Abstract
A controlled-environment membrane model for use in vitro was developed and employed in an attempt to mimic the environment of the vagina in order to study yeast-vaginal cell adhesion. Adhesion in vitro of four strains of Candida albicans (NIH 3181A, NIH 526B, ATCC 18804, and MCO 2400) to vaginal epithelial cells (VEC) appeared to be affected by the pH and the level of carbon dioxide that have been found to be present in the vagina in vivo. Strain 3181A had a greater adhesion ability than 526B when the concentration of yeast cells was increased and when the yeast cells were incubated with VEC at pH 5 in sodium phosphate buffer in ambient air supplemented with 10% CO2. Of the four strains of C. albicans used, 3181A had the greatest adhesion ability, with strains 2400, 18804, and 526B ranked in order of decreasing adhesion ability. Also, an enhanced, electron-dense, matted outer region of the cell walls of the yeasts was observed frequently when they were incubated in ambient air supplemented with 10% CO2. In addition, of the vaginal cells that had yeast cells attached to them, an average of 94.4% of the total yeast cells were attached to the microridge side of the VEC, whereas an average of only 5.6% of the total were found on the nonmicroridge side of the VEC. The results from this study indicate that adhesion of C. albicans to the VEC surface was affected by the strain of yeast used, by the side of the vaginal cell exposed, and by the pH and CO2 levels present in the adhesion assay.
Full text
PDF

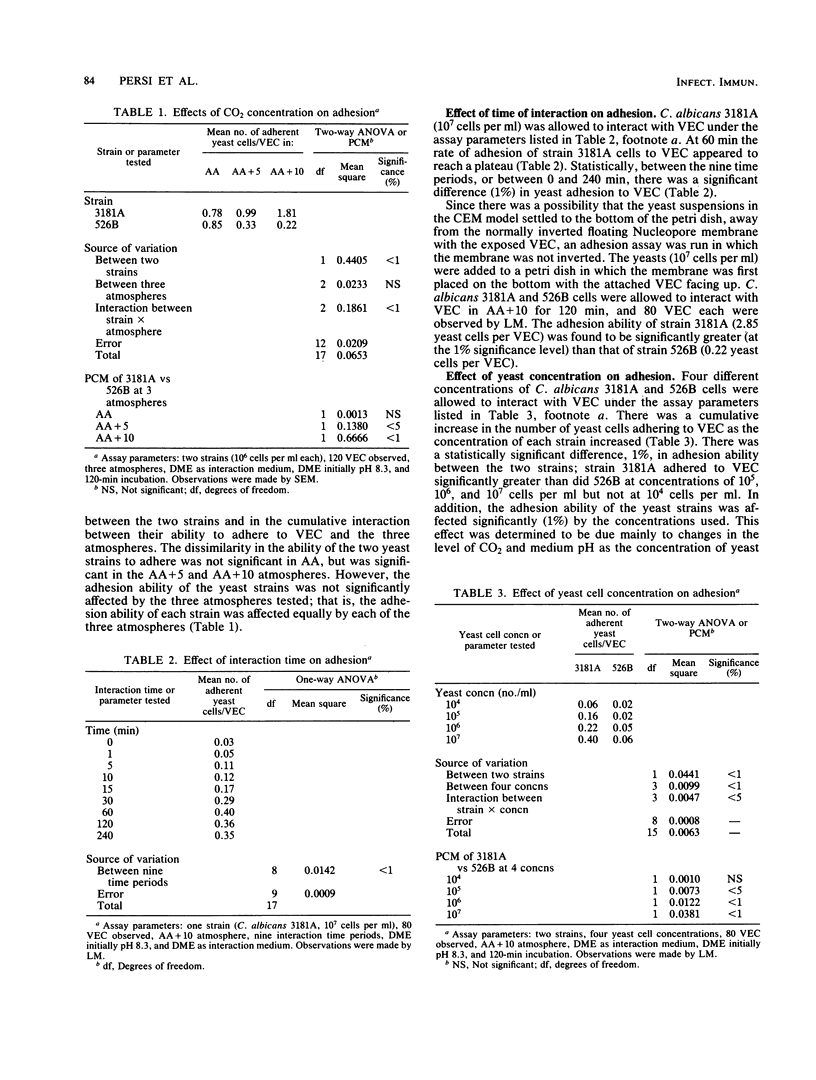




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
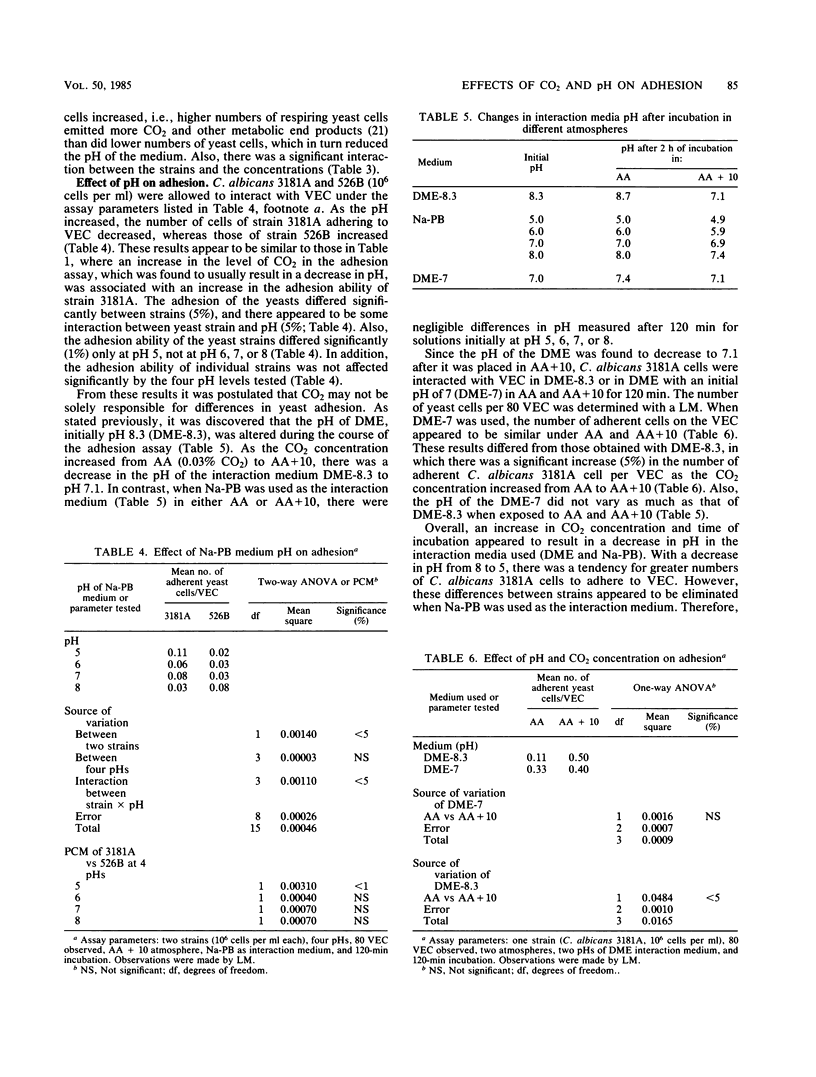
- Cawson R. A., Rajasingham K. C. Ultrastructural features of the invasive phase of Candida albicans. Br J Dermatol. 1972 Nov;87(5):435–443. doi: 10.1111/j.1365-2133.1972.tb01591.x. [DOI] [PubMed] [Google Scholar]
- Centeno A., Davis C. P., Cohen M. S., Warren M. M. Modulation of Candida albicans attachment to human epithelial cells by bacteria and carbohydrates. Infect Immun. 1983 Mar;39(3):1354–1360. doi: 10.1128/iai.39.3.1354-1360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham R. G., McKeehan W. L. Media and growth requirements. Methods Enzymol. 1979;58:44–93. doi: 10.1016/s0076-6879(79)58126-9. [DOI] [PubMed] [Google Scholar]
- Kearns M. J., Davies P., Smith H. Variability of the adherence of Candida albicans strains to human buccal epithelial cells: inconsistency of differences between strains related to virulence. Sabouraudia. 1983 Jun;21(2):93–98. doi: 10.1080/00362178385380161. [DOI] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect Immun. 1980 May;28(2):464–468. doi: 10.1128/iai.28.2.464-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Lee J. C., Morris A. L. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun. 1980 Feb;27(2):667–674. doi: 10.1128/iai.27.2.667-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., King R. D. Characterization of Candida albicans adherence to human vaginal epithelial cells in vitro. Infect Immun. 1983 Sep;41(3):1024–1030. doi: 10.1128/iai.41.3.1024-1030.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. F., Reiss E. Comparison by ELISA of serum anti-Candida albicans mannan IgG levels of a normal population and in diseased patients. Mycopathologia. 1980 Mar 17;70(2):89–93. doi: 10.1007/BF00443073. [DOI] [PubMed] [Google Scholar]
- Lehmann P. F., Reiss E. Detection of Candida albicans mannan by immunodiffusion, counterimmunoelectrophoresis, and enzyme-linked immunoassay. Mycopathologia. 1980 Mar 17;70(2):83–88. doi: 10.1007/BF00443072. [DOI] [PubMed] [Google Scholar]
- Mardon D., Balish E., Phillips A. W. Control of dimorphism in a biochemical variant of Candida albicans. J Bacteriol. 1969 Nov;100(2):701–707. doi: 10.1128/jb.100.2.701-707.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Westtöm L. Adherence of bacterial to vaginal epithelial cells. Infect Immun. 1976 Mar;13(3):661–666. doi: 10.1128/iai.13.3.661-666.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persi M. A., Burnham J. C. Use of tannic acid as a fixative-mordant to improve the ultrastructural appearance of Candida albicans blastospores. Sabouraudia. 1981 Mar;19(1):1–8. doi: 10.1080/00362178185380021. [DOI] [PubMed] [Google Scholar]
- Samaranayake L. P., MacFarlane T. W. Factors affecting the in-vitro adherence of the fungal oral pathogen Candida albicans to epithelial cells of human origin. Arch Oral Biol. 1982;27(10):869–873. doi: 10.1016/0003-9969(82)90043-7. [DOI] [PubMed] [Google Scholar]
- Segal E., Lehrer N., Ofek I. Adherence of Candida albicans to human vaginal epithelial cells: inhibition by amino sugars. Exp Cell Biol. 1982;50(1):13–17. doi: 10.1159/000163121. [DOI] [PubMed] [Google Scholar]
- Sobel J. D., Myers P. G., Kaye D., Levison M. E. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J Infect Dis. 1981 Jan;143(1):76–82. doi: 10.1093/infdis/143.1.76. [DOI] [PubMed] [Google Scholar]
- WELD J. T. Candida albicans; rapid identification in cultures made directly from human materials. AMA Arch Derm Syphilol. 1953 May;67(5):473–478. doi: 10.1001/archderm.1953.01540050037008. [DOI] [PubMed] [Google Scholar]
- WELD J. T. Candida albicans; rapid identification in pure cultures with carbon dioxide on modified eosin-methylene blue medium. AMA Arch Derm Syphilol. 1952 Dec;66(6):691–694. doi: 10.1001/archderm.1952.01530310029003. [DOI] [PubMed] [Google Scholar]
- Wagner G., Ottesen B. Vaginal physiology during menstruation. Ann Intern Med. 1982 Jun;96(6 Pt 2):921–923. doi: 10.7326/0003-4819-96-6-921. [DOI] [PubMed] [Google Scholar]
- Wilks M., Thin R. N., Tabaqchali S. Quantitative methods for studies on vaginal flora. J Med Microbiol. 1982 Feb;15(1):141–147. doi: 10.1099/00222615-15-1-141. [DOI] [PubMed] [Google Scholar]