the other species of Gnomoniaceae on Alnus. Gnomonia alnea lacks an elongated neck unlike O. alni-viridis, O. ischnostyla and O. trientensis. Ophiognomonia trientense has an ascospore l:w less than 3 while O. alni-viridis and O. ischnostyla both have an ascospore l:w greater than 3.
For a detailed description, see Monod (1983).
PLAGIOSTOMA Fuckel, Jb. Nassau Ver. Naturk. 23–24: 118. 1870. Lectotype designated by Höhnel (1917): Plagiostoma euphorbiae Fuckel
= Cryptodiaporthe Petr., Ann. Mycol. 19: 118. 1921. Lectotype designated by Clements & Shear (1931): Cryptodiaporthe aesculi (Fuckel) Petr. now recognised as Plagiostoma aesculi (Fuckel) Sogonov, comb. nov.
= Rostrocoronophora Munk, Dansk Bot. Arkiv 15: 98. 1953. Type: R. geranii Munk, now recognised as Plagiostoma geranii (Hollos) Sogonov, comb. nov.
Perithecia solitary, on fallen leaves, epiphyllous or on petioles, on dead but still attached pedicels of trees and shrubs, or on dead parts of herbaceous plants, in groups of 5–15 perithecia with or without a rudimentary stroma on twigs of trees and shrubs. Perithecia black, remaining immersed in substrate, oblate to globose when moist, convex, sometimes with irregular dents when dry, round in top view, with one neck. Necks central to marginal, never truly lateral, mostly length 0.5–2 times perithecial diam but varying from almost lacking to 3–4 perithecial diam long. Asci fusiform, with an apical ring, with eight spores arranged irregularly multiseriate or obliquely uniseriate. Ascospores mostly two-celled, rarely one-celled, oval to fusiform, l:w 2.5–6; ends mostly rounded, rarely pointed; appendages mostly absent or less commonly present, subulate, navicular or whip-shaped, to 30 μm long.
Cultures: Colonies fast growing, often reaching edges of 90 mm Petri plates after 2 wk at 23 °C l/d or at least 60–70 mm diam. Colonies floccose or lanose all over surface or in lobes or concentric rings intermingled with glabrous or velvety areas. Colonies whitish, grey, orange-grey, brownish orange, dark brown, olive. Some species produce fertile perithecia in culture after 5–6 mo at 2/10 Cl/d. Conidiomata often produced after 2–4 wk at 23 °C l/d.
Hosts: In diverse taxonomic groups (Aceraceae, Ericaceae, Euphorbiaceae, Fagaceae, Geraniaceae, Hippocastanaceae, Oleaceae, Platanaceae, Polygonaceae, Salicaceae). Most species are specific at the level of plant host species or genus, however, a few species occur on a wide diversity of plants.
Type species of Plagiostoma and synonymous genus, Cryptodiaporthe
Plagiostoma euphorbiae (Fuckel) Fuckel, Jb. Nassau Ver. Naturk. 23–24: 118. 1870. Figs 43A–C; 44A; 45A–F.
Fig. 43.
Morphology on natural substrates, perithecia. A–C. Plagiostoma euphorbiae, lectotype Fungi Rhenani 863, BPI bound. D–I. P. aesculi. D, E, I. Epitype BPI 748430. F–H. BPI 840942. J, K. P. barriae, holotype BPI 877717B. A, D, E, J. Intact air-dry perithecia on stems, twigs, leaves and petioles. B. Air-dry perithecium on fragment of bark, bottom view. C, H, I, K. Extracted and rehydrated perithecia. F. Air-dry perithecium on fragment of bark, side view. G. Air-dry perithecium on fragment of outer layers of stem, bottom view. Scale 200 μm.
Fig. 44.
Morphology on natural substrates, asci and ascospores. A. Plagiostoma euphorbiae, lectotype Fungi Rhenani 863, BPI bound. B, C. P. aesculi, epitype BPI 748430. D–F. P. barriae, holotype BPI 877717B. Scale 10 μm.
Fig. 45.
Culture morphology, colony habit. A–F. Plagiostoma euphorbiae CBS 817.79. G–R. P. barriae CBS 121249. S–X. P. devexum BPI 843489. A–F, M–X. 40 d, 23 °C. G–L. 14 d, 23 °C. A, C, E, G, I, K, M, O, Q. Surface. B, D, F, H, J, L, N, P, R. Reverse. A, B, G, H, M, N. PDA. C, D, I, J, O, P. MEA. E, F, K, L, Q, R. MYA. Scale 1 cm.
≡ Sphaeria euphorbiae Fuckel, Enumeratio fungorum Nassoviae: 69. 1860.
≡ Gnomonia euphorbiae (Fuckel) Sacc., Michelia 2: 312. 1881.
≡ Gnomoniella euphorbiae (Fuckel) Sacc., Syll. Fung. 1: 418. 1882.
= Gnomonia tithymalina Sacc. & Briard, Revue mycol. 7: 209. 1885 fide Monod 1983.
Perithecia solitary, without stroma, randomly scattered on dead stems, black, suboblate to oblate-spheroidal when moist, 230–350 μm high × 290–430 μm diam, convex when dry. Necks central or eccentric, short, only slightly projecting from plant tissue, 70–95 μm long, 80–90 μm diam. Asci oval, (33–)37.5–41(–52.5) × 10.5–12.5(–13.5) μm (mean = 41 × 12, SD 7.5, 1.5, n=5), apical ring 2.5–3 μm diam, with eight ascospores arranged obliquely uniseriate, obliquely biseriate ot irregularly multiseriate. Ascospores ellipsoidal, straight or inequilateral, (12–)13–13.5(–15.5) × (3–)3.5(–4) μm (mean = 13.5 × 3.5, SD 0.5, 0.2, n=33), l:w (3.1–)3.7–4(–4.5) (mean = 3.9, SD 0.3), two-celled, not constricted at septum; septum located at (42–)45–49(–56) % (mean = 47, SD 3) of ascospore length; cells with parallel walls, rounded at ends, each cell with two large guttules; appendages absent.
Cultures: Colonies on PDA attaining 60 mm diam after 40 d at 23 °C, flat, glabrous to velvety, dark brown to greyish brown; margins submerged, orange-grey; margin irregular; reverse dark brown to brownish orange. Colonies on MEA attaining 90 mm after 40 d at 23 °C, flat, superficial and partly submerged, with no aerial mycelium, thin, consisting of dendroid branches, shades of orange-grey, brownish orange, brownish grey; margin irregular; reverse of same colours as surface. Colonies on MYA attaining 90 mm after 40 d at 23 °C, flat, short felty orange-grey in central part, glabrous to velvety, dark brown; margin submerged, greyish orange, irregular; reverse dark brown to brownish orange.
Habitat: On dead stems of Euphorbia palustris L. and E. pannonica Host (Euphorbiaceae).
Distribution: Europe (Germany, Hungary, The Netherlands, Russia, Switzerland).
Lectotype designated here: Germany, Freienweinheim, 1860 or before, K.W.G.L. Fuckel, Fungi Rhenani 863 (BPI bound).
Additional cultures examined: The Netherlands, Baarn, 12 May 1978, W. Gams, H.A. van der Aa 6449 (CBS 340.78); Switzerland, Vaud, lake shore between Yverdon and Yvonand, 14 Jun. 1978, M. Monod 466 (CBS 817.79).
Notes: Plagiostoma euphorbiae is distinguished from the other species of Gnomoniaceae on Euphorbia by the short neck, less than 100 μm long, on each perithecium.
Plagiostoma aesculi (Fuckel) Sogonov, comb. nov. MycoBank MB 512193. Figs 43D–I; 44B,C. Basionym: Cryptospora aesculi Fuckel, Jb. Nassau Ver. Naturk. 23–24: 193. 1870.
≡ Cryptosporella aesculi (Fuckel) Sacc., Michelia 1: 30. 1877.
≡ Diaporthe aesculi (Fuckel) Höhn., Ann. Mycol. 16: 116. 1918.
≡ Cryptodiaporthe aesculi (Fuckel) Petr., Ann. Mycol. 19: 119. 1921.
Perithecia in groups of 3–10, with loose stroma, on fresh dead twigs. Perithecia black, oblate spheroidal when moist, convex, usually with irregular dents on top when dry, 300–450 μm high × 380–600 μm diam. Necks converged with others in group, eccentric to marginal, slightly curved, 420–700 μm long, 100–150 μm wide at base, 60–150 μm wide at apex. Asci fusiform, (45.5–)48.5–67(–78.5) × (10–)12.5–16(–21.5) μm (mean = 58.5 × 14.5, SD 11, 3, n=16), apical ring absent, with eight ascospores arranged obliquely biseriate to irregularly multiseriate. Ascospores variable in size and shape, ellipsoidal to fusiform, (13–)17.5–20(–23.5) × (3.5–)4–5(–6.5) μm (mean = 18.5 × 4.5, SD 2, 0.7, n=109), l:w (2.6–)3.5–4.4(–5.7) (mean = 4, SD 0.7), two-celled, constricted or not constricted at septum, ends rounded to tapering, distal cell often slightly wider than basal, septum located at (37–)46–50(–57) % (mean = 48, SD 4, n=98) of ascospore length; appendages usually absent, if present, subulate, length to 5 μm.
Cultures: Not observed.
Habitat: On overwintered twigs of Aesculus hippocastanum L. (Sapindaceae).
Distribution: Europe (Austria, Czech Republic, Germany, United Kingdom).
Lectotype designated here: Germany, Reichartshausen, on dry twigs of Aesculus hippocastanum, winter 1894 or before, K.W.G.L. Fuckel, Fungi Rhenani 2003 (BPI 601244).
Epitype designated here: Austria, Vienna, 19th district, Krapfenwaldgasse, Grinzing, Aesculus hippocastanum, 11 Nov. 2000, W. Jaklitsch 1695 (BPI 748430, ex-type epiculture CBS 109765).
Additional specimens examined: Austria, Trieblach, St. Margareten im Rosental, Kaertnen, on dead twigs of A. hippocastanum, 14 Apr. 2001, W. Jaklitsch 1732 (BPI 840942, culture CBS121905) GenBank EU254994; Czech Republic, Moravia, Hranice na Moravě, Aesculus sp., March 1913, F. Petrak (Flora Moravica, Missouri Bot. Gard. Herb. 43417 (BPI 617579); Moravia, Hranice na Moravě, Teplice, A. hippocastanum, May 1914, F. Petrak (BPI 617580); Germany, Saxony, near Köningstein, A. hippocastanum, 04 May 1907, W. Krieger Fungi Saxonici 2022 (BPI bound).
New species of Plagiostoma
Plagiostoma barriae Sogonov, sp. nov. MycoBank MB 512194. Figs 43J,K; 44D–F; 45G–R.
Perithecia 140–170 μm alta × 180–240 μm diam, in sicco convexae. Rostrum 800–130 μm longum, basi 45–52 μm diam, apice 30–38 μm diam. Ascosporae ellipsoideae vel ovales, rectae, (11.5–)14–15.5(–17.5) × (2.5–)3.5–4(–4.5) μm, L:l (3–)3.6–4.1(–5.1). Ad aliis Plagiostomae speciebus morphologiae characteribus combinatis differt. Holotypus: BPI 877717B.
Etymology: Names for the Canadian mycologist Margaret E. Barr Bigelow in recognition of her contribution to the taxonomy of the Diaporthales.
Perithecia solitary, without stroma, randomly scattered on overwintered petioles, black, suboblate when moist, 140–170 μm high × 180–240 μm diam, convex when dry. Necks central, straight, 80–130 μm long, 45–52 μm wide at base, 30–38 μm wide at apex. Asci fusiform, (47.5–)48.5–53(–56.5) × (8.5–)9.5–10.5(–11) μm (mean = 51 × 10, SD 4, 1.2, n=4), apical ring 2.5–4 μm diam, with eight ascospores arranged biserate to irregularly multiseriate. Ascospores ellipsoidal to oval, straight to oval, (11.5–)14–15.5(–17.5) × (2.5–)3.5–4(–4.5) μm (mean = 15 × 4, SD 1.5, 0.5, n=58), l:w (3–)3.5–4(–5) (mean = 4, SD 0.5), two-celled, constricted at septum; septum located at (44–)47–49(–55) % (mean = 48, SD 3, n=20) of ascospore length; cells slightly tapering, at ends blunt, rounded, each cell with 2 large and sometimes 1–2 small guttules where the largest guttule close to septum; appendages absent or indistinct.
Cultures: Colonies on PDA and MYA attaining 90 mm after 40 d at 23 °C, flat, woolly to floccose, with indistinct concentric zones, with areas of tint of orange in central part, with scarce-grey soft sclerotium-like bodies; reverse pale brown in centre to greyish orange at margin. Colonies on MEA attaining 90 mm after 40 d at 23 °C, flat, thin, semitransparent, shortly felty, colourless or whitish with tint of brown in central part, with scattered flocks of white aerial mycelium, with dark brown matt or covered with pale grey felty mycelium soft sclerotium-like bodies; margin diffuse; reverse brownish orange to brown-grey. Neither perithecia nor conidiomata observed in cultures at 2/10 °C after 8 mo.
Habitat: On overwintered petioles of Acer macrophyllum Pursh (Aceraceae).
Holotype: U.S.A., Washington, Pierce Co., Gig Harbor, Narrows Park, 16 May 2006, M.V. Sogonov MS0367a (BPI 877717B, ex-holotype culture CBS 121249).
Additional GenBank nucleotide sequence: U.S.A., Washington, Klickitat, young non-mildewed leaf of Acer macrophyllum, date unknown, C. Nischwitz, G. Newcombe, nrDNA ITS1–5.8S–ITS2 (AY961407).
Notes: Plagiostoma barriae having central necks on the perithecia differs from other species of Plagiostoma on Acer, specifically P. inclinatum (Desm.) Barr, P. petiolophilum, and P. pseudobavarica M. Monod, all species that have lateral necks.
Additional species accepted in Plagiostoma
Plagiostoma amygdalinae (Fuckel) Sogonov, comb. nov. MycoBank MB 512195. Basionym: Gnomonia amygdalinae Fuckel, Jb. Nassau Ver. Naturk. 23–24: 121. 1870.
≡ Gnomoniella amygdalinae (Fuckel) Sacc., Syll. Fung. 1: 418. 1882.
= Gnomoniella amygdalinae (Fuckel) Sacc. f. euphorbiae-stepposae Sandu-Ville, Studii Cerc. Biol., Bot. 18: 18. 1966 fide Monod 1983.
Habitat: On overwintered leaves of Euphorbia amygdaloides L. and E. stepposa Zoz (Euphorbiaceae).
Distribution: Europe (Bulgaria, France, Germany, Romania, Switzerland).
Notes: Among the species of Plagiostoma on Euphorbia, Plagiostoma amygdalinae as well as P. euphorbiaceum and P. euphorbiae-verrucosae have perithecial necks longer than 100 μm and thus are distinct from P. euphorbiae having a shorter neck. Plagiostoma amygdalinae and P. euphorbiaceum have one-septate ascospores while those of P. euphorbiae-verrucosae are non-septate. Plagiostoma amygdalinae has ascospores that are 13–15.5 × 2.3–3 μm that are narrower than those of P. euphorbiae-verrucosae. See Monod (1983) for a detailed description of P. amygdalinae as Gnomonia amygdalinae.
Plagiostoma devexum (Desm.) Fuckel, Jb. Nassau Ver. Naturk. 23–24: 119. 1870. Figs 45S–X; 46A–E; 47A,B.
Fig. 46.
Morphology on natural substrates, perithecia. A–E. Plagiostoma devexum. A, B, E. Plantes Cryptogames de France 367, BPI bound. C, D. BPI 843489. F, G. P. euphorbiae-verrucosae, BPI 877685. H. P. euphorbiaceum, BPI 871053. I. P. fraxini, BPI 877686. J. P. rhododendri, BPI 877701. A, C, D, F, H, J. Intact air-dry perithecia on stems, twigs, petioles and pedicels. B. Extracted air-dry perithecia. E, G, I. Extracted and rehydrated perithecia. Scale 200 μm.
Fig. 47.
Morphology on natural substrates, asci and ascospores. A, B. Plagiostoma devexum, BPI 843489. C. P. euphorbiae-verrucosae, BPI 877685. D. P. euphorbiaceum, BPI 871053. E. P. fraxini, BPI 877687. F. P. rhododendri, BPI 877701. Scale 10 μm.
≡ Sphaeria devexa Desm., Cryptog. de France, Edit. II, Sér. II, No 367. 1856.
≡ Gnomonia devexa (Desm.) Auersw. in Gonn. & Rabenh., Mycol. Europ. 5/6: 23. 1869.
≡ Gnomoniella devexa (Desm.) Sacc., Syll. Fung. 1: 417. 1881.
≡ Gnomonopsis devexa (Desm.) Moesz & Smarods, Bot. Közl. 38: 68. 1941.
= Sphaeria euphorbiae f. polygoni Fuckel, Fungi Rhenani 864. 1864 fide Monod 1983.
= Sphaeria excentrica Cooke & Peck, Ann. Rep. New York State Museum 25: 105. 1873 fide Monod 1983.
≡ Gnomoniella excentrica (Cooke & Peck) Sacc., Syll. Fung. 1: 418. 1881.
= Diaporthe sechalinensis Sacc., Atti del Congr. bot. di Palermo 1902: 52. 1902 fide Monod 1983.
= Ceriosporella polygoni A.L. Sm. & Ramsb., Trans. Br. mycol. Soc. 4: 325. 1914 fide Monod 1983.
Habitat: On overwintered stalks and leaves of Persicaria amphibium (L.) Delarbre, P. lapathifolia (L.) Gray, P. maculosa Gray., Polygonum sp. and Rumex longifolius DC. (Polygonaceae), and Vitis vitifera L. (Vitaceae).
Distribution: Europe (Denmark, France, Germany, Sweden, Switzerland, United Kingdom) and U.S.A. (NY).
Notes: Barr (1978) and Monod (1983) provide detailed descriptions of this species.
Plagiostoma euphorbiaceum (Sacc. & Briard) Sogonov, comb. nov. MycoBank MB 512196. Figs 46H; 47D; 48A–F. Basionym: Gnomonia euphorbiacea Sacc. & Briard, Revue Mycol. 7: 208. 1885.
Fig. 48.
Culture morphology. A–F. P. euphorbiaceum CBS 121241. G–L. P. fraxini CBS 121258. M–R. P. rhododendri CBS 847.79. A, C, E, G, I, K, M, O, Q. Surface. B, D, F, H, J, L, N, P, R. Reverse. A, B, G, H, M, N. PDA. C, D, I, J, O, P. MEA. E, F, K, L, Q, R. MYA. Scale 1 cm.
Habitat: On dead branches of Euphorbia amygdaloides L. and E. palustris L. (Euphorbiaceae).
Distribution: Europe (Germany, Switzerland).
Specimen examined: Switzerland, Vaud, Arzier, on overwintered stems of Euphorbia amygdaloides, 25 May 2005, coll. M.V. Sogonov (BPI 871053) GenBank EU255004.
Notes: Among the species of Plagiostoma on Euphorbia, P. euphorbiaceum as well as P. amydalinae and P. euphorbiae-verrucosae have perithecial necks longer than 100 μm and thus are distinct from P. euphorbiae having a shorter neck. Plagiostoma euphorbiaceum and P. amygdalinae have one-septate ascospores while those of P. euphorbiae-verrucosae are non-septate. Plagiostoma euphorbiaceum has ascospores that are 14–17.5 × 3.5–4.5 μm and wider than those of P.amygdalinae. For a detailed description, see Monod (1983 as G. euphorbiacea).
Plagiostoma euphorbiae-verrucosae (M. Monod) Sogonov, comb. nov. MycoBank MB 512197. Figs 15F,G; 29D. Basionym: Gnomoniella euphorbiae-verrucosae M. Monod, Beih. Sydowia 9: 42. 1983.
Habitat: On overwintered stalks of Euphorbia verrucosa L. (Euphorbiaceae).
Distribution: Europe (Switzerland).
Specimen examined: Switzerland, Les Plans sur Bex, Pont de Nant, on overwintered stems of Euphorbia verrucosa, 29 May 2005, coll. M.V. Sogonov (BPI 877685) GenBank EU255006.
Notes: Plagiostoma euphorbiae-verrucosae as well as P. amygdalinae and P. euphorbiaceum have perithecial necks longer than 100 μm and thus are distinct from P. euphorbiae having a shorter neck. The ascospores of P. euphorbiae-verrucosae are non-septate while those Plagiostoma amygdalinae and P. euphorbiaceum are one-septate. For a detailed description of P. euphorbiae-verrucosae, see Monod (1983 as G. euphorbiae-verrucosae).
Plagiostoma fraxini (Redlin & Stack) Sogonov, comb. nov. MycoBank MB 512198. Figs 46I; 47E; 48G–L. Basionym: Gnomoniella fraxini Redlin & Stack, Mycotaxon 32: 185. 1988.
Habitat: On living and overwintered leaves of Chionanthus retusus Lindl. & Paxton, Fraxinus americana L., and F. pennsylvanica Marshall (Oleaceae)
Distribution: Canada (Manitoba, Saskatchewan) and U.S.A. (CA, DE, IA, IL, LA, KY, MI, MD, MS, NC, ND, NY, OK, OR, SD, VA, WI)
Specimens examined: U.S.A., Maryland, Prince George's Co., Riverdale, Anacostia, on overwintered leaves of Fraxinus americana, 12 Jun 2006, coll. M.V. Sogonov (BPI 877687) GenBank EU255008; Howard Co. Centennial Park, on overwintered leaves of Fraxinus americana, 9 Apr 2005, coll. M.V. Sogonov (BPI 877686) GenBank EU255007.
Notes: The anamorph of Plagiostoma fraxini is Discula fraxinea (Peck) Redlin & Stack under which this species has been reported to cause ash anthracnose (Holcomb 1998, Rossman et al. 2004) and anthracnose of fringetree (Gregory et al. 2004). For a detailed description, see Redlin & Stack (1988 as G. fraxini).
Plagiostoma geranii (Hollós) Sogonov, comb. nov. MycoBank MB 512199. Basionym: Gnomonia geranii Hollós, Annls Mus. nat. hung. 7: 52. 1909.
= Rostrocoronophora geranii Munk, Dansk Bot. Arkiv 15(2): 98. 1953.
Habitat: On overwintered stalks of Geranium sanguineum L., G. sylvaticum L. (Geraniaceae).
Distribution: Europe (Bulgaria, Denmark, Germany, Hungary, Sweden, Switzerland)
Specimen examined: Bulgaria, Sredna Gory Mt (western), Lozenska Planina, along track to Vlakovete, on overwintered petioles and stems of Geranium sanguineum, 2 May 2005, coll. D. Stoykov (BPI 877688) GenBank EU255010.
Note: For a detailed description see Monod (1983) and Müller & Arx (1962) as G. geranii.
Plagiostoma petiolophilum (Peck) Sogonov, comb. nov. MycoBank MB 512200. Basionym: Sphaeria petiolophila Peck, Ann. Rep. New York State Museum 35: 144. 1884.
≡ Gnomonia petiolophila (Peck) Berl. & Voglino, Syll. Fung. Addit. 1–4: 90. 1886.
≡ Cryptodiaporthe petiolophila (Peck) Barr, Mycol. Mem. 7: 136. 1978.
Habitat: On overwintered leaves, petioles, and twigs of Acer negundo, A. pensylvanicum, A. rubrum, A. saccharum, A. spicatum, and Acer sp. (Aceraceae)
Distribution: Canada (Ontario) and U.S.A. (GA, MD, MI, NH, NY, TN).
Specimens examined: U.S.A., Maryland, Prince George's Co., Paint Branch Park, on overwintered petioles of Acer rubrum, 17 Mar 2006, coll. M.V. Sogonov (BPI 877699) GenBank EU255040; Tennessee, Great Smoky Mountain National Park, on overwintered petioles of Acer sp., 10 May 2006, coll. L. Vasilyeva (BPI 878448, culture CBS 121254) GenBank EU255050.
Notes: Plagiostoma petiolophilum has lateral necks on the perithecia unlike P. barriae with one central neck. Barr (1978 as C. petiolophila) provides a detailed description of this species.
Plagiostoma rhododendri (Auersw.) Sogonov, comb. nov. MycoBank MB 512201. Figs 46J; 47F; 48M–R. Basionym: Gnomonia rhododendri Auersw. in Gonn. & Rabenh., Mycol. Europ. 5/6: 26. 1869.
≡ Apiognomonia rhododendri (Auersw.) Remler, Bibiotheca Mycologica 68: 74. 1979.
Habitat: On overwintered branches, flowers, and leaves of Rhododendron ferrugineum L., R. hirsutum L. (Ericaceae)
Distribution: Europe (Austria, Germany, Italy, Switzerland)
Specimens examined: Switzerland, Vaud, Pont de Nant, Botanical Garden, on dead inflorescenes of Rhododendron hirsutum, 29 May 2005, M. Monod (BPI 877701) GenBank EU255045.
Note: Remler (1979 as A. rhododendri) and Monod (1983 as G. rhododendri) provide detailed descriptions of this species.
Plagiostoma robergeanum (Desm.) Sogonov, comb. nov. MycoBank MB 512202. Basionym: Sphaeria robergeana Desm., Ann. sci. nat., Ser. 3, 16: 306. 1851.
≡ Diaporthe robergeana (Desm.) Niessl in Rabenh., Fungi Europ. 2222. 1882.
≡ Cryptodiaporthe robergeana (Desm.) Wehm., The Genus Diaporthe: 200. 1933.
Habitat: On overwintered, still attached branches of Staphylea colchica Steven and S. pinnata L. (Staphyleaceae)
Distribution: Europe (Austria, Czech Republic, France, Germany, Poland, Russia, Switerland, United Kingdom)
Note: Wehmeyer (1933) provides a detailed description of this species as Cryptodiaporthe robergeana.
Plagiostoma salicellum (Fr.) Sogonov, comb. nov. MycoBank MB 512203. Basionym: Sphaeria salicella Fr., Syst. Mycol. 2: 377. 1823.
[≡ Cryptodiaporthe salicella (Fr.) Wehm., The Genus Diaporthe, p. 193. 1933 non Petrak 1921].
= Diaporthe spina Fuckel, Jb. Nassau Ver. Naturk. 23–24: 210. 1870 fide Wehmeyer 1933.
≡ Gnomonia spina (Fuckel) Feltg., Vorst. Pilz. Lux., Nachtr. I: 214. 1899.
= Valsa populina Fuckel, Jb. Nassau. Ver. Naturk. 25–26: 314. 1871 fide Wehmeyer 1933.
≡ Cryptodiaporthe populina (Fuckel) Petr., Ann. Mycol. 19: 119. 1921 fide Wehmeyer 1933.
= Cryptodiaporthe apiculata (Wallr.) Petr., Ann. Mycol. 19: 177. 1921 fide Wehmeyer 1933.
Habitat: On overwintered, still attached branches of Populus nigra L., P. tremula L. Salix appendiculata Vill., S. aurita L., S. caprea L., S. fragilis L., S. triandra L., and S. vitellina L., (Salicaceae).
Distribution: Canada (Ontario, Quebec), Europe (Austria, Belgium, Bulgaria, Czech Republic, Germany, Poland, Sweden, Switzerland, United Kingdom) and U.S.A. (MA, NY).
Notes: Wehmeyer (1933) suggests the relationship of this species to the Gnomoniaceae and provides a detailed description as Cryptodiaporthe salicella.
Genera not included in this study or excluded from the Gnomoniaceae
The genus Anisogramma Theiss. & Syd. includes two species pathogenic on woody plants, A. virgultorum (Fr.: Fr.) Theiss. & Syd., the type of the genus and cause of a disease of Betula in Europe (Froidevaux & Müller 1976), and A. anomala (Peck) E. Müll., cause of eastern filbert blight, a serious disease of Corylus avellana in North America (Gottwald & Cameron 1979, Johnson et al. 1996). Recent research on the phylogeny of these species has demonstrated that Anisogramma is sister to the Gnomoniaceae (DeSilva et al. 2008).
The genus Apioplagiostoma M.E. Barr was established for species similar to Plagiostoma in having perithecia bearing an eccentric or lateral neck but with unequally septate ascospores (Barr 1978). Included in Apioplagiostoma is the type species, A. populi (E.K. Cash & Waterman) M.E. Barr, a species not included in this study. One species, Apioplagiostoma carpinicola, is herein transferred to Gnomonia carpinicola. One isolate of A. aceriferum was sequenced and determined to belong in Pleuroceras (Fig. 1). One additional species, A. hilberovae Schmid-Heckel (Schmid-Heckel 1988) is included in this genus.
The genus Bagcheea E. Müll. & Menon includes only the type species, B. albomaculans (Fukui) Hino & Katumoto (= B. castaneae E. Müll. & Menon). It occurs on living leaves of Castaneopsis in India and Japan. Kobayashi (1970) collected this species but was unable to obtain it in culture.
Clypeoporthella Petr. is based on C. brencklei Petr. occurring on Solidago in North America. However, based on the associated Phomopsis asexual state, it appears likely to be a synonym of Diaporthe. A specimen identified as this taxon (BPI 843482), grown in culture, and sequenced was determined to be a Diaporthe, thus Clypeoporthella is considered a synonym of Diaporthe.
Dicarpella P. Syd. & Syd. based on D. bina (Harkn.) P. Syd. & Syd. (≡ Physalospora bina Harkn.) is known only from the type collection on Quercus agrifolia Nee in California. The anamorphic state of Dicarpella, Tubakia B. Sutton is relatively common on oak leaves.
Diplacella Syd., D. paullinae (Frag. & Cif.) Syd. is parasitic on leaves of Paullinia and other members of the Sapindaceae in Central and South America. Arx & Müller (1954) provide a description of this species.
The genus Gaeumannomyces Arx & D.L. Olivier was included in the Gnomoniaceae by a number of authors such as Barr (1978) and Monod (1983), and considered as a synonym of Linocarpon by Kobayashi (1970). However, molecular data obtained from the type species and serious pathogen G. graminis (Sacc.) Arx & D.L. Olivier confirmed that this genus is not a member of the Diaporthales but that it belongs to the Magnaporthaceae (Castlebury et al. 2002, Zhang et al. 2008).
The genus Gnomoniella Sacc., based on the type species, G. tubaeformis (Fr.: Fr.) Sacc., was not included in this study for lack of living material. The type species occurs on overwintered leaves and petioles of Alnus spp. in Europe and North America. Several fresh collections were obtained of the non-type species G. alnobetulae Volkart, a species having four-spored asci but otherwise morphologically close to G. tubaeformis. Attempts to prepare a pure culture were not successful. Based on the ITS sequence of G. alnobetulae obtained by direct amplication from asci, this species may represent a distinct genus in the Gnomoniaceae.
Hypospilina bifrons (DC.: Fr.) Traverso, type of the genus Hypospilina (Sacc.) Traverso, occurs on dead leaves of Quercus spp. in Europe and was not collected for this study.
Lambro Racib. based on L. insignis Racib. produces necrotic spots in leaves of Sterculia subpeltata Blume in Indonesia (Müller & Arx 1962). Monod (1983) examined the type specimen and suggested that this species is related to Stegophora, thus it may belong in the Sydowiellaceae.
The genus Linospora Fuckel based on L. capreae (DC.: Fr.) Fuckel) was distinguished by ascomata covered with a rudimentary stroma and elongate ascospores. Linospora capreae groups with Pleuroceras. Because Linospora was published after Pleuroceras, Linospora is considered a synonym of Pleuroceras. Fifty-seven names have been placed in Linospora but most of them have been little studied and are infrequently encountered.
Mamiania Ces. & De Not. based on M. fimbriata (Fr.) Ces. & De Not. occurs on living leaves of Carpinus spp. in Europe, Asia, and North America. This species has a conspicuous stroma surrounding the perithecia and ascospores with a distinct, submedian septum.
The genus Mamianiella Höhn., based on the species, M. coryli (Batsch: Fr.) Höhn., occurs on living leaves of Corylus. We have not been able to grow this species in culture.
Mazzantia Mont. (syn. Clypeocarpus Kirschst., Paramazzantia Petr.) is based on M. galii (Fr.) Mont. Paramazzantia is based on Laestadia biennis Dearness on overwintered leaves of Solidago in North America. Clypeocarpus is based on C. alpinus Kirschst. occurs on Veratrum album and is considered a synonym of M. napelli by Von Arx & Müller (1954). Mazzantia napelli was sequenced and determined to belong in the Diaporthaceae by Castlebury et al. (2002).
Phylloporthe Syd., based on P. vernoniae Syd., is parasitic on living leaves of Vernonia triflosculosa H.B.K. in Costa Rica. It is only known from the type specimen. A second species, P. orbiculata (Syd.) E. Müll. is now placed in Uleoporthe (Cannon 2001).
Plagiosphaera Petr. based on P. immersa (Trail) Petr. (≡ Ophiobolus immersus Trail) occurs on overwintered stalks of Campanula and Urtica in Europe and was not collected during the course of this study.
The genus Pleuroceras Riess includes Gnomonia-like fungi having ascomata with eccentric, lateral necks and elongated ascospores and was placed in the Valsaceae by Barr (1978). The type species is P. cryptoderis (Lév.) Höhn., which occurs on overwintered leaves of Populus alba. Many of the 23 species currently recognised in Pleuroceras were transferred from Gnomonia by Barr (1978) and Monod (1983). Most of the species included in Pleuroceras are found on overwintered leaves of hardwood trees in temperate regions.
Although several species of Pleuroceras are included in the multigene phylogeny in this paper (Fig. 1), this genus is not treated in detail, because its type species, P. cryptoderis, was not available for sequencing. Several species of Pleuroceras were included in this study and form a well-supported monophyletic genus. One species of Pleuroceras, P. sassafras, is transferred to Ophiognomonia.
Sphaerognomonia Potebnia based on S. carpinea (Fr.) Potebnia includes the synonym, Apiosporopsis (Traverso) Mariani based on the same type species. Sequences of this species (CBS 617.72 and CBS 738.6) place this genus in the Diaporthales but outside of the Gnomoniaceae and Melanconidaceae (Castlebury, unpublished).
Stegophora P. Syd. & Syd. based on S. ulmea (Schw.: Fr.) P. Syd. & Syd. is parasitic on living leaves of Ulmus spp. in North America. A second species, S. oharana (Y. Nisik. & H. Matsumoto) Petr. occurs in Japan. Molecular data suggests that S. ulmea belongs to the Sydowiellaceae (Castlebury, unpub. data) as circumscribed by Rossman et al. (2007),Rossman et al. (2007).
Uleoporthe Petr., typified by U. orbiculata (Syd.) Petr., was redescribed by Cannon (2001) based on a fresh specimen from Guyana. His redesciption, the occurrence of this species as a leaf parasite, and the presence of a distinct well-developed stroma suggest an affiliation with the Sydowiellaceae.
Acknowledgments
This work was funded by NSF PEET grant 0328634 for research on the systematics of the Gnomoniaceae, Diaporthales for which we are most grateful. The following individuals contributed significantly to this project: Michel Monod, Lausanne, Switzerland, was generous in sharing his knowledge and providing useful advice and comments as well as arranging the logistics for collecting in Switzerland. Jean-Louis Moret, Lausanne, Switzerland, allowed the first author access to specimens housed in the Botanical Garden Museum of University of Lausanne. Margaret Barr, recently deceased, Sidney, Canada, and Larissa Vassiljeva, Vladivostok, Russia, both experts in the Gnomoniaceae, willingly assisted in identifications and helped with collecting and providing fresh specimens. Walter Jaklitsch, Vienna, Austria, and Dimitar Stoykov, Bulgaria, sent numerous accurately identified fresh collections from which cultures were obtained. Dmitriy Maykov, Kholm, Novgorod oblast, Russia, also provided fresh collections and assistance with logistics while in Russia. Gary Samuels, Drew Minnis, John Wiersema, and Joseph Kirkbride, USDA-ARS, Beltsville, Maryland, provided advice on nomenclatural issues. Christian Feuillet kindly provided the Latin diagnoses. David Farr assisted with use of the microscope, digital photography, and computer aspects of this research. A number of technicians at the Systematic Mycology & Microbiology Laboratory were essential to this work, specifically Brandon Dyson, Franklin Hendrick, Aimee Hyten, Cindy Park, Suganda Patibanda, and Tunesha Phipps. Erin McCray, Collections Manager of the U.S. National Fungus Collections (BPI), was invaluable in locating, obtaining and accessioning the many specimens from other herbaria as well as those at BPI. The curators and directors of the numerous herbaria listed in the Material and Methods are thanked for providing reference collections essential for this research. The Centraalbureau voor Schimmelcultures especially Pedro Crous, Gerard Verkley and Trix Merkx were extremely cooperative in providing and accessioning the numerous cultures derived from specimens used in this study. Finally, the authors would like to acknowledge the thorough scrutiny given to this manuscript by two anonymous reviewers who provided many useful suggestions for improvements.
Taxonomic novelties: New genus: Ambarignomonia. New species: Gnomonia incrassata, G. monodii, G. neognomon, G. orcispora, G. pendulorum, G. rodmanii, G. skokomishica, G. virginianae, Gnomoniopsis paraclavulata, Ophiognomonia balsamiferae, O. pseudoclavulata, O. vasiljevae, Plagiostoma barriae. New combinations: Ambarignomonia petiolorum; Apiognomonia hystrix; Gnomonia alnea, G. carpinicola, Gnomoniopsis clavulata, G. comari, G. fructicola, G. macounii, G. racemula, G. tormentillae; Ophiognomonia alni-viridis, O. gei-montani, O. intermedia, O. ischnostyla, O. leptostyla, O. micromegala, O. nana, O. rubi-idaei, O. setacea, O. trientensis; Plagiostoma aesculi, P. amygdalinae, P. robergeanum, and P. salicellum.
References
- Anagnostakis SL (1987). Chestnut blight: The classical problem of an introduced pathogen. Mycologia 79: 23–37. [Google Scholar]
- Arx JA von, Müller E (1954). Die Gattungen der amerosporen Pyrenomyceten. Beiträge Kryptogamenflora der Schweiz 11(1): 1–434. [Google Scholar]
- Barr ME (1978). The Diaporthales in North America with emphasis on Gnomonia and its segregates. Mycologia Memoirs 7: 1–232. [Google Scholar]
- Barr ME (1990). Prodromus to nonlichenised, pyrenomycetous members of class hymenoascomycetes. Mycotaxon 39: 43–184. [Google Scholar]
- Berry, Frederick H. 1981. Walnut Anthracnose Forest Insect & Disease Leaflet 85. U.S. Dept. of Agriculture, Forest Service, Northern Area State & Private Forestry, [Broomall, PA].
- Braun, U. 1991. Studies on Ramularia and allied genera (IV). Nova Hedwigia 53: 291–305. [Google Scholar]
- Cannon PF (2001). Rediscovery and redescription of the genus Uleoporthe (Melanconidaceae). Fungal Diversity 7: 17–25. [Google Scholar]
- Carbone I, Kohn LM (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN (2002). A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. [PubMed] [Google Scholar]
- Clamp M, Cuff J, Searle SM, Barton GJ (2004). The Jalview Java Alignment Editor. Bioinformatics 20: 426–427. [DOI] [PubMed] [Google Scholar]
- Clements FE, Shear C (1931). The Genera of Fungi. H.W. Wilson Company.
- Cohen SD (1999). Technique for large scale isolation of Discula umbrinella and other foliar endophytic fungi from Quercus species. Mycologia 91: 917–922. [Google Scholar]
- Cohen SD (2004). Endophytic-host selectivity of Discula umbrinella on Quercus alba and Quercus rubra characterised by infection, pathogenicity and mycelial compatability. European Journal of Plant Patholology 110 713–721. [Google Scholar]
- Danti R, Sieber TN, Sanguineti G (2002). Endophytic mycobiota in bark of European beech (Fagus sylvatica) in the Apennines. Mycological Research 106: 1343–1348. [Google Scholar]
- De Silva H, Castlebury LA, Green S, Stone J (2008). The phylogenetic relationship between Anisogramma virgultorum and A. anomala within the Diaporthales (Ascomycota). Mycological Research: in press. [DOI] [PubMed]
- Eriksson OE, Baral H-O, Currah RS, Hansen K, Kurtzman CP, Rambold G, Laessøe T (eds.) (2001). Outline of Ascomycota-2001. Myconet 7: 1–88. [Google Scholar]
- Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 6 227–242. [DOI] [PubMed] [Google Scholar]
- Froidevaux L, Müller E (1972). [Anisogramma virgultorum, a pathogenic ascomycete on Betula pubescens]. European Journal of Forest Patholology 2: 185–187. [Google Scholar]
- Fuckel KWGL (1869) [1870]. Symbolae mycologicae. Jahrbücher des Nassauischen Vereins für Naturkunde. 23–24: 1–459. (Reprint Cramer 1966). [Google Scholar]
- Gottwald TR, Cameron HR (1979). Studies in the morphology and life history of Anisogramma anomala. Mycologia 71: 1107–1126. [Google Scholar]
- Green S (2004). Fungi associated with shoots of silver birch (Betula pendula) in Scotland. Mycological Research 108: 1327–1336. [DOI] [PubMed] [Google Scholar]
- Green S, Castlebury LA (2007). Connection of Gnomonia intermedia to Discula betulina and its relationship to other taxa in Gnomoniaceae. Mycological Research 111: 62–69. [DOI] [PubMed] [Google Scholar]
- Gregory NF, Mulrooney RP, Rossman AY, Castlebury LA (2004). Anthracnose caused by Discula fraxinea on the new host Chinese fringetree and white ash in Delaware. Plant Disease 88 427. [DOI] [PubMed] [Google Scholar]
- Gryzenhout M, Myburg H, Wingfield BD, Wingfield MJ (2006). Cryphonectriaceae (Diaporthales), a new family including Cryphonectria, Chrysoporthe, Endothia and allied genera. Mycologia 98: 239–249. [DOI] [PubMed] [Google Scholar]
- Hawksworth DL, Eriksson O (1988). (895) – (906) Proposal to conserve 11 family names in the Ascomycotina (Fungi). Taxon 37: 190–193. [Google Scholar]
- Höhnel F von (1917). System der Diaportheen. Berichte der Deutschen Botanischen Gesellschaft 35: 631–638. [Google Scholar]
- Holcomb GE (1998). First report of anthracnose of velvet ash caused by Discula fraxinea in Louisiana. Plant Disease 82: 1401. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Johnson KB, Mehlenbacher SA, Stone JK, Pscheidt JW, Pinkerton JN (1996). Eastern filbert blight of European hazelnut. Plant Disease 80: 1308–1316. [Google Scholar]
- Juhasova G, Ivanova H, Spisak J (2006). Biology of fungus Gnomonia leptostyla in agro-ecological environments of Slovakia. Mikologica Fitopatologie 40: 538–547. [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers JA (eds) (2001). Ainsworth and Bisby's Dictionary of the Fungi. Ninth Edition. CAB International, Kew.
- Klebahn H (1918). Haupt- und Nebenfruchtformen der Askomyzeten. Verlag von Gebruder Borntraeger.
- Kobayashi T (1970). Taxonomic studies of Japanese Diaporthaceae with special reference to their life-histories. Bulletin of the Government Forest Experiment Station 226: 1–242. [Google Scholar]
- Kornerup A, JH Wanscher (1978). Methuen Handbook of Colour. 3rd Ed. Methuen London Ltd, London.
- Krause RA, Webster RK (1972). The morphology, taxonomy, and sexuality of the rice stem rot fungus, Magnaporthe salvinii (Leptosphaeria salvinii). Mycologia 64: 103–114. [Google Scholar]
- Maas JL (1998). Compendium of Strawberry Diseases, Second Edition. American Phytopathology Society, St. Paul, Minnesota.
- McNeill J, Barrie FR, Burdet HM, Demoulin V, Hawksworth DL, Marhold K, Nicolson DH, Prado J, Silva PC, Skog JE, Wiersema JH, Turland NJ (eds.) (2006). International Code of Botanical Nomenclature (Vienna Code). A. R. G. Gantner Verlag, Ruggell, Liechtenstein.
- Mejia LC, Castlebury LA, Rossman AY, Sogonov MV, White JF (2008). Phylogenetic placement and taxonomic review of the genus Cryptosporella and its synonyms Ophiovalsa and Winterella (Gnomoniaceae, Diaporthales). Mycological Research 112: 23–35. [DOI] [PubMed] [Google Scholar]
- Monod M (1983). Monographie taxonomique des Gnomoniaceae. Beihefte sur Sydowia 9: 1–315. [Google Scholar]
- Moricca S, Ragazzi A (2008). Fungal endophytes in Mediterranean oak forests: A lesson from Discula quercina. Phytopathology 98: 380–386. [DOI] [PubMed] [Google Scholar]
- Moročko I, Fatehi J (2007). Molecular characterisation of strawberry pathogen Gnomonia fragariae and its genetic relatedness to other Gnomonia species and members of Diaporthales. Mycological Research 111: 603–614. [DOI] [PubMed] [Google Scholar]
- Moročko I, Fatehi J, Gerhardson B (2006). Gnomonia fragariae, a cause of strawberry root rot and petiole blight. European Journal of Plant Pathology 114: 235–244. [Google Scholar]
- Müller E, Arx JA von (1962). Die Gattungen der didymosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz 11(2): 1–922. [Google Scholar]
- Neely D, Black WM (1976). Anthracnose of black walnuts in the midwest. Plant Disease Reporter 60: 519–521. [Google Scholar]
- Ostry ME (1996). Butternut canker: history, biology, impact, and resistance. Unknown publisher: 1–9.
- Paulus B, Gadek P, Hyde K (2007). Successional patterns of microfungi in fallen leaves of Ficus pleurocarpa (Moraceae) in an Australian tropical rain forest. Biotropica 38 42–51. [Google Scholar]
- Pennycook SR (2007). Discula betulae comb. nov., correct name for the anamorph of Gnomonia intermedia. Mycotaxon 101: 361–364. [Google Scholar]
- Podlahova R, Svrček M (1970). Three new species of Pyrenomycetes from alders. Česka Mykologie. 24: 129–133. [Google Scholar]
- Posada D, Crandall KA (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14 (9): 817–818. [DOI] [PubMed] [Google Scholar]
- Redlin SC (1991). Discula destructiva sp. nov., cause of dogwood anthracnose. Mycologia 83: 633–642. [Google Scholar]
- Redlin SC, Rossman AY (1991). Cryptodiaporthe corni (Diaporthales) cause of Cryptodiaporthe canker of pagoda dogwood. Mycologia 83: 200–209. [Google Scholar]
- Redlin SC, Stack RW (1988). Gnomoniella fraxini sp. nov. teleomorph of the ash anthracnose fungus and its connection to Discula fraxinea comb. nov. Mycotaxon 32: 175–198. [Google Scholar]
- Reeb V, Lutzoni F, Roux C (2004). Contribution of RPB2 to multilocus phylogenetic studies of the Pezizomycotina (euascomycetes, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Molecular Phylogenetics and Evolution 32: 1036–1060. [DOI] [PubMed] [Google Scholar]
- Rehner S (2001) Primers for Elongation Factor 1-a (EF1-a). Available from: http://ocid.nacse.org/research/deephyphae/EF1primer.pdf
- Remler P (1979). Ascomyceten auf Ericaceen in den Ostalpen. Bibliotheca Mycologica 68: 1–321. [Google Scholar]
- Rossman AY, Farr DF, Castlebury LA (2007). A review of the phylogeny and biology of the Diaporthales. Mycoscience 48: 135–144. [Google Scholar]
- Rossman AY, Castlebury LA, Farr DF, Stanosz GR (2007). Sirococcus conigenus, Sirococcus piceicola sp. nov. and Sirococcus tsugae sp. nov. on conifers: anamorphic fungi in the Gnomoniaceae, Diaporthales. Forest Pathology 38: 47–60. [Google Scholar]
- Rossman AY, Castlebury LA, Putnam ML (2004). First report of ash anthracnose caused by Discula fraxinea in Oregon. Plant Disease 88: 222. [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Blackwell M (2001). Pyrenomycetes – fungi with perithecia. In: McLaughlin D, McLaughlin E. The Mycota VII Part A. Systematics and Evolution. Springer-Verlag, Berlin, 221–255.
- Schmid-Heckel H (1988). Pilze in den Berchtesgadener Alpen. Forschungsberichte Nationalpark Berchtesgaden 15: 1–136. [Google Scholar]
- Sinclair WA, Lyon HH (2005). Diseases of Trees and Shrubs, 2nd Edition. Cornell University, Ithaca, NY.
- Sogonov MV (2005). Software for morphological and molecular taxonomic studies of fungi. Inoculum 56(4): 54. [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, Farr DF, White JF (2005). The type species of genus Gnomonia, G. gnomon, and the closely related G. setacea. Sydowia 57: 102–119. [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman A, White JF (2007). The type of species of Apiognomonia, Apiognomonia veneta, with its Discula anamorph is distinct from Apiognomonia errabunda. Mycological Research 111: 693–709. [DOI] [PubMed] [Google Scholar]
- Stoykov DY, Denchev CM (2006). Current knowledge of Diaporthales (Ascomycota) in Bulgaria. Mycologia Balanica 3: 179–185. [Google Scholar]
- Sutton BC (1980). The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata. Commonwealth Mycological Institute, Kew, Surrey, England.
- Swofford D L (2002). PAUP v. 4.0b10. Phylogenetic Analysis Using Parsimony. – Sinauer Associates, Sunderland, Massachusetts.
- Vasilyeva LN (1998). Pyrenomycetidae et Loculomycetidae. Plantae non Vasculares, Fungi et Bryopsidae. Orientis extremi Rossica. Fungi 4 1–418 (in Russian). [Google Scholar]
- Viret O, Petrini O (1994). Colonisation of beech leaves (Fagus sylvatica) by the endophyte Discula umbrinella (teleomorph: Apiognomonia errabunda). Mycological Research 98: 423–432. [Google Scholar]
- Voglmayr H, Jaklitsch WM (2008). Prosthecium species with Stegonsporium anamorphs on Acer. Mycological Research 112: 885–905. [DOI] [PubMed] [Google Scholar]
- Vujanovic V, Britton J (2002). A comparative study of endophytic mycobiota in leaves of Acer saccharum in eastern North America. Mycological Progress 1: 147–154. [Google Scholar]
- Wallroth KFW (1833). Flora Cryptogamica Germaniae. Vol. 2. Nuremburg.
- Wehmeyer LE (1933). The genus Diaporthe Nitschke and its segregates. University of Michigan Studies, Science Series 9: 1–349. [Google Scholar]
- Wehmeyer LE (1975). The pyrenomycetous fungi. Mycologia Memoirs 6 1–250. [Google Scholar]
- White TJ, T Bruns S Lee, J Taylor (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA & al. (eds.). PCR Protocols: A Guide to Methods and Applications. Academic Press, San Diego: 315–322.
- Wilson D, Barr ME, Faeth SH (1997). Ecology and description of a new species of Ophiognomonia endophytic in the leaves of Quercus emoryi. Mycologia 89: 537–546. [Google Scholar]
- Winter G (1886). Die Pilze Deutschlands, Osterreichs und der Schweiz. II. Abteilung: Ascomyceten: Gymnoasceen. Rabenhorst's Kryptogamen-Flora, 1(2). Verlag E. Kummer, Leipzig.
- Zhang N, Blackwell M (2001). Molecular phylogeny of dogwood anthracnose fungus (Discula destructiva) and the Diaporthales. Mycologia 93: 355–365. [Google Scholar]
- Zhang N, Castlebury LA, Miller AN, Schoch C, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung G-H (2006). An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98: 1076–1087. [DOI] [PubMed] [Google Scholar]