Abstract
The development of nonsteroidal anti-inflammatory drugs (NSAIDs) selective for cyclooxygenase (COX)-2 (named coxibs) has been driven by the aim of reducing the incidence of serious gastrointestinal (GI) adverse events associated with the administration of traditional (t) NSAIDs – mainly dependent on the inhibition of COX-1 in GI tract and platelets. However, their use has unravelled the important protective role of COX-2 for the cardiovascular (CV) system, mainly through the generation of prostacyclin. In a recent nested-case control study, we found that patients taking NSAIDs (both coxibs and tNSAIDs) had a 35% increase risk of myocardial infarction. The increased incidence of thrombotic events associated with profound inhibition of COX-2-dependent prostacyclin by coxibs and tNSAIDs can be mitigated, even if not obliterated, by a complete suppression of platelet COX-1 activity. However, most tNSAIDs and coxibs are functional COX-2 selective for the platelet (ie, they cause a profound suppression of COX-2 associated with insufficient inhibition of platelet COX-1 to translate into inhibition of platelet function), which explains their shared CV toxicity. The development of genetic and biochemical markers will help to identify the responders to NSAIDs or who are uniquely susceptible at developing thrombotic or GI events by COX inhibition. We will describe possible strategies to reduce the side effects of etoricoxib by using biochemical markers of COX inhibition, such as whole blood COX-2 and the assessment of prostacyclin biosynthesis in vivo.
Keywords: etoricoxib, nonsteroidal antiinflammatory drugs, COX-2, gastrointestinal toxicity, cardiovascular toxicity, prostacyclin
Nonsteroidal antiinflammatory drugs (NSAIDs) are commonly used in the general population for treating pain and inflammatory conditions (Burke et al 2006). They comprise traditional (t) NSAIDs and NSAIDs selective for cyclooxygenase (COX)-2 (named coxibs) which were developed to reduce the risk of serious gastrointestinal (GI) complications – dependent, at least in part, on the inhibition of COX-1 (FitzGerald and Patrono 2001). The therapeutic effects (analgesic and anti-inflammatory) of NSAIDs, both traditional and coxibs, are mostly due to the inhibition of COX-2-dependent prostanoids (Figure 1A). In placebo-controlled randomized clinical trials (RCTs), coxibs (rofecoxib [Vioxx®], celecoxib [Celebrex®, Artilog®, Solexa®, Artrid®] and valdecoxib [Bextra®]) were associated with an increase in the relative risk (RR) of cardiovascular (CV) events by 1- to 2.7-fold (Ott et al 2003; Bresalier et al 2005; Solomon et al 2005; Pfizer 2005; Nussmeier et al 2005). However, the results of observational studies and a meta-analysis of data derived from trials with coxibs have shown that the CV hazard is not restricted to NSAIDs selective for COX-2 but also applies to some tNSAIDs, such as diclofenac (Hernandez-Diaz et al 2006; Kearney et al 2006). In a recent nested-case control study, we found that patients taking NSAIDs (both coxibs and tNSAIDs) had a 35% increased risk of myocardial infarction (Patrignani et al 2008a). Clinical results suggest that the CV hazard associated with the administration of NSAIDs is dose-dependent (Patrignani et al 2008a; Solomon et al 2008). In addition, the genetic background of the individual may play a role in increased susceptibility to inhibition of NSAIDs (Arehart et al 2008). To limit the possible detrimental effects, associated with the administration of this efficacious class of drugs, is necessary to develop strategies of risk management through the identification of genetic and biochemical markers to select the responders to NSAIDs or who are uniquely susceptible to developing thrombotic or GI events by COX inhibition.
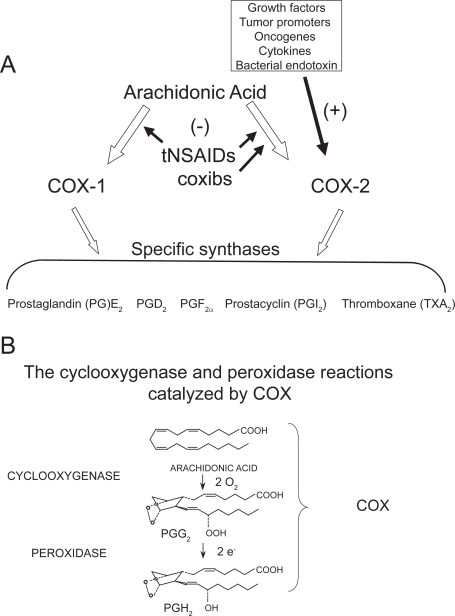
Figure 1.
Pathways of prostanoid biosynthesis. (A) Prostanoids (PGE2, PGD2, PGF2α, PGI2, TXA2) are produced by COX-1 and COX-2 and specific synthases; (B) PGH2, generated by cyclooxygenase and peroxydase activity of COX, is then converted to prostanoids by the activity of different synthases.
Differential COX pathways are effective in health and disease
Biology of COX-1 and COX-2
Prostanoids are lipid autacoids – including prostaglandin (PG) E2, PGF2α, PGD2, prostacyclin (PGI2), and thromboxane(TX) A2 – that are immediately released outside the cell after intracellular biosynthesis and modulate a wide variety of physiologic and pathologic processes via the interaction with specific receptors expressed mostly on the surface of target cells (Narumiya et al 1999; Breyer et al 2001).
Under normal physiologic conditions, prostanoids play an essential homeostatic role in the GI cytoprotection, hemostasis, renal physiology, gestation, and parturition (Funk 2001; Patrono et al 2001; FitzGerald 2003). Moreover, they play important roles in pathophysiologic processes such as inflammation, cancer, and thrombosis (Funk 2001; Patrono et al 2001; FitzGerald 2003). Two isoforms of COX (COX-1 and COX-2) have been cloned and characterized (Simmons et al 2004). COX-1 and COX-2 are the products of different genes. COX-1 is considered a “housekeeping gene” by virtue of constitutive low-levels of expression in most cell types. However, high levels of constitutive expression of COX-1 have been detected in the stomach, platelets, and the kidney. In addition, COX-1 can be regulated during development (Rocca et al 1999). In contrast, the gene for COX-2 is a primary response gene with many regulatory sites; thus, COX-2 expression can be rapidly induced by bacterial endotoxin (LPS), cytokines, such as interleukin (IL)-1β and tumor necrosis factor-α, growth factors, and the tumor promoter phorbol myristate acetate (PMA) (reviewed by Kang et al 2007). However, COX-2 is constitutively expressed in some cells in lung (Asano et al 1996), brain (Yamagata et al 1993; Kaufmann et al 1997), kidney (Harris et al 1994), pancreatic β-cells (Robertson 1998), and GI carcinomas (Ristimaki et al 1997; Shao et al 2000; Carlson et al 2003).
COX-1 and COX-2 share the same catalytic activities: the cyclooxygenase which oxidizes arachidonic acid (AA) to PGG2 and the peroxidase which reduces PGG2 to the unstable endoperoxide PGH2, precursor of prostanoids (Smith et al 2000) (Figure 1B). Although the peroxidase reaction is considered the second step in the formation of PGH2, the COX reaction is absolutely dependent on peroxidase activity for its activation (Malkowski et al 2000). However, the two COX-isozymes require different levels of hydroperoxides to initiate cyclooxygenase catalysis, ie, COX-2 requires considerably lower levels of hydroperoxides than COX-1 (Kulmacz and Wang 1995; Marnett et al 1999). Elegant work performed by Funk’s group reveals that COX-1 can partially compensate for COX-2 function but that this is limited by the differential ability of these two isoforms to metabolize low concentrations of arachidonate (Yu et al 2007). In fact, COX-2 catalysis occurs at lower levels of free AA than COX-1 (Swinney et al 1997).
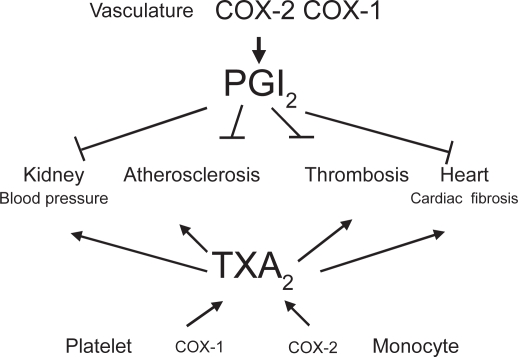
These differences in the regulation of COX-1 and COX-2 catalysis contribute to the development of cell-specific pathways of prostanoid biosynthesis dominated by one or the other enzyme in vivo even in the presence of the concurrent expression of the 2 isozymes (Capone et al 2007). As an example, in endothelial cells where different antioxidant defence systems are operative to maintain a reductive cytosolic environment, COX-2 is the dominant pathway turned on to generate prostacyclin, an important anti-atherogenic and anti-thrombotic mediator (Grosser et al 2006) (Figure 2).
Figure 2.
Vascular prostacyclin counteracts the cardiovascular effects of platelet TXA2.
COX-2 is the major pathway in pain
Other than in inflammation and pyresis, COX-2 plays an important role in nociception (Vane et al 1998). Noxious stimuli (eg, physical, chemical, thermal) in peripheral tissues cause damage and the inflammatory response leads to the release of pain-producing substances that activate nociceptors on the terminals of sensory nerve fibers. This pain is the hallmark of protective response to adverse stimuli and subsides with removal of the stimulus. In this context, the induction of COX-2, leading to enhanced prostanoid release, translates into sensitization of peripheral nociceptor terminals and produces localized pain hypersensitivity. In fact, prostanoids increase neuronal activity in nociceptive nerve fibers by raising cAMP levels and lowering the activation threshold for opening of tetrodotoxin-resistant sodium channels in the neuronal membrane. PGE2 and prostacyclin, produced in the peripheral terminals of sensory nerve endings, are hyperalgesic and enhance nociception produced by other mediators (such as bradykinin) (Murata et al 1997; Nakao et al 2007). Moreover, peripheral inflammation also generates pain hypersensitivity in neighboring uninjured tissue (secondary hyperalgesia), because of increased neuronal excitability in the spinal cord (central sensation) and a syndrome comprising diffuse muscle and joint pain, fever, lethargy, and anorexia (Samad et al 2001). NSAIDs play an antinociceptive action by acting both at peripheral sites and at central sites mainly through COX-2 inhibition. Both COX-1 mRNA and COX-2 mRNA as well as COX-1 and COX-2 proteins are expressed constitutively in the spinal cord but only COX-2 can be induced in response to exogenous and endogenous cytokines (Beiche et al 1996; Inoue et al 1999; Tonai et al 1999). Like nociceptive pain, hyperalgesia is linked to an adverse stimulus and diminishes with healing and decreased inflammation. Prolonged acute pain and hyperalgesia, however, can evolve into chronic pain.
The results of RCTs showing a comparable pain relief by NSAIDs selective for COX-2 and tNSAIDs both in acute clinical pain models (for example, oral surgery) and chronic clinical pain models (such as osteoarthritis [OA] and inflammatory arthropaties), support a major role of inhibition of COX-2 in their clinical efficacy (Fitzgerald and Patrono 2001; Sciulli et al 2005). However, it should be pointed out that often the RCTs, comparing tNSAIDs and coxibs, had inadequate power to detect small differences possibly associated with inhibition of COX-1. Mostly, they were sized to detect equivalence, not superiority, of either (Patrono et al 2001).
COX-1 is the major pathway in GI protection
Prostanoids (PGE2 and prostacyclin) are key mediators to maintain gastric mucosal blood flow and increase protective mucus as well as bicarbonate production (Wilson 1991). Several lines of evidence show that prostanoids produced in normal GI tissue which are required for normal physiological functioning are derived from the COX-1 isoform (Kargman et al 1996). However, upregulation of COX-2 in the margin of healing ulcers and evidence from the use of COX inhibitors in rodents has raised concerns that COX-2 might be involved in the healing of existing ulcers (Shigeta et al 1998; Takahashi et al 1998). However, COX-2 up-regulation in the GI tract has been observed in cancer development (Romano et al 1998; Prescott and Fitzpatrick 2000; Nardone et al 2004; Wang and Dubois 2006).
COX-1 is involved in TXA2-dependent platelet function
Platelets generate TXA2 from AA through the activity of COX-1 (Patrignani et al 1999). TXA2 generated in response to a variety of stimuli (for example, collagen, thrombin, and adenosine diphosphate) participates in the control of hemostasis through the induction of irreversible platelet aggregation triggered by the interaction with G-protein–coupled receptors, the TXA2 receptors (Alfranca et al 2006). Recent findings show that tiny concentrations of TXA2 can cause platelet activation (Minuz et al 2002; Pulcinelli et al 2005; Minuz et al 2006). Thus, 10 nM of the TXA2 mimetic U46619 induces platelet adhesion and shape change (Minuz et al 2002), and in the presence of a subthreshold concentration of collagen it causes platelet aggregation (Pulcinelli et al 2005). Moreover, TXA2 causes vasoconstriction and vascular proliferation; thus, it is involved in atherogenesis and increase in blood pressure (Figure 2) (Grosser et al 2006).
COX-2-dependent prostacyclin is the major pathway in vascular protection
In vascular endothelial cells, COX-2 plays a dominant role in producing important prostanoids to regulate the functions of underlying vascular smooth muscle cells and circulating cells. Although endothelial cells may generate a different array of the prostanoids PGD2, PGE2, and prostacyclin along the vascular beds, there is robust evidence that prostacyclin is the dominant prostanoid produced in the macrocirculation (Moncada et al 1977; Grosser et al 2006).
Prostacyclin potently inhibits aggregation of platelets induced by TXA2 and other agonists, vascular smooth muscle cell proliferation and vascular tone, leukocyte–endo-thelial cell interactions, and cholesteryl ester hydrolase (Grosser et al 2006). As shown in Figure 2, most of the deleterious effects of TXA2 in the CV system are countered by prostacyclin, mainly through the activity of COX-2. Thus, inhibition of vascular COX-2 leads to loss of the protective function associated with prostacyclin. Recently, it has been reported an antioxidant role for prostacyclin through the induction of hemoxygenase-1 (Egan et al 2004). For all these biological actions, prostacyclin has on the distinctive features of a cardioprotective mediator. Studies in knockout (KO) mice for prostacyclin receptor (IP) and the recent findings of acceleration of CV disease in humans by a dysfunctional IP mutation convincingly support the protective role of prostacyclin for the CV system (Arehart et al 2008; Patrignani et al 2008b).
Biochemical biomarkers of COX inhibition
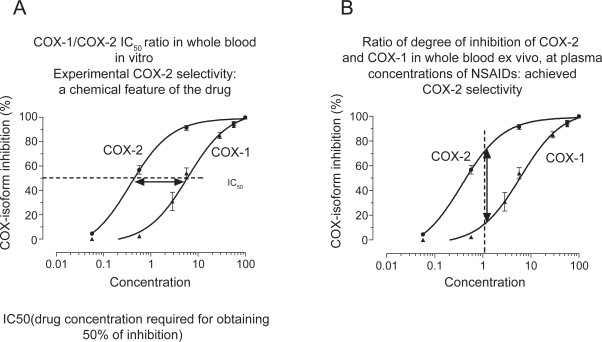
NSAIDs are distinguished on the basis of their COX-isozyme selectivity in vitro, described as the ratio of the concentrations required to inhibit the activity of the isozymes by 50% (IC50 for COX-1/IC50 for COX-2) (Figure 3A). The assessment of COX-1/COX-2 ratios in vitro describes an experimental COX-isozyme selectivity which mirrors the chemical features of the different NSAIDs. This is assessed using the human whole blood assays which evaluate the effects of drugs on platelet COX-1 and monocyte COX-2 activities (Patrono et al 1980; Patrignani et al 1994). They are capacity indexes of COX-isozyme activities to generate prostanoids from endogenous sources of AA and their pharmacological inhibition is not influenced by different pathological conditions. These assays are based on the measurement of PGE2 production, in response to lipopolysaccharide (LPS) added to heparinized human blood samples for 24 h which reflects the time-dependent induction of COX-2 in circulating monocytes (Patrignani et al 1994). The parallel measurement of TXB2 production during whole blood clotting is used as an index of platelet COX-1 activity (Patrono et al 1980). When the COX-1/COX-2 IC50 ratio is higher than 1 the drug is more potent in inhibiting COX-2. When the ratio is approximately 1, the drug is a non-selective inhibitor of both COX-1 and COX-2. When the ratio is lower than 1, the drug is more potent towards COX-1. We assessed COX-1/COX-2 IC50 values of several COX inhibitors. Interestingly, we have found that the biochemical selectivity is a continuous variable preventing the separation of tNSAIDs from coxibs (Patrignani et al 2008c). However, we can identify some drugs more selective for COX-1 than COX-2 (such as aspirin, naproxen and ibuprofen) from the wide cluster of agents more selective for COX-2 than COX-1. This includes: i) piroxicam and indomethacin which are approximately 3-fold more selective for COX-2; ii) etodolac, meloxican, diclofenac, celecoxib, and valdecoxib which are 6- to 60-fold more selective for COX-2; iii) the highly selective COX-2 inhibitors such as, etoricoxib, rofecoxib, and lumiracoxib which have a COX-1/COX-2 IC50 ratio higher than 100 (Table 1).
Figure 3.
Assessment of COX-isoform selectivity in whole blood in vitro (A) and ex vivo (B).
Table 1.
Biochemical selectivity, assessed as COX-1/COX-2 IC50 values of several COX inhibitors (derived from Patrignani et al 2008a)
| COX-inhibitors | COX-1/COX-2 IC50 values |
|---|---|
| naproxen | 0.49 |
| ibuprofen | 0.56 |
| indomethacin | 2.9 |
| piroxicam | 3.1 |
| etodolac | 5.6 |
| meloxicam | 13.8 |
| diclofenac | 24.4 |
| celecoxib | 32 |
| valdecoxib | 61a |
| etoricoxib | 162 |
| rofecoxib | 255 |
| lumiracoxib | 400b |
COX-1/COX-2 IC50 ratio value from Capone et al 2008.
COX-1/COX-2 IC50 ratio value from Patrignani et al 2004.
The whole blood assays also enable estimation of achieved COX-isozyme selectivity in humans, which is the ratio of isozyme inhibition at a given plasma concentration (Figure 3B). Importantly, achieved selectivity of NSAIDs varies as a consequence of the dose administered.
There is evidence that the whole blood assays for COX-2 and COX-1 may be candidate surrogate end-points of efficacy and GI toxicity of NSAIDs, respectively. It has been shown that COX-2 inhibition, as determined by PGE2 levels in LPS-stimulated whole blood, can be used as a marker to predict drug efficacy in humans (Huntjens et al 2005). In fact, (concentration required to inhibit the activity of IC80 COX-2 by 80% in vitro) values have been found to correlate directly with the analgesic/anti-inflammatory plasma concentrations of different COX inhibitors (Huntjens et al 2005). Thus, COX-2 inhibition, by assessing PGE2 levels in the whole blood assay in vitro and ex vivo, can be used as a marker to predict drug effects (analgesia, antiinflammatory effects) in humans. Similarly, platelet COX-1 inhibition, by assessing TXB2 levels in the whole blood assay in vitro and ex vivo, can be used as a marker to predict toxicity or efficacy (GI toxicity or antiplatelet effects) in humans by NSAIDs. In fact, Cryer and Feldman (1998) reported that the inhibitory effects of tNSAIDs on gastric PGE2 synthesis correlate with COX-1 inhibitory potency in clotting blood.
Now it is critical to establish the CV safety profile of specific individual NSAIDs, be it tNSAIDs or coxibs, and to find valid surrogate biochemical predictors of that risk.
Studies of clinical pharmacology have shown discordant functional implications of inhibition of COX-1 and COX-2. A linear relationship exists between the extent of inhibition of COX-2 and that of prostacyclin in vivo (Patrignani et al 2008a). In contrast, inhibition of TXA2-dependent platelet function in vivo occurs when platelet COX-1-dependent capacity to synthesize TXA2 (as assessed by measuring serum TXB2 levels) is reduced ≥95% (Reilly and FitzGerald 1987). In fact, a non-linear relationship of inhibition of platelet TXA2 generation with inhibition of TXA2-mediated platelet aggregation has been found (Sciulli et al 2006). This is explained by the fact that even tiny concentrations of TXA2 may activate the platelets. Minuz et al (2006) have shown that low concentrations of TXA2 activate the tyrosine-kinase-based signaling pathway which may translate into a full platelet activation in the presence of weak platelet agonists or subthreshold concentrations of stronger agonists.
These findings lead to the concept of functional COX-2 selectivity by NSAIDs, ie, inhibition of COX-2 in the presence of an insufficient reduction of platelet COX-1 activity to translate into inhibition of platelet function. It has to be pointed out that most NSAIDs are functionally selective for COX-2 at therapeutic doses similarly to coxibs. The only tNSAID that has been shown to be functionally non-selective for the platelet is naproxen at high doses, in some individuals (Capone et al 2004). This depends on the fact that naproxen usually administered at high doses bid is more potent for COX-1 than COX-2, and it is characterized by a pharmacokinetic half-life of approx 14 h (Burke et al 2006). Thus, naproxen is associated with profound inhibition of prostacyclin but the possible CV hazard associated with this effect may be mitigated by a parallel profound and persistent suppression of platelet TXA2 (Capone et al 2004), which translates into a small CV protection or neutral effect (Hernández-Díaz et al 2006; Kearney et al 2006).
Recent findings suggest that for functional non-selective NSAIDs the extent of inhibition of COX-2-dependent pros-tacyclin may predict the increased incidence of myocardial infarction (MI) (Patrignani et al 2008a). Individual NSAIDs with a degree of COX-2 inhibition <90% at therapeutic concentrations presented a RR of 1.18 (95% CI, 1.02–1.38) while those with a greater COX-2 inhibition had a RR of 1.60 (95% CI, 1.41–1.81).
Thus, it has been proposed that the assessment of whole blood COX-2 ex vivo – alone or in combination with the measurement of urinary levels of 2,3-dinor-6- keto-PGF1α [a biomarker of prostacyclin biosynthesis in vivo (Figure 4)] – in association with genetic biomarkers (such as polymorphisms in the prostacyclin receptor IP) (Arehart et al 2008) may be surrogate end-points of CV hazard by pharmacological inhibition of COX-2 (Patrignani et al 2008b).
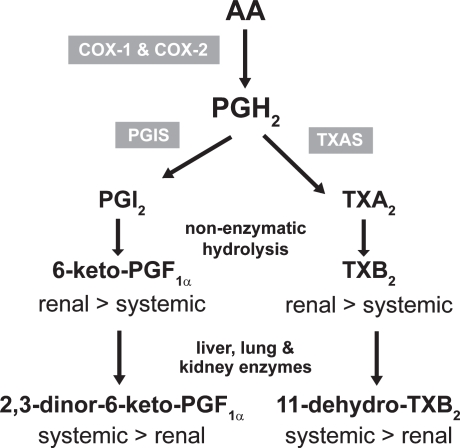
Figure 4.
Biosynthesis and metabolism of prostacyclin (PGI2) and thromboxane (TX) A2.
Abbreviations: PGIS, prostacyclin synthase; TXS, TX synthase.
Etoricoxib
Pharmacology
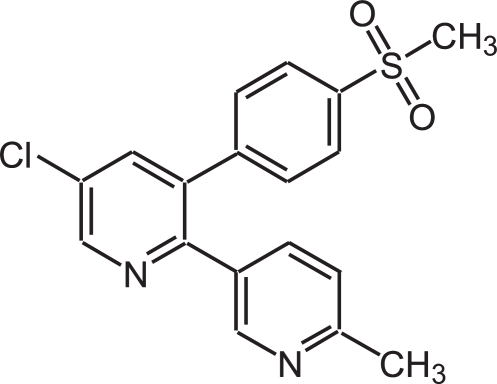
The NSAID selective for COX-2 etoricoxib, 5-chloro-6′-methyl-3-[4-(methylsulfonyl) phenyl]-2,3′-bipyridine (Figure 5) (Riendeau et al 2001) (Arcoxia®, Tauxib®, Algix®) has been approved in Europe, Latin America, and Asia-Pacific region for the treatment of OA, rheumatoid arthritis (RA), ankylosing spondylitis, acute gouty arthritis, acute pain, and chronic musculoskeletal pain, at doses ranging between 60 and 120 mg daily depending on the different indications.
Figure 5.
Chemical structure of etoricoxib: bipyridine with a methyl sulfone side chain 5-chloro-6′-methyl-3-[4-(methylsulfonyl) phenyl]-2,3′-bipyridine.
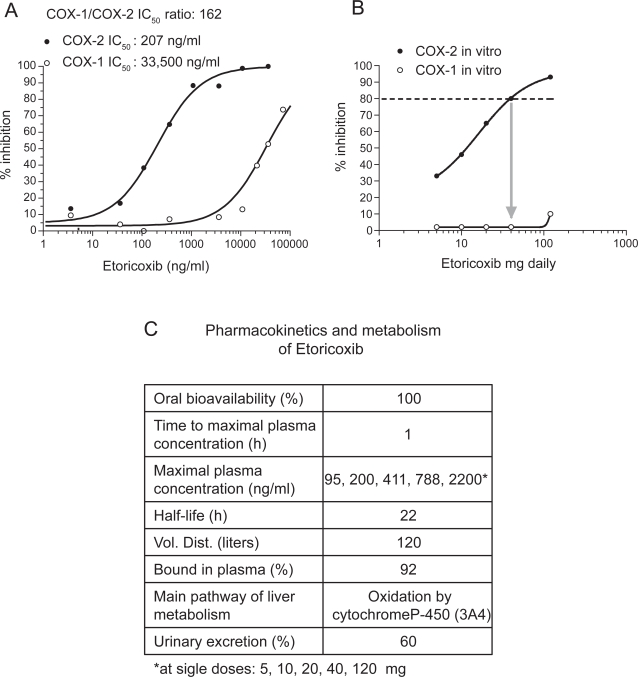
As shown in Figure 6A, etoricoxib is 162-fold more potent towards COX-2 than COX-1 in the whole blood assay in vitro. The pharmacokinetics features of etoricoxib are reported in Figure 6C (Agrawal et al 2003). Importantly, it has a long half-life of 22 h and is metabolized via 6’-methyl hydroxylation in human liver microsomes. The reaction is catalyzed by a number of P450 forms, although CYP3A4 accounts for the majority (40%–90%) of the activity. The remainder of the activity is equally divided between a number of other P450s (eg, CYP2D6, CYP2C9, CYP1A2, and possibly CYP2C19). In this regard, the P450 reaction phenotype of etoricoxib is unique and differs from that of other COX inhibitors (Kassahun et al 2001).
Figure 6.
COX-2 selectivity of etoricoxib in human whole blood. (A) Concentration-response curves assessed in vitro; (B) degree of inhibition of COX-2 and COX-1 by peak plasma concentrations obtained after dosing with etoricoxib 5, 10, 20, 40, and 120 mg; (C) pharmacokinetics features of etoricoxib.
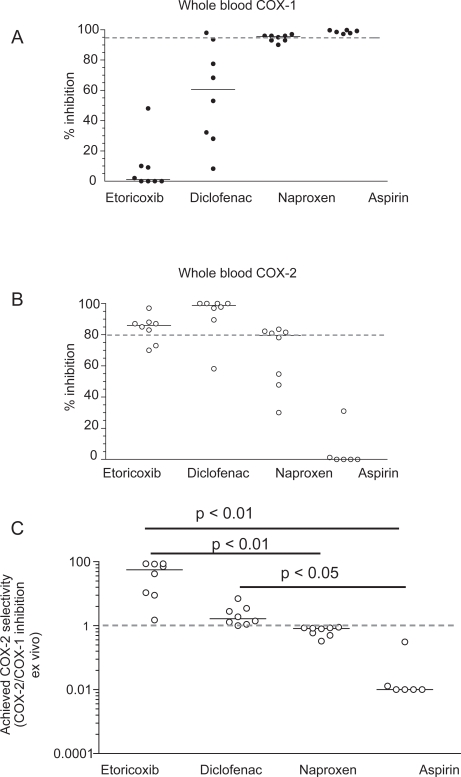
As shown in Figure 6B, etoricoxib administered up to 120 mg is a selective COX-2 inhibitor. This result has been obtained by the extrapolation of COX-1 and COX-2 inhibition in vitro in the human whole blood assays by the maximal plasma concentration achieved after the administration of etoricoxib at single doses of 5, 10, 20, 40, 120 mg. Interestingly, an inhibition of COX-2 by 80% may be reached after the administration of 40 mg, suggesting that the dosage of the drug could be reduced without affecting efficacy. We performed studies of clinical pharmacology to assess achieved COX-2 selectivity ex vivo by the chronic administration of etoricoxib 90 mg daily versus the tNSAIDs diclofenac 75 mg bid, naproxen 500 mg bid, and aspirin 100 mg daily (Figure 7 A–C). As shown in Figure 7A the administration of etoricoxib (at 12 h after dosing), diclofenac (at 4 h after dosing), naproxen (at 12 h after dosing), and low-dose aspirin (at 24 h after dosing) caused a median inhibition of COX-1 of 1%, 60%, 95%, and 98%, respectively. These results show that inhibition of platelet COX-1 ≥95% (which translates into inhibition of platelet function) occurs in all subjected treated with low-dose aspirin and in some of those treated with high-dose naproxen, at the interval between doses, sustaining an anti-platelet effect of high-dose naproxen. In contrast, in all subjects treated with diclofenac and etoricoxib, the degree of inhibition of platelet COX-1 was not adequate to affect significantly TXA2-dependent platelet function. However, our results showed a marked inter-subject variability in the inhibition of platelet COX-1 after the administration of diclofenac (range 8–98%) and to a lesser extent also for etoricoxib (range 0%–48%). As shown in Figure 7B, whole blood COX-2 was profoundly affected by etoricoxib, diclofenac, and naproxen, but not by low-dose aspirin (median inhibition and range: 86% [70–90], 98% [58–100], 79% [30–83], and 0% [0–31], respectively]. These data show that both diclofenac and etoricoxib were administered at supratherapeutic doses, in fact most of individuals had a COX-2 inhibition higher than 80%, considered sufficient to translate into efficacy. From these values we calculated the achieved selectivity (ie the ratio of COX-2 inhibition and COX-1 inhibition ex vivo). As shown in Figure 7C, for etoricoxib and diclofenac the individual values of achieved COX selectivity were placed above 1 (which represents a balanced inhibition of COX-2 and COX-1) showing that circulating concentrations of the two drugs caused a more profound inhibition of COX-2 than COX-1. In contrast, naproxen and aspirin caused a more profound inhibition of COX-1 than COX-2; in fact the ratio of COX-2 /COX-1 inhibition ex vivo was always lower than 1. These data clearly show that diclofenac and naproxen, despite being considered tNSAIDs, are significantly different in selectivity compared with COX-isozymes at therapeutic doses. Interestingly, the achieved selectivity was not different, in a statistically significant fashion, between etoricoxib and diclofenac. These results will be relevant for interpretation of the clinical results, for both efficacy and toxicity.
Figure 7.
Whole blood COX-1 (A) and COX-2 (B) inhibition ex vivo after the chronic administration of etoricoxib 90 mg, diclofenac 75 mg bid, naproxen 500 mg bid, and aspirin 100 mg daily to humans; (C) achieved COX-2 selectivity by the drugs.
Prostanoids are autocoids that act in the proximity of the cell where they are generated. In fact, they are subjected to an intense metabolism which leads to inactivation of prostanoids. It proceeds through β-oxidation ω-oxidation, 15-hydroxy dehydrogenation, and double-bond reduction through enzymatic pathways in liver, kidney, and lung (Ramwell et al 1980; Roberts 1987) (Figure 4). The assessment of major enzymatic metabolites of prostanoids represents an index of their systemic biosynthesis. Several lines of evidence support a dominant platelet origin (mostly COX-1-derived) of a major urinary enzymatic metabolite of TXB2, ie 11-dehydro-TXB2, while vasculature (mostly COX-2 derived) represents the dominant origin of a major urinary enzymatic metabolite of prostacyclin, ie, 2,3-dinor-6-keto-PGF1α (Capone et al 2007).
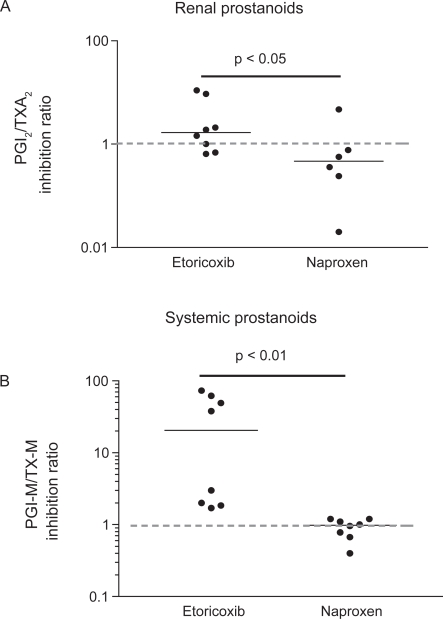
In susceptible patients, inhibition of prostanoid generation by tNSAIDs and coxibs causes sodium retention with resulting edema and hypertension (Grosser et al 2006). Similarly to thrombogenesis, COX-1 inhibition may mitigate the consequence of COX-2 inhibition in the kidney. This is compatible with a COX-2-dependent source of vasodilatory prostacyclin and COX-1-dependent origin of vasoconstrictors, like TXA2 in the kidney (Qi et al 2002; Grosser et al 2006). We studied the impact of etoricoxib and naproxen on renal and systemic prostanoid biosynthesis by assessing 6-keto-PGF1α/TXB2 inhibition ratio and 2,3-dinor-6-keto-PGF1α/11-dehydro-TXB2 inhibition ratio, respectively, after the administration of the drugs (Figure 8).
Figure 8.
Effects of the chronic administration of etoricoxib 90 mg daily and naproxen 500 mg bid to humans on prostacyclin/TXA2 inhibition ratio in the kidney (by assessing their urinary non-enzymatic metabolites: 6-keto-PGF1α and TXB2, respectively) (A), versus systemic biosynthesis (by assessing their urinary enzymatic metabolites: PGIM = 2,3-dinor-6-keto-PGF1α [a major urinary enzymatic metabolite of prostacyclin] and TX-M = 11-dehydro-TXB2 [a major enzymatic metabolite of TXA2], respectively) (B).
As shown in Figure 8 A and B, etoricoxib caused a more profound inhibition of COX-2 than COX-1 both at renal and extrarenal sites. This is predictive of a CV hazard but of reduced GI toxicity by etoricoxib. In contrast, naproxen reduced more profoundly COX-1 both at renal and extrarenal sites (Figure 8A and B), which may translate into reduced CV but higher GI side effects.
Etoricoxib and GI toxicity
The GI safety of etoricoxib has been assessed in the MEDAL (Multinational Etoricoxib vs Diclofenac Arthritis Long Term) Programme (Laine et al 2007). It was prospectively designed to pool data from 3 randomized, double-blind clinical trials: the MEDAL study, the Etoricoxib vs Diclofenac Sodium Gastrointestinal Tolerability and Effectiveness (EDGE) study, and the EDGE II study. Patients with OA or RA aged 50 years or over were eligible for enrollment if they had a clinical diagnosis of OA of the knee, hip, hand, or spine, or a clinical diagnosis of RA that satisfied at least 4 of 7 of the American Rheumatism Association 1987 revised criteria (Arnett et al 1988), and in the judgment of the investigator, would require chronic therapy with an NSAID. Thus, etoricoxib (60 or 90 mg daily) and diclofenac (150 mg daily) were compared in 34,701 patients with OA or RA for upper GI clinical events (bleeding, perforation, obstruction, or ulcer) and the subset of complicated events (perforation, obstruction, witnessed ulcer bleeding, or significant bleeding). As shown in Table 2, there were significantly fewer upper GI clinical events with the COX-2 selective inhibitor etoricoxib than with the tNSAID diclofenac due to a decrease in uncomplicated events, but not in the more serious complicated events. Less inhibition of gastric COX-1 by etoricoxib than diclofenac (etoricoxib is a more COX-2 selective drug than diclofenac in vitro and ex vivo [Patrignani et al 1997; Tacconelli et al 2002] [Figure 7]) may explain the reduced incidence of uncomplicated GI events. In contrast, the comparable rates of GI bleeding by the two drugs could be due to a similar impact on platelet COX-1, in some individuals, and on healing of gastric lesions. In fact, it has been shown that COX-2 plays an important role in the healing of gastric ulcers and that inhibition of COX-2 delays ulcer healing (Konturek et al 2005). As shown in Figure 7B, etoricoxib and diclofenac have a comparable profound impact on COX-2 (>80%).
Table 2.
Risk of GI events with etoricoxib and diclofenac in MEDAL study (derived from Laine et al 2007)
| Type of GI events | Hazard ratio with etoricoxib vs diclofenac | 95% CI | p |
|---|---|---|---|
| All upper-GI events | 0.69 | 0.57–0.83 | 0.0001 |
| Uncomplicated upper-GI events | 0.57 | 0.45–0.74 | <0.0001 |
| complicated upper-GI events | 0.91 | 0.67–1.24 | 0.0001 |
Abbreviations: GI, gastrointestinal; MEDAL, Multinational Etoricoxib vs Diclofenac Arthritis Long Term.
In MEDAL, the use of low-dose aspirin and proton pump inhibitors (PPIs) was allowed. Analyzing the data in aspirin and non-aspirin populations, it has been found that etoricoxib reduced the risk of uncomplicated upper GI events compared with diclofenac in patients taking low-dose aspirin regularly, but the magnitude of the GI benefit decreased with low-dose-aspirin use (aspirin: hazard ratio 0.73, 95% CI 0.64–1.07 versus no-aspirin: 0.52, 0.38–0.71, p = 0.021) (Laine et al 2007). PPIs were used concomitantly for at least 75% of the study period by 40% of patients and treatment effects did not differ significantly in these individuals; however, significantly fewer patients discontinued etoricoxib than diclofenac due to dyspepsia, and this decrease was similar in patients who took PPIs, suggesting that the COX-2-selective inhibitor provided symptomatic benefit even in patients already taking a PPI (Laine et al 2007).
Etoricoxib and CV toxicity
In placebo-controlled RCTs, coxibs (rofecoxib, celecoxib, and valdecoxib) were associated with increased RR of CV events (1.92–3.7) (Ott et al 2003; Bresalier et al 2005; Nussmeier et al 2005; Pfizer 2005; Solomon et al 2005). An overview of data derived from trials with coxibs suggests that MI predominates over stroke (Kearney et al 2006). These results led the regulatory authorities such as the Food and Drug Administration (FDA) and the European Agency for the Evaluation of Medicinal Products (EMEA) to restrict the use of these drugs (Patrignani et al 2008c). However, the results of observational studies and a meta-analysis of data derived from trials with coxibs have shown that the CV hazard is also associated with some tNSAIDs, such as diclofenac (Hernandez-Diaz et al 2006; Kearney et al 2006). A plausible mechanism in increased risk of vascular events in individuals treated with NSAIDs selective for COX-2 and some tNSAIDs is the inhibition of COX-2-dependent prostacyclin unaccompanied by inhibition of COX-1-dependent TXA2 at functional range, ie >95%. In fact, most tNSAIDs are as COX-2 selective as coxibs for platelet function. In addition, coxibs and tNSAIDs inhibit profoundly prostacyclin generation in vivo, as assessed by the measurement of urinary levels of a major enzymatic metabolite 2,3-dinor-6-keto-PGF1α (Catella-Lawson et al 1999; McAdam et al 1999).
The CV hazard associated with the use of coxibs led to the voluntarily withdrawal from the US and EU market of rofecoxib and valdecoxib, in 2004 and 2005, respectively. Recently, the FDA has formally rejected manufacturer application for approval of etoricoxib as a prescription pain reliever. The FDA decision was influenced by the results of safety analyses from the MEDAL Program (Cannon et al 2006). The authors reported that etoricoxib (60 or 90 mg daily, mean duration of therapy was 18 months) and diclofenac (150 mg daily, ie 50 mg tid or 75 mg bid) administered to OA or RA patients have comparable rates, constant over time, of thrombotic CV events (Cannon et al 2006). Despite etoricoxib and diclofenac having different COX-2 selectivity in vitro, they are functional COX-2 selective for platelet function. The shared pharmacodynamic translates into comparable incidence of thrombotic events detected in MEDAL.
Aside from the platelet, the relationship between inhibition of prostaglandin formation and prostaglandin dependent function appears to be roughly linear in other cell types. In this context, even an incomplete inhibition of COX-1 might attenuate the functional effect derived from inhibition of COX-2-dependent prostacyclin. This may occur in the kidney where COX-2 is the source of vasodilatory prostacyclin and COX-1 is the source of vasoconstrictor TXA2 (Qi et al 2002; Grosser et al 2006). This is sustained by the results of MEDAL where the selective COX-2 inhibitor etoricoxib caused a higher incidence of blood pressure than the less selective tNSAID diclofenac. However, the more pronounced signal from MI rather than stroke showed by the use of NSAIDs (traditional and selective for COX-2) suggests that thrombosis is the dominant component of the CV hazard and thus lesser degrees of selectivity are likely only to mitigate the hazard to a marginal extent.
Both EMEA and FDA and the American Heart Association (Antman et al 2007) issued the recommendations that selective COX-2 inhibitors are contraindicated in patients with ischemic heart disease and/or stroke, that they have to be avoided in patients with risk factors for coronary heart disease, and that all patients have to take the lowest effective dose for the shortest time necessary to control symptoms. Importantly, we have recently shown that the degree of COX-2 inhibition achieved by individual NSAIDs (an index of drug potency/exposure), that do not show functional suppression of platelet COX-1, is a determinant of the CV hazard associated with them (Patrignani et al 2008a, c).
Thus, a possible approach to reduce the CV risk associated with etoricoxib will be to use the lowest effective dose. Studies of efficacy with lower doses of etoricoxib than those used in MEDAL seem to support this possible strategy. Etoricoxib 30 mg/day has been shown efficacious in OA compared with either ibuprofen 800 mg tid (Wiesenhutter et al 2005) or diclofenac 50 mg tid (Leung et al 2002). Moreover, it has been recently assessed the efficacy of etoricoxib 30 mg daily versus the recommended therapeutic dose of celecoxib, 200 mg daily, in the treatment of OA patients (Bingham et al 2007). The primary hypothesis was that etoricoxib 30 mg would be at least as effective as celecoxib 200 mg for Western Ontario and McMaster (WOMAC) Pain Subscale, WOMAC Physical Function Subscale and Patient Global Assessment of Disease Status. Etoricoxib was non-inferior to celecoxib and both were superior to placebo (p < 0.001) for all three outcomes. The authors suggested that etoricoxib 30 mg was at least as effective as celecoxib 200 mg and had similar safety in the treatment of OA of knee and hip. As shown in Figure 6B, etoricoxib 30 mg seems adequate to reduce whole blood COX-2 at a degree appropriate to translate into clinical efficacy. However, further studies of clinical pharmacology in patients with OA and RA should be performed to verify the impact of etoricoxib 30 mg on biochemical markers of COX inhibition, such as whole blood COX-2 and systemic and renal biosynthesis of prostacyclin and TXA2.
Conclusions
NSAIDs are an important and efficacious class of drugs for the management of musculoskeletal symptoms. The development of NSAIDs selective for COX-2 – to reduce the incidence of serious GI adverse effects compared with tNSAIDs mainly dependent on the inhibition of COX-1 in GI tract and platelets – has unravelled the important protective role of COX-2 for the CV system, mainly through the generation of prostacyclin. In a recent nested-case control study, we found that patients taking NSAIDs (both coxibs and tNSAIDs) had a 35% increase risk of MI. The increased incidence of thrombotic events associated with profound inhibition of COX-2-dependent prostacyclin can be mitigated, even if not removed, by a complete suppression of platelet COX-1 activity. In fact, inhibition of TXA2-dependent platelet function occurs when platelet COX-1 activity, assessed ex vivo, is reduced ≥95% (Reilly and FitzGerald 1987). We have introduced the concept of functional COX-2 selectivity (ie, a profound suppression of COX-2 associated with insufficient inhibition of platelet COX-1 to cause inhibition of platelet function) which is a feature shared by most tNSAIDs and coxibs. We have recently shown that inhibition of COX-2 and the functional selectivity with which it is achieved is relevant to CV hazard from NSAIDs and relates to drug potency (exposure) (Patrignani et al 2008a, c).
The development of genetic and biochemical markers will help to identify the responders to NSAIDs or who are uniquely susceptible at developing thrombotic or GI events by COX inhibition. This will lead to the development of individual responder approaches. We propose that the assessment of whole blood COX-2 ex vivo (alone or in combination with urinary 2,3-dinor-6-keto-PGF1α) may represent a valid surrogate end-point to predict CV risk for functionally selective COX-2 inhibitors. The use of these biochemical markers together with genetic biomarkers will help to select patients uniquely susceptible to developing CV risk through inhibition of COX-2-dependent-prostacyclin when exposed to NSAIDs. Their use can lead to a rational selection of doses for efficacy and will help to make decisions in risk management of tNSAIDs and NSAIDs selective for COX-2.
Footnotes
Disclosures
None of the authors has any conflicts of interest to disclose.
References
- Agrawal NG, Porras AG, Matthews CZ, et al. Single- and multiple-dose pharmacokinetics of etoricoxib, a selective inhibitor of cyclooxygenase-2, in man. J Clin Pharmacol. 2003;43:268–76. doi: 10.1177/0091270003251122. [DOI] [PubMed] [Google Scholar]
- Alfranca A, Iñiguez MA, Fresno M, et al. Prostanoid signal transduction and gene expression in the endothelium: role in cardiovascular diseases. Cardiovasc Res. 2006;70:446–56. doi: 10.1016/j.cardiores.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–42. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- Arehart E, Stitham J, Asselbergs FW, et al. Acceleration of cardiovascular disease by a dysfunctional prostacyclin receptor mutation: potential implications for cyclooxygenase-2 inhibition. Circ Res. 2008;102:986–93. doi: 10.1161/CIRCRESAHA.107.165936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatiod arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Asano K, Lilly CM, Drazen JM. Prostaglandin G/H synthase-2 is the constitutive and dominant isoform in cultured human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 1996;271:L126–31. doi: 10.1152/ajplung.1996.271.1.L126. [DOI] [PubMed] [Google Scholar]
- Beiche F, Scheuerer S, Brune K, et al. Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett. 1996;390:165–9. doi: 10.1016/0014-5793(96)00604-7. [DOI] [PubMed] [Google Scholar]
- Bingham CO, 3rd, Sebba AI, Rubin BR, et al. Efficacy and safety of etoricoxib 30 mg and celecoxib 200 mg in the treatment of osteoarthritis in two identically designed, randomized, placebo-controlled, non-inferiority studies. Rheumatology (Oxford) 2007;46:496–507. doi: 10.1093/rheumatology/kel296. [DOI] [PubMed] [Google Scholar]
- Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, et al. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–90. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Burke A, Smyth E, FitzGerald GA. Analgesic-antipyretic agents; Pharmacotherapy of gout. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman‘s The Pharmacological Basis of Therapeutics. 11th ed. New York, USA: McGRAW-HILL, Medical Publishing Division; 2006. pp. 673–715. [Google Scholar]
- Cannon CP, Curtis SP, FitzGerald GA, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet. 2006;368:1771–81. doi: 10.1016/S0140-6736(06)69666-9. [DOI] [PubMed] [Google Scholar]
- Capone ML, Tacconelli S, Francesco LD, et al. Cardiovascular effects of valdecoxib: transducing human pharmacology results into clinical read-outs. Expert Opin Drug Saf. 2008;7:29–42. doi: 10.1517/14740338.7.1.29. [DOI] [PubMed] [Google Scholar]
- Capone ML, Tacconelli S, Di Francesco L, et al. Pharmacodynamic of cyclooxygenase inhibitors in humans. Prostaglandins Other Lipid Mediat. 2007;82:85–94. doi: 10.1016/j.prostaglandins.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Capone ML, Tacconelli S, Sciulli MG, et al. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation. 2004;109:1468–71. doi: 10.1161/01.CIR.0000124715.27937.78. [DOI] [PubMed] [Google Scholar]
- Carlson ML, Wilson ET, Prescott SM. Regulation of COX-2 transcription in a colon cancer cell line by Pontin52/TIP49a. Mol Cancer. 2003;2:42. doi: 10.1186/1476-4598-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catella-Lawson F, McAdam B, Morrison BW, et al. Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735–41. [PubMed] [Google Scholar]
- Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:413–21. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- Egan KM, Lawson JA, Fries S, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306:1954–7. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GA, Patrono C. The Coxibs, selective inhibitors of Cyclooxygenase-2. N Engl J Med. 2001;345:433–42. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- FitzGerald GA. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov. 2003;2:879–90. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RC, McKanna JA, Akai Y, et al. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–10. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Díaz S, Varas-Lorenzo C, García Rodríguez LA. Non-steroidal antiinflammatory drugs and the risk of acute myocardial infarction. Basic Clin Pharmacol Toxicol. 2006;98:266–74. doi: 10.1111/j.1742-7843.2006.pto_302.x. [DOI] [PubMed] [Google Scholar]
- Huntjens DR, Danhof M, Della Pasqua OE. Pharmacokinetic-pharmacodynamic correlations and biomarkers in the development of COX-2 inhibitors. Rheumatology (Oxford) 2005;44:846–59. doi: 10.1093/rheumatology/keh627. [DOI] [PubMed] [Google Scholar]
- Inoue A, Ikoma K, Morioka N, et al. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem. 1999;73:2206–13. [PubMed] [Google Scholar]
- Kang YJ, Mbonye UR, DeLong CJ, et al. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog Lipid Res. 2007;46:108–25. doi: 10.1016/j.plipres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargman S, Charleson S, Cartwright M, et al. Characterization of prostaglandin G/H synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology. 1996;111:445–54. doi: 10.1053/gast.1996.v111.pm8690211. [DOI] [PubMed] [Google Scholar]
- Kassahun K, McIntosh IS, Shou M, et al. Role of human liver cytochrome P4503A in the metabolism of etoricoxib, a novel cyclooxygenase-2 selective inhibitor. Drug Metab Dispos. 2001;29:813–20. [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Taylor CV, et al. Cyclooxygenase-2 expression during rat neocortical development and in Rett syndrome. Brain Dev. 1997;19:25–34. doi: 10.1016/s0387-7604(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–8. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek SJ, Konturek PC, Brzozowski T. Prostaglandins and ulcer healing. J Physiol Pharmacol. 2005;56:5–31. [PubMed] [Google Scholar]
- Kulmacz RJ, Wang LH. Comparison of hydroperoxide initiator requirements for the cyclooxygenase activities of prostaglandin H synthase-1 and -2. J Biol Chem. 1995;270:24019–23. doi: 10.1074/jbc.270.41.24019. [DOI] [PubMed] [Google Scholar]
- Laine L, Curtis SP, Cryer B, et al. Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomized comparison. Lancet. 2007;369:465–73. doi: 10.1016/S0140-6736(07)60234-7. [DOI] [PubMed] [Google Scholar]
- Leung AT, Malmstrom K, Gallacher AE, et al. Efficacy and tolerability profile of etoricoxib in patients with osteoarthritis: a randomized, double-blind, placebo and active-comparator controlled 12-week efficacy trial. Curr Med Res Opin. 2002;18:49–58. doi: 10.1185/030079902125000282. [DOI] [PubMed] [Google Scholar]
- Malkowski MG, Ginell SL, Smith WL, et al. The productive conformation of arachidonic acid bound to prostaglandin synthase. Science. 2000;289:1933–7. doi: 10.1126/science.289.5486.1933. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Rowlinson SW, Goodwin DC, et al. Arachidonic acid oxygenation by COX-1 and COX-2: mechanisms of catalysis and inhibition. J Biol Chem. 1999;274:22903–6. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- McAdam BF, Catella-Lawson F, Mardini IA, et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A. 1999;96:272–7. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuz P, Fumagalli L, Gaino S, et al. Rapid stimulation of tyrosine phosphorylation signals downstream of G-protein-coupled receptors for thromboxane A2 in human platelets. Biochem J. 2006;400:127–34. doi: 10.1042/BJ20061015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuz P, Gaino S, Zuliani V, et al. Functional role of p38 mitogen activated protein kinase in platelet activation induced by a thromboxane A2 analogue and by 8-iso-prostaglandin F2alpha. Thromb Haemost. 2002;87:888–98. [PubMed] [Google Scholar]
- Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977;1:18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- Murata T, Ushikubi F, Matsuoka T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–82. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- Nakao K, Murase A, Ohshiro H, et al. CJ-023,423, a novel, potent and selective prostaglandin EP4 receptor antagonist with antihyperalgesic properties. J Pharmacol Exp Ther. 2007;322:686–94. doi: 10.1124/jpet.107.122010. [DOI] [PubMed] [Google Scholar]
- Nardone G, Rocco A, Vaira D, et al. Expression of COX-2, mPGE-synthase1, MDR-1 (P-gp), and Bcl-xL: a molecular pathway of H pylori-related gastric carcinogenesis. J Pathol. 2004;202:305–12. doi: 10.1002/path.1512. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–91. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- Ott E, Nussmeier NA, Duke PC, et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;125:1481–92. doi: 10.1016/s0022-5223(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Patrignani P, Capone ML, Tacconelli S. NSAIDs and cardiovascular disease. Heart. 2008c;94:395–7. doi: 10.1136/hrt.2007.136002. [DOI] [PubMed] [Google Scholar]
- Patrignani P, Campestrini J, Branson J, et al. Lumiracoxib is a selective inhibitor of cyclooxygenase-2 in patients with osteoarthritis. Ann Rheum Dis. 2004;63:368. [Google Scholar]
- Patrignani P, Di Febbo C, Tacconelli S, et al. Differential association between human prostacyclin receptor polymorphisms and the development of venous thrombosis and intimal hyperplasia: a clinical biomarker study. Pharmacogenetics and Genomics. 2008b;18:611–20. doi: 10.1097/FPC.0b013e328301a774. [DOI] [PubMed] [Google Scholar]
- Patrignani P, Panara MR, Greco A, et al. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J Pharmacol Exp Ther. 1994;271:1705–12. [PubMed] [Google Scholar]
- Patrignani P, Panara MR, Sciulli MG, et al. Differential inhibition of human prostaglandin endoperoxide synthase-1 and -2 by nonsteroidal anti-inflammatory drugs. J Physiol Pharmacol. 1997;48:623–31. [PubMed] [Google Scholar]
- Patrignani P, Sciulli MG, Manarini S, et al. COX-2 is not involved in thromboxane biosynthesis by activated human platelets. J Physiol Pharmacol. 1999;50:661–7. [PubMed] [Google Scholar]
- Patrignani P, Tacconelli S, García Rodríguez LA. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. Arterioscler Thromb Vasc Biol. 2008a;28:e–45. doi: 10.1016/j.jacc.2008.08.041. [DOI] [PubMed] [Google Scholar]
- Patrono C, Ciabattoni G, Pinca E, et al. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb Res. 1980;17:317–27. doi: 10.1016/0049-3848(80)90066-3. [DOI] [PubMed] [Google Scholar]
- Patrono C, Patrignani P, García Rodríguez LA. Cyclooxygenase-selective inhibition of prostanoid formation: transducing biochemical selectivity into clinical read-outs. J Clin Invest. 2001;108:7–13. doi: 10.1172/JCI13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer. A double-blind randomised placebo-controlled comparative study of celecoxib (SC-58635) for the inhibition of progression of Alzheimer’s disease, protocol IQ5-97-02-001.2000 [online]. Accessed 21 November 2005. URL: http://www.clinicalstudyresults.org/documents/company-study_76_0.pdf
- Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta. 2000;1470:M69–78. doi: 10.1016/s0304-419x(00)00006-8. [DOI] [PubMed] [Google Scholar]
- Pulcinelli FM, Riondino S, Celestini A, et al. Persistent production of platelet thromboxane A2 in patients chronically treated with aspirin. J Thromb Haemost. 2005;3:2784–9. doi: 10.1111/j.1538-7836.2005.01633.x. [DOI] [PubMed] [Google Scholar]
- Qi Z, Hao C-M, Langenbach RI, et al. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–9. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramwell PW, Foegh M, Loeb R, et al. Synthesis and metabolism of prostaglandins, prostacyclin, and thromboxanes: the arachidonic acid cascade. Semin Perinatol. 1980;4:3–13. [PubMed] [Google Scholar]
- Reilly IA, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood. 1987;69:180–6. [PubMed] [Google Scholar]
- Riendeau D, Percival MD, Brideau C, et al. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther. 2001;296:558–66. [PubMed] [Google Scholar]
- Ristimaki A, Honkanen N, Jankala H, et al. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–80. [PubMed] [Google Scholar]
- Roberts LJ., II . Handbook of eicosanoids: prostaglandins and related lipids. Boca Raton, FL: CRC; 1987. pp. 233–44. [Google Scholar]
- Robertson RP. Dominance of cyclooxygenase-2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes. 1998;47:1379–83. doi: 10.2337/diabetes.47.9.1379. [DOI] [PubMed] [Google Scholar]
- Rocca B, Spain LM, Pure E, et al. Distinct roles of prostaglandin H synthases 1 and 2 in T-cell development. J Clin Invest. 1999;103:1469–77. doi: 10.1172/JCI6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M, Ricci V, Memoli A, et al. Helicobacter pylori up-regulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J Biol Chem. 1998;273:28560–3. doi: 10.1074/jbc.273.44.28560. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–5. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Sciulli MG, Capone ML, Tacconelli S, et al. The future of traditional nonsteroidal antiinflammatory drugs and cyclooxygenase-2 inhibitors in the treatment of inflammation and pain. Pharmacol Rep. 2005;57:66–85. [PubMed] [Google Scholar]
- Sciulli MG, Renda G, Capone ML, et al. Heterogeneity in the suppression of platelet cyclooxygenase-1 activity by aspirin in coronary heart disease. Clin Pharmacol Ther. 2006;80:115–25. doi: 10.1016/j.clpt.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Shao J, Sheng H, Inoue H, et al. Regulation of constitutive cyclooxygenase-2 expression in colon carcinoma cells. J Biol Chem. 2000;275:33951–6. doi: 10.1074/jbc.M002324200. [DOI] [PubMed] [Google Scholar]
- Shigeta J, Takahashi S, Okabe S. Role of cyclooxygenase-2 in the healing of gastric ulcers in rats. J Pharmacol Exp Ther. 1998;286:1383–90. [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Smith W, DeWitt D, Garavito R. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Wittes J, Finn PV, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials. The Cross Trial Safety Analysis. Circulation. 2008;117:2104–13. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinney DC, Mak AY, Barnett J, et al. Differential allosteric regulation of prostaglandin H synthase 1 and 2 by arachidonic acid. J Biol Chem. 1997;272:12393–8. doi: 10.1074/jbc.272.19.12393. [DOI] [PubMed] [Google Scholar]
- Tacconelli S, Capone ML, Sciulli MG, et al. The biochemical selectivity of novel COX-2 inhibitors in whole blood assays of COX-isozyme activity. Curr Med Res Opin. 2002;18:503–11. doi: 10.1185/030079902125001335. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shigeta J, Inoue H, et al. Localization of cyclooxygenase-2 and regulation of its mRNA expression in gastric ulcers in rats. Am J Physiol. 1998;275:G1137–45. doi: 10.1152/ajpgi.1998.275.5.G1137. [DOI] [PubMed] [Google Scholar]
- Tonai T, Taketani Y, Ueda N, et al. Possible involvement of interleukin-1 in cyclooxygenase-2 induction after spinal cord injury in rats. J Neurochem. 1999;72:302–9. doi: 10.1046/j.1471-4159.1999.0720302.x. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–22. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenhutter CW, Boice JA, Ko A, et al. Evaluation of the comparative efficacy of etoricoxib and ibuprofen for treatment of patients with osteoarthritis: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2005;80:470–9. doi: 10.4065/80.4.470. [DOI] [PubMed] [Google Scholar]
- Wilson DE. Role of prostaglandins in gastroduodenal mucosal protection. J Clin Gastroenterol. 1991;13:S65–71. doi: 10.1097/00004836-199112001-00011. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, et al. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–86. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yu Y, Fan J, Hui Y, et al. Targeted cyclooxygenase gene (ptgs) exchange reveals discriminant isoform functionality. J Biol Chem. 2007;282:1498–506. doi: 10.1074/jbc.M609930200. [DOI] [PubMed] [Google Scholar]