Abstract
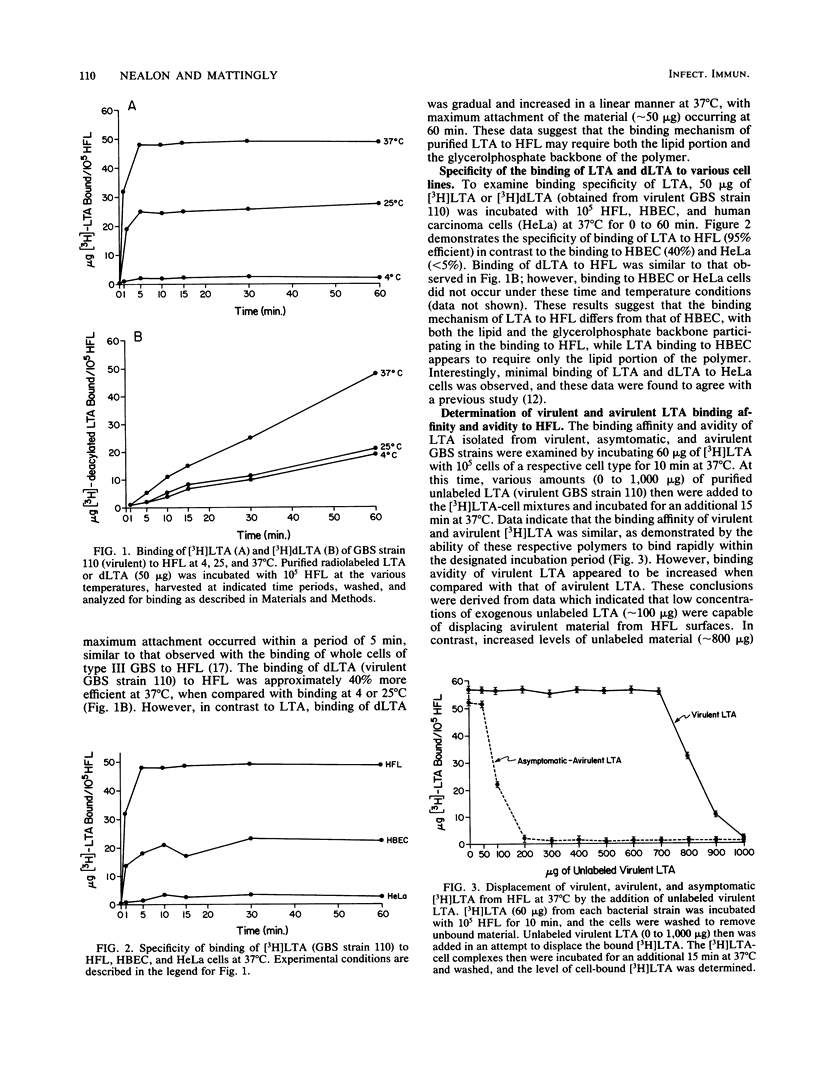
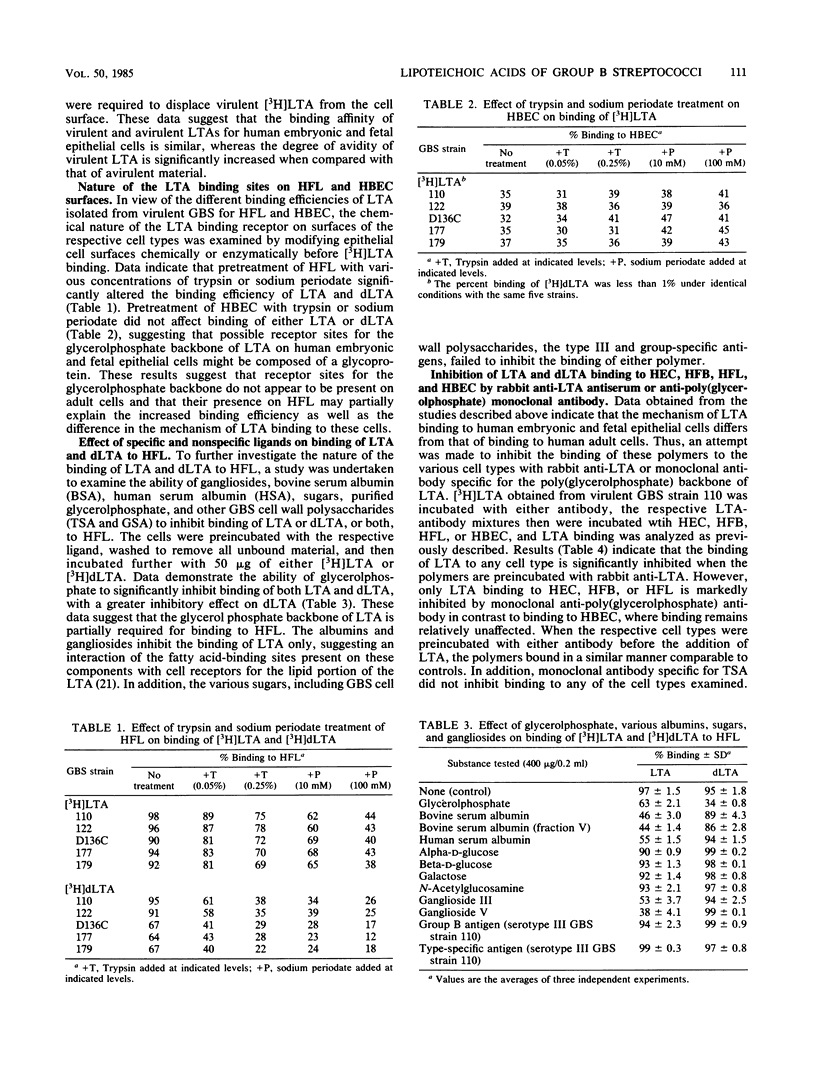
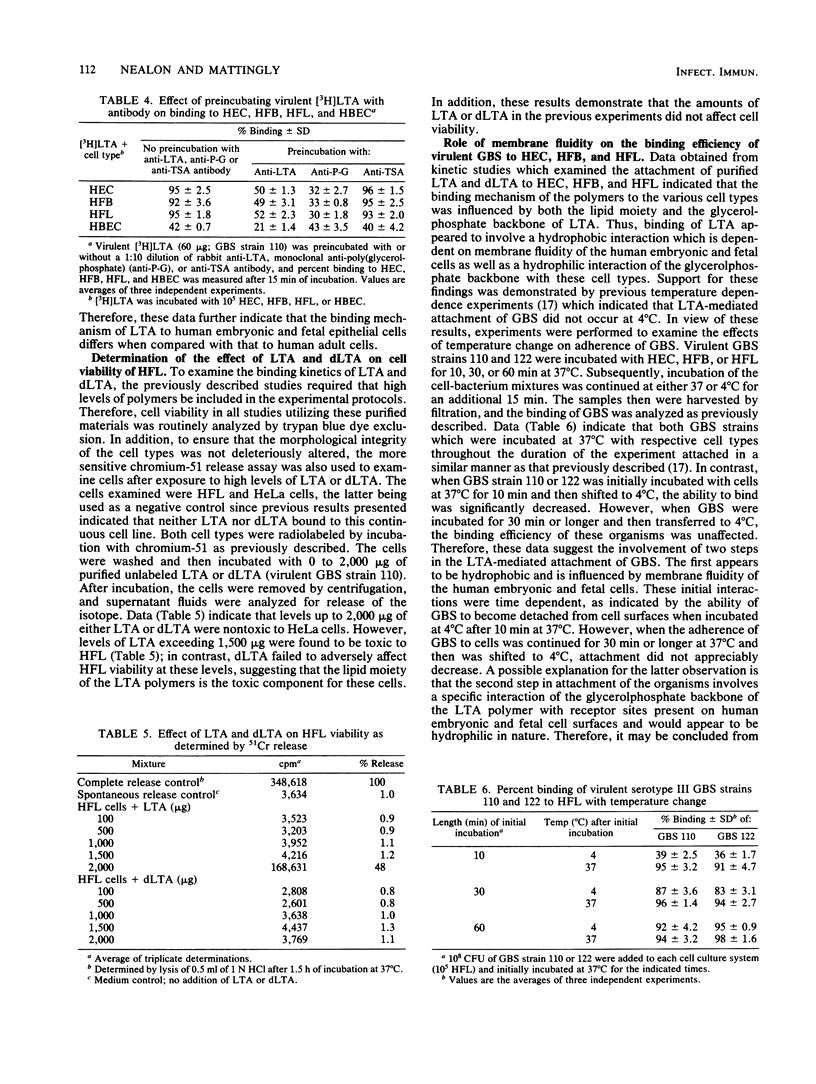
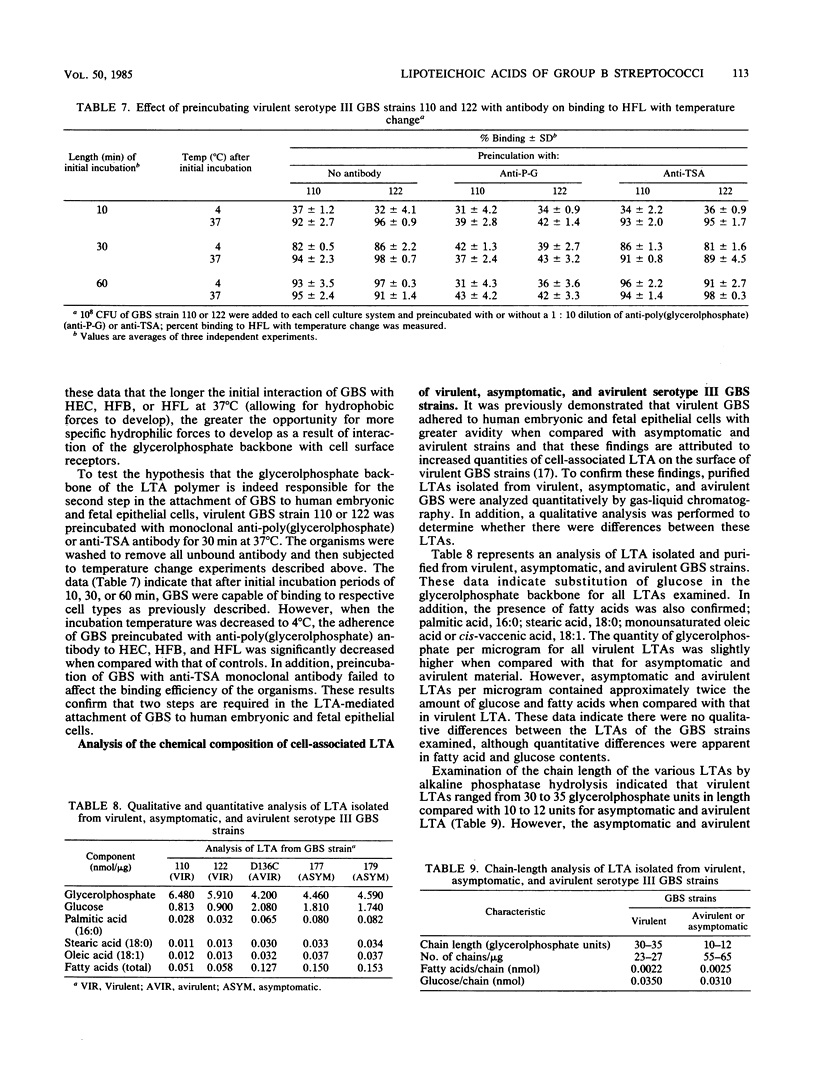
The mechanism(s) involved in the binding of lipoteichoic acid (LTA), isolated from virulent, asymptomatic, or avirulent serotype III strains of group B streptococci, to human embryonic epithelial cells (HEC), human fetal epithelial cells (HFC), and human adult buccal epithelial cells was investigated. It was determined that the binding of purified [3H]LTA to human adult buccal epithelial cells differed from the binding to HEC and HFC. LTA from all group B streptococcus strains bound to human adult buccal epithelial cells in a similar manner and was enhanced by the lipid portion of the polymer; in contrast, [3H]LTA binding to HEC and HFC was mediated by hydrophobic as well as specific interactions due to the glycerolphosphate backbone of LTA. Binding avidity of the LTAs to HEC and HFC varied depending on the bacterial strain. Polymers from asymptomatic and avirulent strains were easily dissociated from cell surfaces with unlabeled virulent LTA through competitive interactions; however, 10-fold greater levels of the same material were required to displace virulent [3H]LTA from HEC and HFC surfaces. These observed differences in binding avidity were shown to be due to longer LTA chains (30 to 35 glycerolphosphate units) in virulent strains when compared with LTA chains (10 to 12 glycerolphosphate units) of asymptomatic and avirulent strains. Thus, LTA appears to enhance the ability of virulent group B streptococci to bind to HEC and HFC with stronger avidity by virtue of the increased length of the cell-associated polymers synthesized by these strains. Mild enzymatic treatment of HEC and HFC with trypsin or periodate abolished LTA binding, which suggests the presence of a certain glycoprotein receptor(s) for LTA which does not appear to be present on human adult buccal epithelial cells. These data may therefore partially explain the increased susceptibility of newborn infants to group B streptococcal infections.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony B. F., Okada D. M. The emergence of group B streptococci in infections of the newborn infant. Annu Rev Med. 1977;28:355–369. doi: 10.1146/annurev.me.28.020177.002035. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Barrett F. F., Gordon R. C., Yow M. D. Suppurative meningitis due to streptococci of Lancefield group B: a study of 33 infants. J Pediatr. 1973 Apr;82(4):724–729. doi: 10.1016/s0022-3476(73)80606-7. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Binding of group A streptococci to human oral mucosal cells by lipoteichoic acid. Trans Assoc Am Physicians. 1975;88:285–292. [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Carruthers M. M., Kabat W. J. Mediation of staphylococcal adherence to mucosal cells by lipoteichoic acid. Infect Immun. 1983 Apr;40(1):444–446. doi: 10.1128/iai.40.1.444-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran T. I., Straus D. C., Mattingly S. J. Factors influencing release of type III antigens by group B streptococci. Infect Immun. 1981 Feb;31(2):615–623. doi: 10.1128/iai.31.2.615-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Enzymatic modification of bacterial receptors on saliva-treated hydroxyapatite surfaces. Infect Immun. 1982 Apr;36(1):52–58. doi: 10.1128/iai.36.1.52-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt J. C., Jr, Panos C. Teichoic acids of Streptococcus agalactiae: chemistry, cytotoxicity, and effect on bacterial adherence to human cells in tissue culture. Infect Immun. 1984 Feb;43(2):670–677. doi: 10.1128/iai.43.2.670-677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo W., Sato M., Ozawa H. Haemagglutinating activity of Leptotrichia buccalis cells and their adherence to saliva-coated enamel powder. Arch Oral Biol. 1976;21(6):363–369. doi: 10.1016/s0003-9969(76)80004-0. [DOI] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Association of elevated levels of cellular lipoteichoic acids of group B streptococci with human neonatal disease. Infect Immun. 1983 Mar;39(3):1243–1251. doi: 10.1128/iai.39.3.1243-1251.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Role of cellular lipoteichoic acids in mediating adherence of serotype III strains of group B streptococci to human embryonic, fetal, and adult epithelial cells. Infect Immun. 1984 Feb;43(2):523–530. doi: 10.1128/iai.43.2.523-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Eyal F., Morrison J. C. Postnatal development of binding of streptococci and lipoteichoic acid by oral mucosal cells of humans. J Infect Dis. 1977 Feb;135(2):267–274. doi: 10.1093/infdis/135.2.267. [DOI] [PubMed] [Google Scholar]
- Ofek I., Simpson W. A., Beachey E. H. Formation of molecular complexes between a structurally defined M protein and acylated or deacylated lipoteichoic acid of Streptococcus pyogenes. J Bacteriol. 1982 Feb;149(2):426–433. doi: 10.1128/jb.149.2.426-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes A., Wong P., Mason E. O., Jr, Taber L. H., Barrett F. F. Nosocomial transmission of group B Streptococci in a newborn nursery. Pediatrics. 1977 May;59(5):679–682. [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M. K., Mattingly S. J. Biosynthesis of cell wall peptidoglycan and polysaccharide antigens by protoplasts of type III group B Streptococcus. J Bacteriol. 1983 Apr;154(1):211–220. doi: 10.1128/jb.154.1.211-220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



