Abstract
Bendamustine is an alkylating agent which also shows properties of a purine analog. Because of its unique mechanism of action it shows activity in relapsed indolent lymphomas which are resistant to alkylating agents, purine analogs, and rituximab. Bendamustine has a favorable toxicity profile causing no alopecia and only a moderate hematotoxicity and gastrointestinal toxicity. Combinations of bendamustine with mitoxantrone and rituximab and with rituximab alone have been shown to be highly active in relapsed/refractory indolent lymphomas and mantle cell lymphomas achieving long lasting complete remissions. Because of only moderate toxicity these combinations can be applied safely in elderly patients who can be treated in an outpatient setting.
Keywords: bendamustine, relapsed-indolent, non-Hodgkin’s lymphoma
Introduction
Indolent B-cell lymphomas are a heterogenous group of lymphoproliferative malignancies with a relatively good prognosis and a median survival of up to 10 years (Armitage et al 1993). Standard first-line chemotherapy results in high response rates but unfortunately the disease relapses and second and further lines of chemotherapy lead to continously lower remission rates and shorter periods until the next anti-lymphoma therapy is necessary. Therefore new treatments that are highly active in the relapsed situation and result in long term remissions are urgently needed.
Development of bendamustine, mechanism of action, and metabolism
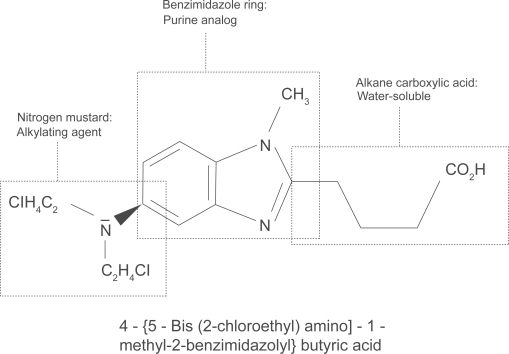
Bendamustine hydrochloride was first synthesized in 1963 by Ozegowski and Krebs in East Germany. It is a water soluble, white, microcrystalline powder with amphoteric properties due to a nitrogen mustard group and a butyric acid side chain. Chemically it is 4-{5-[bis(2-chloro-ethyl)amino]-1-methyl-2-benzimidazolyl}butyric acid (Figure 1). It has a nitrogen mustard moiety, a benzimidazole ring, and an alkane carboxylic acid side chain, which all may be responsible for its cytotoxic activity (Ozegowski and Krebs 1963, 1971; Schnabel et al 1967). Bendamustine acts as an alkylating agent causing intra-strand and inter-strand cross-links between DNA bases (Hartmann et al 1972). Compared with other alkylating agents, bendamustine causes more cross-links and importantly cross-linking seems to be more durable and more difficult to repair than those induced by carmustine and cyclophosphamide (Strumberg et al 1996). Treatment with bendamustine also leads to disruption of the matrix function of DNA in DNA synthesis (Hesse et al 1981). The benzimidazole ring may be responsible for the purine analog activity of bendamustine (Staib et al 1999), although this has not been proven definitely in vivo. It has been shown that bendamustine induces apoptosis in B-chronic lymphocytic leukemia cells (Schwänen et al 2002). Very recently it has been demonstrated that bendamustine has a distinct mechanism of action unrelated to other alkylating agents by activation of DNA damage stress response and apoptosis, inhibition of mitotic checkpoints, and induction of mitotic catastrophe (Leoni et al 2008). Compared with equitoxic concentrations of phosphoramide mustard and chlorambucil, the genes p21 (Cip1/Waf1) and Noxa which are responsible for apoptosis are induced much more strongly by bendamustine. Bendamustine also leads to a unique and marked phosphorylation of Ser15, which is one of the key events of p53-mediated apoptosis. It was shown also in SU-DHL-1 cells that bendamustine, but not phosphoramide or chlorambucil, causes an increase in the protein expression of Bax leading to p53 mediated apoptosis. Furthermore, bendamustine inhibits mitotic checkpoints and induces mitotic catastrophe by the inhibition of several mitosis-related genes like polo-like kinase 1, Aurora Kinase A, and Cyclin B1 (Leoni et al 2008). After intravenous application bendamus-tine undergoes an extensive first-pass metabolism in the liver by cytochrome p450 (Preiss et al 1985). A high percentage of the drug (>95%) is bound to protein, primarily albumin, but only free bendamustine is active (Haase et al 1991). Elimination is biphasic with a half-life of 6–10 minutes and a terminal half-life of approximately 30 minutes. Bendamustine is eliminated primarily by the renal route (Matthias et al 1995). Interestingly it is not necessary to reduce the bendamustine dose in patients with impaired renal function. Bendamustine doses up to 120 mg/m2 on day 1 + 2 can be given safely even to patients on hemodialysis (W. Pönisch, pers comm and own unpublished observation).
Figure 1.
Structure of bendamustine.
Early clinical studies in East Germany
Until 1990 bendamustine was available only in East Germany, the former German Democratic Republic. There it was used for the treatment of different tumors. East German investigators showed that it has considerable cytotoxic activity in chronic lymphocytic leukemia (CLL) (Anger et al 1975), Hodgkin’s disease (Herold et al 1992), non-Hodgkin’s lymphoma (Ruffert et al 1989; Brockmann 1992), multiple myeloma (Anger et al 1967, 1969), and lung cancer (Anger et al 1975).
Bendamustine monotherapy
Apart from case reports, only a few studies have been published that examined the efficacy of bendamustine as a monotherapy in relapsed indolent lymphomas. The first is the study by Heider and Niederle (2001) who treated 58 patients in the relapsed situation with bendamustine 120 mg/m2 day 1 + 2 of a 3-week cycle. The treatment was repeated until complete remission (CR) or partial remission (PR) or stable disease (SD) was confirmed on two consecutive cycles. Fifty-two patients were evaluable for response and toxicity. They reported a CR of 11% and a PR of 62%. SD was seen in 10% and progressive disease (PD) in 17% of the patients. The median duration of remission was 16 months. The main toxicities were a reversible grade 1–4 hematotoxicity and a grade 1 or 2 gastrointestinal toxicity. The second study was reported by Bremer in 2002, who treated 102 relapsed patients with different indolent lymphomas (CLL: 15, immunocytic: 46, multiple myeloma: 25, others: 16). The bendamustine dose was 60 mg/m2, days 1–5 every 4–6 weeks. A median of 4 cycles (1–11) were applied. The main toxicity observed was a grade 3 or 4 hematotoxicity with anemia in 7%, thrombocytopenia in 12%, and leukocytopenia in 25% of the patients treated. Nonhematological toxicity grade 3 or 4 was seen in less than 5% of the patients. Grade 1 or 2 nausea and vomiting was reported in more than 20% of the patients (Bremer 2002). He reported an overall response rate (ORR) of CR + PR of 77% with 20% stable disease and only 4% of patients with progressive disease. Median duration of response in the patients with indolent lymphoma was 39 months (Bremer 2002). Recently, a study examined the efficacy of bendamustine in relapsed patients with rituximab-refractory disease and demonstrated an ORR of 84% (29% CR, 3% CR unconfirmed, and 53% PR) with a bendamustine dose of 120 mg/m2 on day 1 + 2 of a 3-week cycle for 6 cycles (Kahl et al 2007). This study was confirmed by another trial in patients with rituximab resistant disease, in which 20% of the patients had transformed indolent lymphomas (Friedberg et al 2008). In this study, 76 patients were treated with a bendamustine dose of 120 mg/m2 on day 1 + 2 of a 3-week cycle for 6 cycles. The authors reported an ORR of 77% (15% CR, 19% unconfirmed CR, and 43% PR). The median duration of response was 6.7 months with 36% of the responses exceeding 1 year. In this study a grade 3 or 4 hematologic toxcicity included neutropenia (54%), throm-bocytopenia (25%), and anemia (12%).
Bendamustine in combination with other cytotoxic substances
Bendamustine has been combined with different cyto-toxic substances in the past. However, these studies are all small phase I/II studies with 14–82 patients each and no consistent response evaluation (Table 1). The combination with vincristine and prednisone was tested in 4 small trials with a total of 157 patients. The ORR ranged from 66% to 90% with a CR rate of 22%–45% and a PR rate of 41%–52% (Ruffert et al 1989; Blumenstengel et al 1998; Herold et al 1999; Kath et al 2000). Mitoxantrone was combined with bendamustine in one study showed an ORR of 59% (7% CR, 52% PR) (Heck et al 1998). Another study combined mitoxantrone/methotrexate/prednisone with bendamustine in 23 patients and reported an ORR of 48% (13% CR, 35% PR) (Kahl et al 1997). In a small study 14 patients were treated with a combination of bendamustine/idarubicine/dexamethasone with a reported ORR of 79% (29% CR, 50% PR) (König et al 1999). Bendamustine was also combined with oral etoposide in 38 patients with a high ORR of 97% (67% CR, 30% PR) (Ruffert 1999). Most extensively the combination of bendamustin/vincristine/prednisone has been studied by the OSHO group. In a phase III study the BOP regimen (bendamustine 60 mg/m2 days 1–5, vincristine 2 mg on day 1, and prednisolone 100 mg/m2 days 1–5) has been directly compared with the COP (cyclophosphamide, vincristine, and prednisone) regimen. No significant differences were seen for ORR, remission duration, and overall survival, although BOP caused less toxicity. BOP caused significantly less leukocytopenia (19% versus 34%) and alopecia (4% versus 48%) compared with COP (Herold et al 2006).
Table 1.
Bendamustine in combination with other cytotoxic substances
| Author | Bendamustine plus | N | ORR (%) | CR (%) | PR(%) |
|---|---|---|---|---|---|
| Ruffert et al (1989) | Vincistine + prednisone | 31 | 90 | 38 | 52 |
| Blumenstengel et al (1998) | Vincristine + prednisone | 22 | 86 | 45 | 41 |
| Herold et al (1999) | Vincristine + prednisone | 82 | 66 | 22 | 44 |
| Kath et al (2000) | Vincristine + prednisone | 22 | 86 | 45 | 41 |
| Heck et al (1998) | Mitoxantrone | 29 | 59 | 7 | 52 |
| Kahl et al (1997) | Mitoxantrone + MTX + prednisone | 23 | 48 | 13 | 35 |
| König et al (1999) | Dexamethasone + idarubicine | 14 | 79 | 29 | 50 |
| Ruffert (1999) | Etoposide orally | 38 | 97 | 67 | 30 |
| Königsmann et al (2004) | Fludarabine | 29 | 77 | 45 | 32 |
Abbreviations: MTX, methotrexate; CR, complete remission; ORR, overal response rate; PR, partial remission.
In vitro studies suggest that especially combinations of bendamustine with purine analogs could lead to a synergistic cytotoxicity (Chow et al 2001). So far only one phase I/II trial has been published by the OSHO group combining bendamustine with fludarabine. They treated 29 patients with relapsed indolent lymphomas with different dose levels and found a maximal tolerated dose of bendamustine 30 mg/m2 on days 1–3 and fludarabine 30 mg/m2 on days 1–3 when these drugs were combined. The reported ORR rate was 77% with 8 of 15 responders relapsing after a median time of 14 months. Unfortunately, a significant grade 3 or 4 hematotoxicity was observed, and 1 patient died of febrile neutropenia (Königsmann et al 2004).
Bendamustine in combination with rituximab
In 1999, we treated a patient with stage IV indolent lymphoma resistant to alkylating agents, anthracyclines, fludarabine, and rituximab with a combination of bendamustine plus mitoxantrone and rituximab (BMR) as fifth-line therapy. The disease went into CR after the first cycle and remains at CR until today (9+ years). This experience led to a small pilot study, which confirmed the efficacy of the BMR regimen in relapsed/refractory indolent lymphomas and B-cell chronic lymphocytic leukemia (B-CLL) (Weide et al 1999). BMR was applied as follows: bendamustine 90 mg/m2 day 1 + 2, mitoxantrone 10 mg/m2 on day 1, and rituximab 375 mg/m2 on days 8, 15, 22, 29. BM was repeated on day 36 for 3–5 more cycles every 28 days (1 × BMR followed by 3–5 × BM). The pilot phase of BMR was continued and showed a consistent high anti-lymphoma activity with long lasting complete remissions (Weide et al 2002). An ORR of 96% with 41% CR and 55% PR was observed. Importantly the time to next anti-lymphoma therapy was markedly prolonged and a significant proportion of the study population is still in ongoing CR up to more than 9 years after BMR. Interestingly, 46% of the patients received one cycle of BMR only, which means just 5 weeks of cytotoxic therapy, underscoring the potency of this chemoimmunotherapy (Weide et al 2004). The experience of our single center pilot study led to a multicenter phase II trial, which confirmed the efficacy of BMR in rituximab pretreated, relapsed, or refractory indolent lymphomas or mantle cell lymphomas. BMR consisted of bendamustine 90 mg/m2 on day 1 + 2, mitoxantrone 10 mg/m2 on day 1, and rituximab 375 mg/m2 on day 8 every 4 weeks times 4 (4 × BMR). In this trial the ORR was 89% with 35% CR and 54% PR. The main toxicity of BMR was a reversible hematotoxicity grade 3 or 4 (10% anemia, 78% leukocytopenia, 46% granulocytopenia, 16% thrombocytopenia). However, hospitalizations due to therapy were necessary after 4% of BMR applications only (Weide et al 2007). Due to the observed hematotoxicity in pretreated patients, we have reduced the mitoxantrone dose in clinical practice to 6 mg/m2 which results in a significant reduced hematotoxicity without reducing the activity of BMR (unpublished observation). Parallel to the development of BMR, Rummel et al (2002) developed the bendamustine-rituximab regimen (BR). In in vitro studies they could demonstrate in their cell line assay a synergistic cytotoxic effect when they applied bendamustine together with rituximab, leading to a markedly increased cell killing rate (Chow et al 2002; Rummel et al 2002). This led to a multicenter phase II trial in which they could demonstrate a high anti-lymphoma activity of BR in indolent lymphomas and mantle cell lymphomas with an ORR of 90% with a CR rate of 60%. Interestingly they found a remarkably low hematotoxicity grade 3 or 4 (16% leukocytopenia, 3% thrombocytopenia [Rummel et al 2005]). BR consisted of bendamustine 90 mg/m2 days 8 + 9, 36 + 37, 64 + 65, 92 + 93. Rituximab (375 mg/m2) was given on days 1, 7, 35, 63, 91, 120. These results have been confirmed by other groups (Robinson et al 2005; Van der Jagt et al 2006). In a subsequent randomized phase III study the STIL-group (Study Group Indolent Lymphoma, Germany) were able to demonstrate that 6 × BR (bendamustine 90 mg/m2 on day 1 + 2, rituximab 375mg/m2 on day 1) is equally effective concerning overall response rate compared with 6 × R-CHOP in the first-line therapy of indolent lymphomas and mantle cell lymphomas (Rummel et al 2007). Bendamustine has been combined with fludarabine and rituximab (BFR) in the treatment of relapsed indolent lymphomas. In this study bendamustine was applied at a dose of 50 mg/m2 on days 1–3, fludarabine at a dose of 25 mg/m2 days 1–3 and rituximab (375 mg/m2) was given on days 8, 15, 22, 29. The chemotherapy part (bendamustine plus fludarabine) was repeated on day 57 for 4 cycles. BFR proved to be effective with an ORR of 76% (28% CR, 48% PR) (Kirchner et al 2001). Unfortunately, the study could not be continued due to a significant hematotoxicity and a high rate of serious infections (Kirchner et al pers comm). The results of the studies combining bendamustine with rituximab in patients with relapsed or refractory indolent lymphomas are summarized in Table 2. Future directions of bendamustine development could be the sequential application of a chemoimmunotherapy with bendamustine (BMR or BR) followed by radioimmu-notherapy. We have treated so far 10 patients with relapsed or refractory indolent lymphoma and mantle cell lymphoma with 3 × BMR followed by 90Y-ibritumomab tiuxetan (ZEVALINTM) with a high response rate (6 CR, 3 PR, 1 PD) with durable complete remissions in 5 patients. The main toxicity was a reversible grade 3 or 4 hematotoxicity after ZEVALINTM. No blood or platelet transfusions or hospital admissions were necessary. No severe infections were observed (unpublished observation). Other directions could be the combination of bendamustine with bortezomib, thalidomide or revlimide, antiangiogenesis agents like bevacizumab, or new anti-B-cell-antibodies.
Table 2.
Bendamustine in combination with rituximab
| Author | Bendamustine plus | N | ORR(%) | CR(%) | PR(%) |
|---|---|---|---|---|---|
| Kirchner et al (2001) | Fludarabine + rituximab | 25 | 76 | 28 | 48 |
| Weide et al (2004) | Mitoxantrone + rituximab | 54 | 96 | 41 | 55 |
| Rummel et al (2005) | Rituximab | 63 | 90 | 60 | 30 |
| Robinson et al (2005) | Rituximab | 54 | 84 | 21 | 63 |
| Van der Jagt et al (2006) | Rituximab | 66 | 94 | 41 | 53 |
| Weide et al (2007) | Mitoxantrone + rituximab | 57 | 89 | 35 | 54 |
Abbreviations: CR, complete remission; ORR, overal response rate; PR, partial remission.
Conclusions
Bendamustine has enriched our armamentarium against relapsed indolent lymphomas remarkably due to its unique mechanism of action. Especially because of its activity in lymphomas resistant to alkylating agents, purine analogs, and rituximab, it opens up an opportunity for patients who are normally very difficult to treat. Of utmost importance are combinations of bendamustine with rituximab, which have shown to be highly active in achieving long lasting remissions Due to a positive toxicity profile these combinations are especially suitable for the treatment of elderly patients.
Footnotes
Disclosures
The author has declared no conflicts of interest.
References
- Anger G, Hesse P, Köhler P, et al. Erste klinische Erfahrungen mit einem neuen Zytostatikum. Deutsch Gesundheitswes. 1967;22:1079–84. [PubMed] [Google Scholar]
- Anger G, Hesse P, Baufeld H. Behandlung des multiplen Myeloms mit einem neuen Zytostatikum. Dtsch Med Wochenschr. 1969;48:2495–500. doi: 10.1055/s-0028-1110470. [DOI] [PubMed] [Google Scholar]
- Anger G, Fink R, Fleischer J, et al. Vergleichsuntersuchungen zwischen Cytostasan und Cyclophosphamid bei der chronischen Lymphadenose, dem Plasmozytom, der Lymphogranulomatose und dem Bronchialkarzinom. Deutsch Gesundheitswes. 1975;30:1280–5. [Google Scholar]
- Armitage J. Treatment of non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1023–30. doi: 10.1056/NEJM199304083281409. [DOI] [PubMed] [Google Scholar]
- Blumenstengel K, Fricke HJ, Kath R, et al. Bendamustine (B), vincristine (O), prednisone (P) in relapsed and refractory low grade non-Hodgkin’s lymphoma (NHL) [abstract] Ann Hematol. 1998;77(Suppl 11):S149. [Google Scholar]
- Bremer K. High rates of long lasting remissions after 5-day bendamustine chemotherapy cycles in pre-treated low-grade non-Hodgkin’s lymphomas. J Cancer Res Clin Oncol. 2002;128:603–9. doi: 10.1007/s00432-002-0378-6. [DOI] [PubMed] [Google Scholar]
- Brockmann B. Therapy of the recurrence of malignant lymphoma. Z Aerztl Fortbild (Jena) 1992;86:843. [PubMed] [Google Scholar]
- Chow KU, Boehrer S, Geduldig K, et al. In vitro studies of apoptosis of neoplastic cells in low-grade non-Hodgkin’s lymphomas using combinations of established cytotoxic drugs with bendamustine. Haematologica. 2001;86:485–93. [PubMed] [Google Scholar]
- Chow KU, Sommerlad WD, Boehrer S, et al. Anti-CD20 antibody (IDEC-C2B8, rituximab) enhances efficacy of cytotoxic drugs on neoplastic lymphocytes in vitro: role of cytokines, complement, and caspases. Haematologica. 2002;87:33–43. [PubMed] [Google Scholar]
- Friedberg JW, Cohen P, Chen L, et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin’s lymphoma: results from a phase II multicenter, single agent study. J Clin Oncol. 2008;26:204–10. doi: 10.1200/JCO.2007.12.5070. [DOI] [PubMed] [Google Scholar]
- Haase D, Preiss R, Sohr R, et al. Untersuchungen zur Plasmaei-weißbindung von Bendamustin (Cytostasan) und Ambazone. Z Klin Med. 1990;45:1267–71. [Google Scholar]
- Hartmann M, Zimmer CH. Investigation of cross-link formation in DNA by the alkylating cytostatica IMET 3106, 3393 and 3943. Biochim Biophys Acta. 1972;287:386–9. doi: 10.1016/0005-2787(72)90282-1. [DOI] [PubMed] [Google Scholar]
- Heck HK, Preiss JM, Schmidt P. Bendamustine (B) and mitoxantrone (M) in the treatment of low grade non-Hodgkin’s lymphoma (NHL) [abstract] J Cancer Res Clin Oncol. 1998;124(Suppl):R147. [Google Scholar]
- Heider A, Niederle N. Efficacy and toxicity of bendamustine in patients with relapsed low – grade non-Hodgkin’s lymphomas. Anticancer Drugs. 2001;12:725–9. doi: 10.1097/00001813-200110000-00003. [DOI] [PubMed] [Google Scholar]
- Herold M, Keinert K, Anger G, et al. Risk-adapted combined radio-therapy and chemotherapy for Hodgkin’s disease – results of a pilot study. Onkologie. 1992;15:501–5. [Google Scholar]
- Herold M, Schulze A, Mantovani L, et al. BOP versus COP in advanced low-grade non-Hodgkin’s lymphoma – results of a randomized multicenter trial [abstract] Ann Oncol. 1999;10(Suppl 3):125. [Google Scholar]
- Herold M, Schulze A, Niederwieser D, et al. Bendamustine, vincristine and prednisone (BOP) versus cyclophosphamide, vincristine and prednisone (COP) in advanced indolent non-Hodgkin’s lymphoma and mantle cell lymphoma: results of a randomised phase III trial (OSHO# 19) J Cancer Res Clin Oncol. 2006;132:105–12. doi: 10.1007/s00432-005-0023-2. [DOI] [PubMed] [Google Scholar]
- Hesse G, Schulze W, Wachtel E. Zur Methodik der Bestimmung alkylierender Aktivitäten von N-Losten mit Nitrobezylpyridin (NBP) in biologischem Material. Pharmazie. 1981;36:609–12. [PubMed] [Google Scholar]
- Kahl C, Herold M, Höffkes H, et al. Bendamustine, methotrexate, mitoxantrone and prednisone (BMMP) for the treatment of high grade non-Hodgkin’s lymphoma. Onkologie. 1997;20:406–8. [Google Scholar]
- Kahl B, Bartlett NL, Leonard JP, et al. Bendamustine is safe and effective in patients with rituximab-refractory, indolent B-cell non-Hodgkin’s lymphoma [abstract] Blood. 2007;110:1351. [Google Scholar]
- Kath R, Höffken K, Merkle K. Prevention of immunological complications in bendamustine treatment [abstract] Onkologie. 2000;23:171. [Google Scholar]
- Kirchner HH, Gaede B, Steinhauer EU, et al. Chemoimmuno-therapy with Fludarabine, Bendamustine and Rituximab for relapsed low grade malignant non-Hodgkin’s lymphoma [abstract] Blood. 2001;98:568. [Google Scholar]
- König U, Junghass C, Decker S, et al. Response of refractory and relapsed low grade non-Hodgkin’s lymphoma and chronic lymphocytic leukemia to Dexa-BID, a bendamustine hydrochloride-containing regimen [abstract] Ann Oncol. 1999;10(Suppl 3):132. [Google Scholar]
- Königsmann M, Knauf W, Herold M, et al. Fludarabine and bendamustine in refractory and relapsed indolent lymphoma – a multicenter phase I/II trial of the east german society of hematology and oncology (OSHO) Leuk Lymphoma. 2004;45:1821–7. doi: 10.1080/1042819042000223822. [DOI] [PubMed] [Google Scholar]
- Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–17. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- Matthias M, Preiss R, Sohr R, et al. Pharmacokinetics of bendamustine in patients with malignant tumors [abstract] Proc Am Soc Clin Oncol. 1995;14:1476. [Google Scholar]
- Ozegowski W, Krebs D. w-[bis-(chlorethyl)-amino-benzimidazolyl-(2)]-propionic or butyric acids as potential cytostatic agents. J Prakt Chem. 1963;20:178–86. [Google Scholar]
- Ozegowski W, Krebs D. IMET 3393, gamma-(1-methyl-5-bis-(ß-chloräthyl)-amino-benzimidazolyl(2)-buttersäure-hydrochlorid, ein neues Zytostatikum aus der Reihe der Benzimidazol-Loste. Zbl Pharm. 1971;110:1013–19. [Google Scholar]
- Preiss R, Sohr R, Matthias M, et al. The pharmacokinetics of bendamustine (Cytostasan) in humans. Pharmazie. 1985;40:782–4. [PubMed] [Google Scholar]
- Robinson KS, Williams ME, Cohen P, et al. Bendamustine HCl (TREANDATM) treatment in combination with rituximab results in objective responses in patients with refractory/relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma: Results from phase II multicenter study (SDX-105–02) [abstract] Blood. 2005;106:923. [Google Scholar]
- Ruffert K, Jann H, Syrbe G, et al. Cytostasan (bendamustine) as an alternative therapeutic approach to treat malignant non-Hodgkin’s lymphoma. Z Klin Med. 1989;44:671–4. [Google Scholar]
- Ruffert K. Therapy of low grade non-Hodgkin’s lymphoma (NHL) with bendamustine and oral etoposide [abstract] Ann Oncol. 1999;10(Suppl 3):125. [Google Scholar]
- Rummel MJ, Chow KU, Hölzer D, et al. In vitro studies with bendamustine: enhanced activity in combination with rituximab. Semin Oncol. 2002;29(4 Suppl 13):12–14. doi: 10.1053/sonc.2002.34873. [DOI] [PubMed] [Google Scholar]
- Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low – grade non Hodgkin’s lymphoma. J Clin Oncol. 2005;23:3383–9. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- Rummel MJ, von Grünhagen U, Niederle N, et al. Bendamustine plus rituximab versus CHOP plus rituximab in the first line treatment of patients with indolent and mantle cell lymphomas – first interim results of a randomized phase III study of the StiL (Study Group Indolent Lymphomas, Germany) [abstract] Blood. 2007;110:385. [Google Scholar]
- Schnabel R, Jungstand W, Gutsche W, et al. Comparative studies on the cystostatic activity of the new nitrogen mustard derivative IMET 3393 and endoxan in three experimental mouse tumours (Ehrlich-ascites-carcinoma, sarcoma 180 solid, leukaemia LAJ 1) Acta Biol Med. 1967;19:534–58. [PubMed] [Google Scholar]
- Schwänen C, Hecker T, Hübinger G, et al. In vitro evaluation of bendamustine induced apoptosis in B-chronic lymphocytic leukemia. Leukemia. 2002;16:2096–105. doi: 10.1038/sj.leu.2402651. [DOI] [PubMed] [Google Scholar]
- Staib P, Schinkothe T, Dimski T, et al. In vitro modulation of ara-CTP accumulation in fresh AML cells by bendamustine in comparison with fludarabine, 2-CDA and gemcitabine [abstract] Blood. 1999;94:63. [Google Scholar]
- Strumberg D, Harstrick A, Doll K, et al. Bendamustine hydrochloride activity against doxorubicine-resistant human breast cancer cell lines. Anticancer Drugs. 1996;7:415–21. doi: 10.1097/00001813-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Van der Jagt RH, Cohen P, Cheson BD, et al. Phase II Results of bendamustine in combination with rituximab in relapsed indolent and mantle-cell non-Hodgkin’s lymphoma [abstract] Blood. 2006;108:2710. [Google Scholar]
- Weide R, Heymanns J, Köppler H. Successful treatment of alkylating agent resistant low grade B-cell non Hodgkin’s lymphomas with bendamustine/mitoxantrone/rituximab (BMR) [abstract] Onkologie. 1999;22(Suppl 1):644. [Google Scholar]
- Weide R, Heymanns J, Gores A, et al. Bendamustine, mitoxantrone and rituximab (BMR): A new effective regimen for refractory or relapsed indolent lymphomas. Leuk Lymphoma. 2002;43:327–31. doi: 10.1080/10428190290006107. [DOI] [PubMed] [Google Scholar]
- Weide R, Pandorf A, Heymanns J, et al. Bendamustine/Mitoxantrone/ Rituximab (BMR): A very effective, well tolerated outpatient chemoimmunotherapy for relapsed and refractory CD20-positive indolent malignancies. Final results of a pilot study. Leuk Lymphoma. 2004;45:2445–2449. doi: 10.1080/10428190400004521. [DOI] [PubMed] [Google Scholar]
- Weide R, Hess G, Köppler H, et al. High anti – lymphoma activity of bendamustine/mitoxantrone/rituximab in rituximab pretreated relapsed or refractory indolent lymphomas and mantle cell lymphomas. A mul-ticenter phase II study of the German Low Grade Lymphoma Study Group (GLSG) Leuk Lymphoma. 2007;48:1299–306. doi: 10.1080/10428190701361828. [DOI] [PubMed] [Google Scholar]