Abstract
Introduction:
The aim of this study was to investigate whether asymptomatic women with diabetes mellitus (DM) without previous history of ischemic heart disease (IHD) and normal electrocardiogram (ECG) have suffered silent myocardial infarction (MI).
Methods:
The study population consisted of 64-years old women with DM and albuminuria (n = 15) and aged- and body mass index-matched controls (n = 16). The patients were selected after screening of 240 women with previously known or unknown DM. The individuals with previous history of IHD and ECG suggesting the presence of IHD were excluded. All subjects were investigated with magnetic resonance imaging (MRI).
Results:
MRI investigation has revealed the presence of subendocardial MI in the two DM women (13%). No MI was detected in the control group. MR coronary angiography detected the presence of significant stenosis in the proximal segment of left anterior descending (LAD) coronary artery in one DM woman. This patient developed unstable angina 1 week after the MRI investigation. The conventional angiography has confirmed the presence of significant stenosis in LAD demanding invasive revascularization by percutaneous coronary angioplasty. No difference was found in indices of left ventricular (LV) systolic function while diastolic function was disturbed in the DM group. There was a tendency for increased LV mass in the DM group. No difference was found in the LV volumes.
Conclusion:
Clinically significant proportion of the women with DM and albuminuria without previous history of IHD have had silent MI. MRI screening of these high risk female patient is valuable diagnostic tool which may increase diagnostic accuracy and improve prognosis in DM patients with IHD.
Keywords: asymptomatic, diabetes mellitus, ischemic heart disease, silent myocardial infarction
Introduction
Diabetes mellitus (DM) is a major risk factor for coronary artery disease and is associated with higher incidence of myocardial infarction (MI; Kannel and McGee 1979) and sudden death (Haffner 2000) compared to nondiabetics. This is in particular the case for women in whom cardiovascular mortality associated with type 2 is almost doubled in comparison with men (Niskanen et al 1998). Mortality, morbidity and re-infarction rate are higher among DM patients after MI compared to non-DM patients (Abbott et al 1988) with one-year mortality reaching 50% (Miettinen et al 1998). Diabetics have also increased incidence of congestive heart failure (Kannel et al 1974; Poornima et al 2006). Reports from general population have found that unrecognized MI is a common phenomenon and may be associated with similar or even a worse prognosis than clinically diagnosed MI (Kannel and Abbott 1984; Kannel et al 1985; Yano and MacLean 1989; Sigurdsson et al 1995). Corresponding studies in DM population are sparse (Lundblad and Eliasson 2003; Davis et al 2004). Furthermore, these studies may be associated with important limitations if only the traditional method, ie, the presence of Q-wave on ECG, is used for detection of MI (Sheifer et al 2001; Davis et al 2004). Some postmortem observational studies have demonstrated increased prevalence of unrecognized MI in diabetics while clinical evaluation have revealed propensity for induction of silent myocardial ischemia on exercise test during perfusion imaging (Gokcel et al 2003; Valensi et al 2005). Autonomic neuropathy has been proposed to be the major contributing factor for development of “painless myocardial ischemia” in DM (Chico et al 2005). Microalbuminuria is associated with a high risk of future cardiovascular disease in type 2 diabetes (Ibsen et al 2006). The explanation to this association is unknown but hypothetically diabetic patients with microalbuminuria may have microvascular disease causing both renal damage and ischemic myocardial lesions. Accordingly, the aim of this study was to investigate whether women with known or recently diagnosed DM, no previous history of cardiovascular disease, and normal ECG may have suffered silent MI using state-of-the-art magnetic resonance imaging (MRI) methods. Our results show that a clinically significant proportion of DM women have presence of nonviable tissue in their hearts.
Methods
Patient population
The study was approved by the ethics committee at Gothenburg University. The recruitment of the study population was previously described in detail (Brohall et al 2006). Briefly, all 64-year-old women identified through the County Register in Gothenburg, Sweden, were sent an invitation letter to participate in a screening that took place in 2001–2004 and included women born in 1937–1940. Subjects who responded positively but reported a recent cancer diagnosis, chronic inflammatory disease, severe mental disorder, other severe illness, or drug addictions were excluded. Participants were invited to a screening examination including an oral glucose tolerance test (OGTT). Women with known diabetes who were being treated with oral antidiabetic drugs or insulin were examined without preceding OGTTs, whereas women with diet-treated diabetes and fasting blood glucose (FBG) <7.5 mmol/l were examined with OGTTs. The examinations also included a questionnaire regarding previous diseases, current medication, smoking habits, and heredity for diabetes. Anthropometric measurements were made, and blood pressure and heart rate were recorded. Body weight, waist, and hip circumferences were measured according to current guidelines. Blood pressure was measured in the right arm with the patient in the supine position using a cuff of appropriate size after at least 5 min of rest. The mean of two recordings was used. The World Health Organization (WHO) criteria (19) for capillary blood glucose cutoff values were used. Diabetes was defined as FBG ≥ 6.1 (≥110 mg/dl) and/or ≥11.1 mmol/l (≥200 mg/dl) 2 h after glucose load measured on two occasions. Detection of albuminuria was conducted according to the standard procedure (Agewall et al 1997). The patients were asked to bring two overnight (12 h) urine samples for determination of urinary albumin excretion by using an immunonephelometry method. The mean value was used in the analyses. Microalbuminuria was defined as a urinary albumin excretion between 20 and 200 μg/min.
Study design
The screening program resulted in 240 DM individuals with previously known or unknown DM. Inclusion criteria in this study were type 2 diabetes, microalbuminuria, and no indications of cardiovascular disease. The diagnosis of type 2 diabetes was based on the WHO definition and the measurement of GAD antibodies. The healthy control group was matched as in regard to body mass index and waist circumference. Using these criteria 18 patients with DM and albuminuria and 17 healthy volunteers were randomly selected and asked for participation in the MR study. One patient was excluded due to the presence of atrial fibrillation at the diagnostic work-up while two patients in the end did not agree to participate in the study. Table 1 describes the size and characteristics of the study group.
Table 1.
Basic characteristics
| DM n = 15 | Control n = 16 | |
|---|---|---|
| Height (cm) | 163 ± 6 | 164 ± 7 |
| Weight (kg) | 79 ± 16 | 81 ± 13 |
| BMI (kg/m2) | 29.4 ± 4.8 | 30.1 ± 4.5 |
| Waist (cm) | 100.9 ± 12.2 | 96.9 ± 10.9 |
| Waist-to-hip ratio | 0.87 ± 0.51* | 0.94 ± 0.71 |
| Systolic blood pressure (mmHg) | 146 ± 20* | 123 ±11 |
| Smoking | 5 (33%) | 4 (25%) |
| Total-cholesterol (mmol/L) | 5.92 ± 1.13 | 6.12 ± 1.06 |
| Triglycerides (mmol/L) | 1.90 ± 1.06* | 1.18 ± 0.32 |
| HDL (mmol/L) | 1.48 ± 0.34 | 1.61 ± 0.52 |
| LDL (mmol/L) | 3.58 ± 1.1 | 3.98 ± 0.96 |
| P-insulin (mU/L) | 105 ± 57* | 39 ± 16 |
| U-albumin (mg/L) | 82 ± 190* | 5 ± 4 |
| HbA1c (%) | 6.2 ± 1.8* | 4.4 ± 0.4 |
| ACE/ARB | 6 (40%) | – |
| Beta-blocker | 4 (27%) | – |
| Ca-blocker | 3 (20%) | – |
| Statin | 3 (20%) | – |
| Insulin | 2 (13%) | – |
| Sulfonylurea | 3 (20%) | – |
| Metformin | 4 (27%) | – |
| Glitazones | 2 (13%) | – |
| Aspirin | 6 (40%) | – |
Note: *p < 0.05 versus control.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; BMI, body mass index; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Magnetic resonance imaging
All patients were placed supine in a 1.5-T clinical scanner (Intera, Philips, Netherlands) and a phased-array receiver coil was placed on the chest for imaging. Images were acquired during breath-hold and were ECG-gated to end diastole. Cine images were acquired in six to eight short-axis views and two long-axis views for evaluation of cardiac function and morphology. The acquisition of short-axis views started 1 cm below the level of the mitral-valve–insertion plane and continued in 1-cm increments through the left ventricle. A commercially available gadolinium-based contrast agent (Magnevist) was administered intravenously at a dose of 0.2–0.03 mmol per kilogram of body weight and contrast-enhanced images were acquired in the same views as those used for cine MRI. Contrast-enhanced T1 weighting of images were achieved with an inversion-recovery fast low-angle shot (IR-FLASH) pulse sequence according to the previously described general protocol (Kim et al 2000). Typical parameters for imaging were TE = 2 ms, TR = 6 ms, voxel size = 1 × 1 × 6 mm, 300-ms inversion delay, k-space data segmented over 4 cardiac cycles (32 lines per cycle) with data acquired every other cardiac cycle. Whole-heart coronary MR angiography was obtained while patients were free breathing, with the use of a 3-dimensional, segmented steady-state free precession sequence with T2 preparation and radial k-space sampling (repetition time = 4.6 ms, echo time = 2.3 ms, flip angle = 90°, excitations per cardiac cycle = 20 to 50, SENSE factor = 2.0, navigator gating window of = 2.5 mm, no drift correction, field of view = 280 × 280 × 120 mm, acquisition matrices = 256 × 256 × 80, reconstruction matrices = 512 × 512 × 160). Analysis of MRI scans was performed independently by two radiologists blinded to the clinical conditions of the patients.
Invasive coronary angiography
The individuals in whom MRI investigation showed nonviable tissue and/or there was suspicion of significant coronary artery disease on MR angiogram were admitted to further investigation with conventional angiography and patient care services according to the local routines.
Statistics
Computer software (Statview 5.0.1; SAS Institute, Cary, NC) was used to perform standard statistical procedure with calculation of mean value and standard deviation (SD) in the different groups. Unpaired student t-test was used to determine if variables between the groups were statistically significant. All data are presented as mean ± SEM, unless otherwise indicated. Categorical data were tested using a 2 × 2 contingency table and Fisher’s exact test. The p < 0.05 (two-sided) was defined as significant.
Results
Study population
The main characteristic of patients and control are given in Table 1. The patients and controls were comparable regarding most of the basic characteristic, however there were differences in waist-to-hip-ratio, systolic blood pressure, triglycerides, plasma insulin, HbA1c systolic blood pressure, and urine albumin.
Magnetic resonance imaging
DM patients have shown strong tendency for increased LV mass. No difference in stroke volume (SV) and ejection fraction (EF) was found between the groups (Table 2) suggesting preserved systolic function. Delayed-enhancement MRI revealed the presence of subendocardial infarction in two patients (13%) from the DM group. In the first patient there was evidence of small subendocardial infarction located anteriorly while in the second patient subendocardial infarction was located inferiorly. No signs of myocardial damage were detected in the control subjects. In addition MR coronary angiography showed high-grade stenosis in the proximal portion of the left anterior descending (LAD) coronary artery in one DM patient. Interestingly, this patient developed unstable angina expressed as acute chest pain and ST-segment depression one week after MRI investigation and underwent acute revascularization with percutaneous coronary intervention. The conventional coronary angiography confirmed the presence of culprit lesion in the LAD (Figure 4). One DM patient with subendocardial MI have shown presence of significant coronary artery stenosis on MR angiography in LAD and right coronary artery. The other DM patient with presence MI had normal MR coronary angiogram. No significant coronary artery disease was detected in the control group on MR angiogram. If we combine these two findings—ie, presence of myocardial scar and significant coronary artery disease—than our MRI protocol detected important cardiac abnormalities in 20% of asymptomatic DM patients.
Table 2.
LV systolic function and morphology
| DM | Control | |
|---|---|---|
| EF (%) | 63 ± 4 | 60 ± 3 |
| SV (mL) | 75 ± 7 | 77 ± 3 |
| LVD (mL) | 119 ± 7 | 131 ± 4 |
| LVS (mL) | 52 ± 7 | 54 ± 5 |
| LV mass (g) | 92 ± 8 | 75 ± 5 |
Abbreviations: EF, ejection fraction; SV, stroke volume; LVD, left ventricular volume in diastole; LVS, left ventricular volume in systole; LV, left ventricular mass.
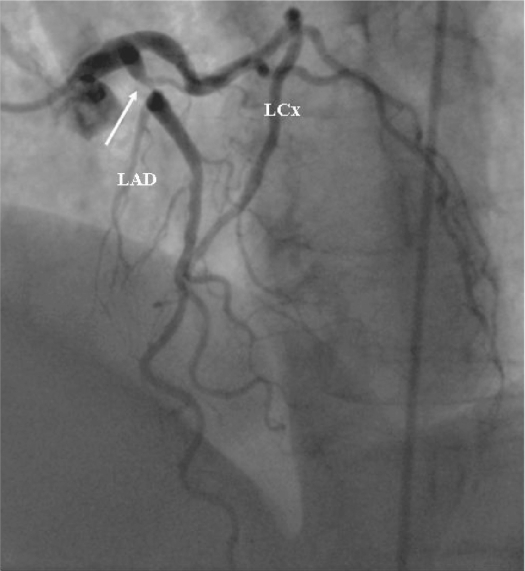
Figure 4.
Coronary angiogram from the patient diabetes mellitus with developed unstable angina one week after that magnetic resonance imaging investigation suggested presence of significant coronary disease. Notice tight narrowing (arrow) in the proximal segment of left anterior descending artery (LAD).
Invasive coronary angiography
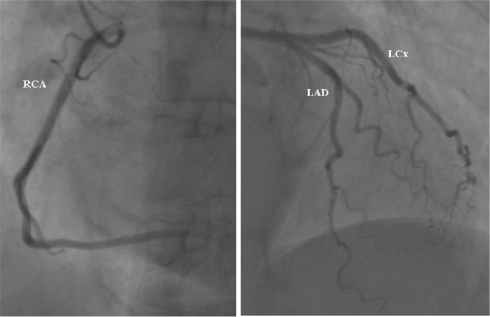
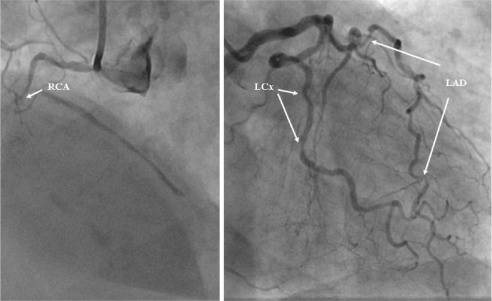
Three patients underwent invasive coronary angiography ie, the DM patient who developed unstable angina one week after MRI and the two DM patients with subendocardial MI. Invasive angiography confirmed the presence of high-grade stenosis in the proximal LAD artery (Figure 4) in the patient that developed unstable angina. In the second patient with small anterior subendocardial infarction no abnormalities could be found on the angiogram (Figure 2). In the third patient with inferior subendocardial infarction there was evidence of multivessel disease with occlusion of right coronary artery (RCA) and multiple stenoses in the LAD and left circumflex (LCx) artery (Figure 3).
Figure 2.
Normal coronary angiogram from the patient with small anterior subendocardial infarction. This patient had recently diagnosed diabetes mellitus. Coronary angiography did not revealed any signs of obstructive atherosclerotic plaque in the large epicardial arteries. (Left panel) left anterior oblique view of the right coronary artery. (Right panel) Right anterior oblique view of left anterior descending artery (LAD) and left circumflex artery (LCx).
Figure 3.
Coronary angiogram from the patient with inferior subendocardial infarction. There is evidence of multivessel disease. The images depicts occluded (arrow) right coronary artery (RCA) (left panel) and multiple significant stenosis (>50 % of reference lumen diameter) (arrows) in left anterior descending artery (LAD) and left circumflex artery (LCx) (right panel).
Discussion
The results from this study showed that a clinically meaningful proportion of 20% of diabetic patients with microalbuminuria might have important cardiac abnormalities such as myocardial infarction and significant coronary artery narrowing in the absence of symptoms. These clinically unrecognized MI may be reliably detected noninvasively by means of cardiac MRI investigation. These results give support to the observations that the prevalence of MI as well as the mortality related to MI is higher in diabetic subjects than in nondiabetics. In addition, the clinical signs of CHD are often not typical and may be absent. Both silent myocardial ischemia and silent MI seems to be more prevalent in diabetics than in nondiabetics (Gokcel et al 2003), and the presence of autonomic neuropathy has been hypothesized as a possible explanation (Chico et al 2005). A significant proportion of DM patients may develop atypical symptoms or may be symptomless during the development of myocardial ischemia and MI, which suggests that many DM individuals may have suffered MI at some point in time without being aware of it.
Due to the difficulty of screening all diabetic subjects for the presence of silent MI, it is very important to establish subgroups of patients with a high risk for silent MI. Microalbuminuria has been suggested as one marker of high cardiovascular risk in diabetic patients. In the present study we did not perform any comparison with diabetic patients without albuminuria. Stress myocardial perfusion imaging (Le Feuvre et al 2005) and recently MRI (Sandstede 2003; Wagner et al 2003b; Elliott and Kim 2005) have been postulated as the best technique for silent MI screening. Our results are based on the latter technique.
Several studies in general population have suggested that silent MI may be associated with similar or worse prognosis compared to clinically recognized MIs (Kannel and Abbott 1984; Yano and MacLean 1989; Kannel et al 1990; Sigurdsson et al 1995). This question was not addressed equally well in the DM population. In the recent study by Davis and colleagues (2004) the prognosis for DM patients with silent MI was reported to be better than in patients with clinically recognized MI. However, in this study the incidence of MI was determined using traditional approach, ie, the presence of Q-waves on ECG tracings. Our data suggest that this approach may be suboptimal since significant proportion of asymptomatic patients without Q-waves may still have suffered silent MI. Inclusion of this patient category may have resulted in different outcome. In the present study we selected women with type 2 DM and concomitant microalbuminuria; the clinical constellation with the highest risk of future cardiovascular events in DM patients (Ibsen et al 2006). Giving the fact that diabetics have higher mortality rates post-MI compared to the nondiabetics, it is imperative to implement all available diagnostic and therapeutic means to decrease this risk.
There are several methodological and clinical aspects of the study. First, although this was a small study it was based on a population sample, and the cohort consisted entirely of women, contrary to the previous studies. Women have been systematically underrepresented in important cardiovascular clinical trials. Second, we have selected only individuals with no previous history of cardiovascular disease and with normal ECG. To our knowledge such a cohort was not previously studied with MRI. Indeed, this technique revealed “true” silent MI without any trace on ECG recordings. Third, patient and physician “unawareness” of MI may lead to delayed implementation of important life-style changes and pharmacological treatment that decreases future cardiovascular events and prolong life.
The statistical analysis did not reach significance for increased incidence of silent MI in the DM group which is most probably the consequence of small sample size and limited statistical power. In our opinion, however, the data may be clinically relevant since 20 % of the asymptomatic diabetic women either may have suffered MI or may be at risk to develop unstable coronary syndrome. MRI is an excellent noninvasive imaging method for evaluation of cardiac morphology and function (Kim et al 2000). Besides excellent morphological assessment of the heart, this unique technique offers opportunity to study myocardial metabolism providing important insights about biochemistry of the diabetic heart (Neubauer 2000; Scheuermann-Freestone et al 2003; Szczepaniak et al 2003). Using the new and well validated technique of delayed-enhancement we can now diagnosed small subendocardial infarction that may be hidden to other methods such as ECG, SPECT (Wagner et al 2003a) and PET (Klein et al 2002; Kuhl et al 2006). Due to improved availability, decreasing costs and improved sensitivity and specificity of this method one can rightfully ask whether this technique should be used for screening of high-risk patient populations. Future studies are needed to confirm this hypothesis and to evaluate the cost-effectiveness of such screening given the expected increase in the incidence of DM and dismal prognosis of MI in DM. The results from the recently published Swedish study using the late enhancement MRI in 70-years old subjects partly support the main findings of our study, ie, that unrecognized MI are relatively frequent in elderly population and that cardiac MRI is a robust diagnostic tool (Ebeling Barbier et al 2007). Coronary angiography that was performed in the two patients with MRI-proved MI reviled different results. While in one woman coronary angiography has shown advanced three-vessel disease, the angiogram from the other patient has shown no angiographic signs of obstructive atherosclerotic plaque. What could be the pathogenesis of the myocardial damage in these two patients? The first explanation is ischemic damage due to the occlusion of some minor coronary vessel; the mechanism that is highly probable in the women with advanced three-vessel disease and previously known DM. However, the second patient did not show any visible atherosclerotic plaque on the angiogram. Although it can not be excluded that MI in this patient was indeed mediated by the same mechanism (ie, vessel occlusion), it is plausible that myocardial damage might have been induced by different mechanism. Recent studies suggest that DM patients have abnormalities in microvascular function and myocardial energy metabolism (Scheuermann-Freestone et al 2003) that may lead to cellular dysfunction and cell death.
Our results indicate that at 2 of 10 middle-aged women with type 2 diabetes and microalbuminuria have suffered a silent MI or have unrecognized severe coronary artery disease. It is necessary to validate these findings and consider screening strategies in order to provide more aggressive treatment for secondary prevention in patients with diabetes.
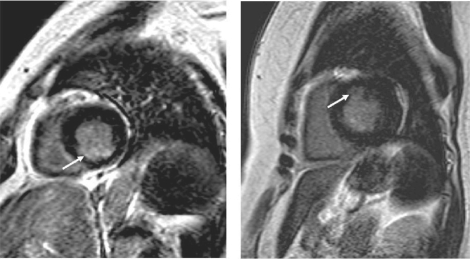
Figure 1.
Short-axis view of LV from the two diabetic women with MRI-visible subendocardial infarction (arrow). In the patient with multi-vessel disease (left panel) the location of subendocardial MI is inferior while the patient with normal coronary angiogram (right panel) had smaller subendocardial MI located in the anterior LV wall.
Abbreviations: LV, left ventricular mass; MI, myocardial infarction; MRI, magnetic resonance imaging.
Acknowledgments
The study was supported by grants from FoU (Research and Development Council) in Western Götaland, Swedish Heart and Lung Foundation, Swedish Scientific Research Council, Gothenburg Medical Society, Medical Faculty at Gothenburg University and Swedish Society for Medical Research.
References
- Abbott RD, Donahue RP, Kannel WB, et al. The impact of diabetes on survival following myocardial infarction in men vs women. The Framingham Study. JAMA. 1988;260:3456–60. [PubMed] [Google Scholar]
- Agewall MDPS, Wikstrand MDPJ, Ljungman MDPS. Usefulness of microalbuminuria in predicting cardiovascular mortality in treated hypertensive men with and without diabetes mellitus. Am J Cardiol. 1997;80:164–9. doi: 10.1016/s0002-9149(97)00312-3. [DOI] [PubMed] [Google Scholar]
- Brohall G, Behre CJ, Hulthe J, et al. Prevalence of diabetes and impaired glucose tolerance in 64-year-old Swedish women: experiences of using repeated oral glucose tolerance tests. Diabetes Care. 2006;29:363–7. doi: 10.2337/diacare.29.02.06.dc05-1229. [DOI] [PubMed] [Google Scholar]
- Chico A, Tomas A, Novials A. Silent myocardial ischemia is associated with autonomic neuropathy and other cardiovascular risk factors in type 1 and type 2 diabetic subjects, especially in those with microalbuminuria. Endocrine. 2005;27:213–17. doi: 10.1385/ENDO:27:3:213. [DOI] [PubMed] [Google Scholar]
- Davis TM, Fortun P, Mulder J, et al. Silent myocardial infarction and its prognosis in a community-based cohort of Type 2 diabetic patients: the Fremantle Diabetes Study. Diabetologia. 2004;47:395–9. doi: 10.1007/s00125-004-1344-4. [DOI] [PubMed] [Google Scholar]
- Ebeling Barbier C, Bjerner T, Hansen T, et al. Clinically unrecognized myocardial infarction detected at MR imaging may not be associated with atherosclerosis. Radiology. 2007;245:103–10. doi: 10.1148/radiol.2451061664. [DOI] [PubMed] [Google Scholar]
- Elliott MD, Kim RJ. Late gadolinium cardiovascular magnetic resonance in the assessment of myocardial viability. Coron Artery Dis. 2005;16:365–72. doi: 10.1097/00019501-200509000-00005. [DOI] [PubMed] [Google Scholar]
- Gokcel A, Aydin M, Yalcin F, et al. Silent coronary artery disease in patients with type 2 diabetes mellitus. Acta Diabetol. 2003;40:176–80. doi: 10.1007/s00592-003-0108-9. [DOI] [PubMed] [Google Scholar]
- Haffner SM. Coronary heart disease in patients with diabetes. N Engl J Med. 2000;342:1040–2. doi: 10.1056/NEJM200004063421408. [DOI] [PubMed] [Google Scholar]
- Ibsen H, Olsen MH, Wachtell K, et al. Does albuminuria predict cardiovascular outcomes on treatment with losartan versus atenolol in patients with diabetes, hypertension, and left ventricular hypertrophy? The LIFE study. Diabetes Care. 2006;29:595–600. doi: 10.2337/diacare.29.03.06.dc05-1724. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the Framingham study. N Engl J Med. 1984;311:1144–47. doi: 10.1056/NEJM198411013111802. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Cupples LA, Gagnon DR. Incidence, precursors and prognosis of unrecognized myocardial infarction. Adv Cardiol. 1990;37:202–14. doi: 10.1159/000418828. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Dannenberg AL, Abbott RD. Unrecognized myocardial infarction and hypertension: the Framingham Study. Am Heart J. 1985;109:581–85. doi: 10.1016/0002-8703(85)90566-6. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- Klein C, Nekolla SG, Bengel FM, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation. 2002;105:162–7. doi: 10.1161/hc0202.102123. [DOI] [PubMed] [Google Scholar]
- Kuhl HP, Lipke CS, Krombach GA, et al. Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography-single photon emission computed tomography imaging protocol. Eur Heart J. 2006;27:846–53. doi: 10.1093/eurheartj/ehi747. [DOI] [PubMed] [Google Scholar]
- Le Feuvre CL, Barthelemy O, Dubois-Laforgue D, et al. Stress myocardial scintigraphy and dobutamine echocardiography in the detection of coronary disease in asymptomatic patients with type 2 diabetes. Diabetes Metab. 2005;31:135–42. doi: 10.1016/s1262-3636(07)70179-9. [DOI] [PubMed] [Google Scholar]
- Lundblad D, Eliasson M. Silent myocardial infarction in women with impaired glucose tolerance: the Northern Sweden MONICA study. Cardiovasc Diabetol. 2003;2:9. doi: 10.1186/1475-2840-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen H, Lehto S, Salomaa V, et al. Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study Group. Diabetes Care. 1998;21:69–75. doi: 10.2337/diacare.21.1.69. [DOI] [PubMed] [Google Scholar]
- Neubauer S. Cardiac magnetic resonance spectroscopy: potential clinical applications. Herz. 2000;25:452–60. doi: 10.1007/s000590050037. [DOI] [PubMed] [Google Scholar]
- Niskanen L, Turpeinen A, Penttila I, et al. Hyperglycemia and compositional lipoprotein abnormalities as predictors of cardiovascular mortality in type 2 diabetes: a 15-year follow-up from the time of diagnosis. Diabetes Care. 1998;21:1861–9. doi: 10.2337/diacare.21.11.1861. [DOI] [PubMed] [Google Scholar]
- Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: The search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- Sandstede JJ. Assessment of myocardial viability by MR imaging. Eur Radiol. 2003;13:52–61. doi: 10.1007/s00330-002-1701-y. [DOI] [PubMed] [Google Scholar]
- Scheuermann-Freestone M, Madsen PL, Manners D, et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040–6. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- Sheifer SE, Manolio TA, Gersh BJ. Unrecognized myocardial infarction. Ann Intern Med. 2001;135:801–11. doi: 10.7326/0003-4819-135-9-200111060-00010. [DOI] [PubMed] [Google Scholar]
- Sigurdsson E, Thorgeirsson G, Sigvaldason H, et al. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med. 1995;122:96–102. doi: 10.7326/0003-4819-122-2-199501150-00003. [DOI] [PubMed] [Google Scholar]
- Szczepaniak LS, Dobbins RL, Metzger GJ, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–23. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003a;361:374–9. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- Wagner A, Mahrholdt H, Sechtem U, et al. MR imaging of myocardial perfusion and viability. Magn Reson Imaging Clin N Am. 2003b;11:49–66. doi: 10.1016/s1064-9689(02)00048-x. [DOI] [PubMed] [Google Scholar]
- Valensi P, Paries J, Brulport-Cerisier V, et al. Predictive value of silent myocardial ischemia for cardiac events in diabetic patients: influence of age in a French multicenter study. Diabetes Care. 2005;28:2722–7. doi: 10.2337/diacare.28.11.2722. [DOI] [PubMed] [Google Scholar]
- Yano K, MacLean CJ. The incidence and prognosis of unrecognized myocardial infarction in the Honolulu, Hawaii, Heart Program. Arch Intern Med. 1989;149:1528–32. [PubMed] [Google Scholar]