Abstract
Cocaine is an addictive psychostimulant that induces immediate early gene (IEG) expression by activating dopamine (DA) D1 and glutamate NMDA receptors in the striatum. In this study, we show that a single cocaine administration (30 mg/kg) time-dependently increases ERK phosphorylation, c-Fos and FosB protein expression, and MKP-1 phosphorylation (p-MKP-1), in the caudate-putamen (CPu) and nucleus accumbens (NAc) of Fischer rats. In the CPu, one hour after cocaine injection, the increase in c-Fos and FosB proteins expression is totally abolished by pre-administration of DA-D1 receptor antagonist, SCH23390. In the NAc, SCH23390 also inhibits cocaine-induced c-Fos protein expression. The pre-treatment of NMDA receptor antagonist, MK801, partially reduces cocaine-activated c-Fos protein expression in the CPu. Furthermore, the escalation of p-MKP-1 after acute cocaine administration is dependent on both DA-D1 and NMDA receptors activation in both brain regions examined. Our data suggest that cocaine may modulate ERK pathway signaling through the activation of DA-D1 and NMDA receptors, subsequently influencing the IEG protein expression.
Keywords: cocaine, ERK, c-Fos, FosB, MKP-1
1. Introduction
Cocaine is a major drug of abuse in Western countries and induces its psychomotor effects by blocking monoamine transporters. Among three monoaminergic systems, the dopaminergic inputs from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and the nigrostriatal projections to the caudate-putamen (CPu) have been postulated to be the main regulator of cocaine’s behavioral and biochemical effects (reviewed in Hyman and Malenka 2001; Koob and Nestler 1997; Spanagel and Weiss 1999). For example, in vitro and in vivo, cocaine administration causes a buildup of synaptic dopamine (DA) levels and increases DA neuronal activity in the CPu and NAc (Carboni et al., 1989; Kalivas and Duffy 1988; Maisonneuve and Kreek 1994; Reith et al., 1997). Cocaine also exerts its influence on the glutamatergic system. For instance, in the CPu and NAc, studies have shown that single or repeated cocaine injection modulates extracellular glutamate concentration (Pierce et al., 1996; Reid and Berger, 1996; Smith et al., 1995; Zhang et al., 2001).
Extracellular signal-regulated kinases (ERK), one of isoforms of mitogen-activated protein kinases, has been characterized to respond to extracellular stimuli and regulates cell proliferation and differentiation (Seger and Krebs, 1995). In both CPu and NAc, acute cocaine administration induces ERK phosphorylation (p-ERK; Corbille et al., 2007; Jenab et al., 2005; Sun et al., 2007; Valjent, et al., 2000; 2005; Zhang et al., 2004; review in Zhai et al., 2008). p-ERK, in turn, translocates to the nucleus and controls gene expression through regulating cAMP response element binding protein (CREB) and ternary complex factor Elk-1 (Adams and Sweatt, 2002; Davis et al., 2000; Hill et al., 1993). Pharmacological inhibition of mitogen-activated protein kinase/ERK kinase (MEK), an upstream activator of ERK, attenuates cocaine-induced immediate early gene (IEG) expression in mesocorticolimbic brain regions (Ferguson et al., 2006; Valjent et al., 2000); suggesting that ERK-mediated cascades are important for cocaine-regulated transcriptional mechanisms.
Mitogen-activated protein kinase phosphatase-1 (MKP-1) belongs to the family of dual specificity phosphatase that is highly regulated by ERK activation. For example, p-ERK directly induces MKP-1 expression in vivo and in vitro (Brondello et al., 1997; Sgambato et al., 1998). After induction, MKP-1 dephosphorylates p-ERK, inactivating it, as a negative feedback mechanism (Duff et al., 1995; Sun et al., 1993). Corticostriatal stimulation coincidently increases MKP-1 and c-fos mRNA in an ERK-dependent manner (Sgambato et al., 1998). In the striatum, MKP-1 mRNA and protein levels were increased after either acute or chronic methamphetamine administration (Takaki et al., 2001; Ujike et al., 2002). In addition, chronic amphetamine injection also induces MKP-1 mRNA in the ventral VTA (Rajadhyaksha et al., 2004). Furthermore, there is evidence that MKP-1 is phosphorylated (p-MKP-1) by p-ERK at two extreme C-terminal Ser residues in vitro (Brondello et al., 1999). The phosphorylation stabilizes MKP-1 protein but dose not influence its ability to dephosphorylate p-ERK.
Recently, our group and others have shown that cocaine induction of p-ERK may be mediated via DA-D1 and NMDA receptors activation (Jenab et al., 2005; Zhang et al., 2004). Although MPK-1 and c-fos mRNA induction in response to ERK activation has been documented, the parallel p-MKP-1 and Fos-related protein expression after acute cocaine administration has not been elucidated. To this end, we examined the activation profile of p-ERK, p-MKP-1 and Fos-related proteins expression after a single cocaine injection. In a second study, we determined the effects of DA-D1 and NMDA receptor blockade on cocaine-increased p-MKP-1 and Fos protein expression in the CPu and NAc.
2. Results
Effects of acute cocaine administration on p-ERK, Fos protein expression and p-MKP-1
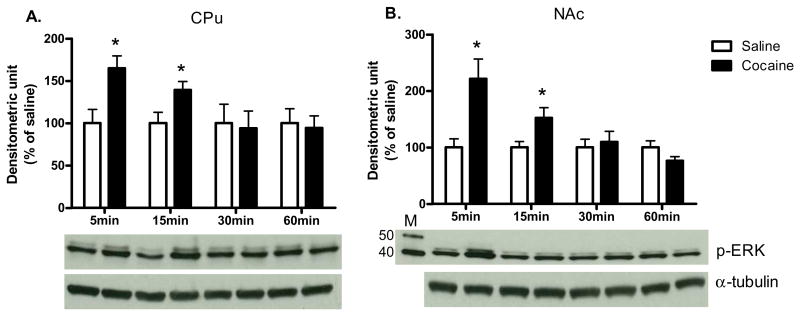
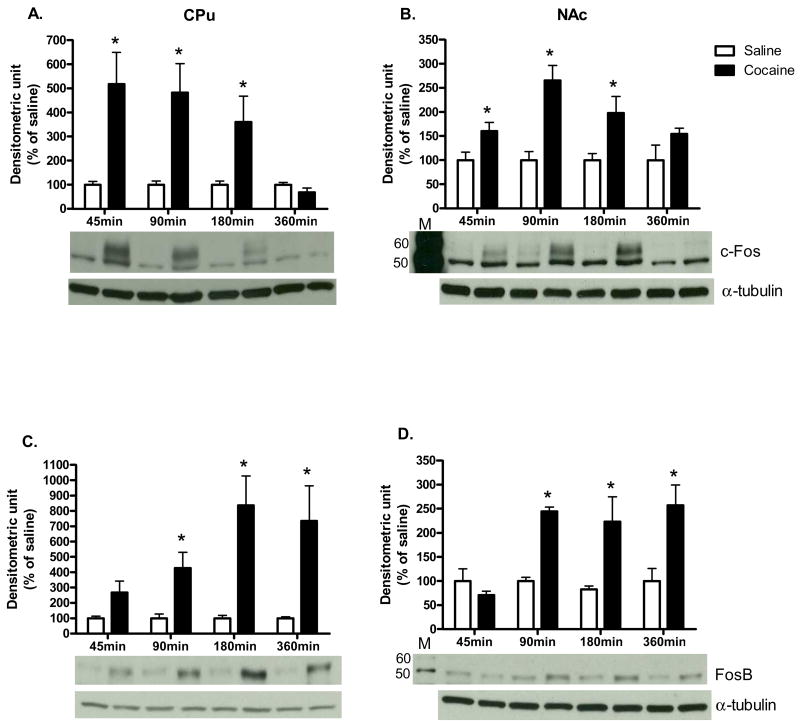
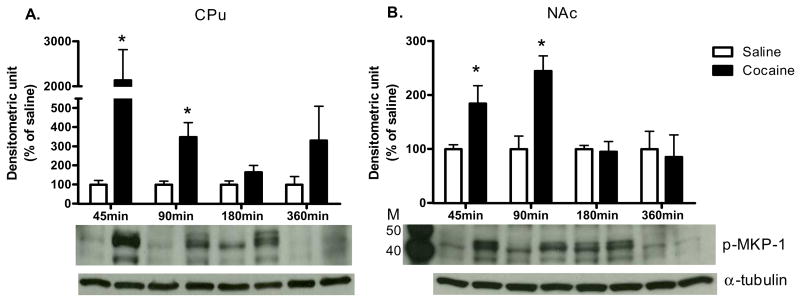
As shown in Fig. 1, acute cocaine administration time-dependently increased p-ERK protein levels in the CPu and NAc [CPu: 5 min: t (6) = 2.98, P<0.05; 15 min: t (6) = 2.45, P<0.05; NAc: 5 min: t (6) = 3.19, P<0.05; 15 min: t (6) = 2.50, P<0.05]. In both CPu and NAc, c-Fos protein levels were also significantly higher after cocaine administration when compared to saline controls [CPu: 45 min: t (6) = 3.16, P<0.05; 90 min: t (6) = 3.17, P<0.05; 180 min: t (6) = 2.44; P<0.05; NAc: 45 min: t (6) = 2.50, P<0.05; 90 min: t (6) = 4.62, P<0.01; 180 min: t (6) = 2.66; P<0.05; Fig. 2A and B respectively]. Furthermore, cocaine induced FosB protein expression from 45 to 360 min in both brain regions [CPu: 90 min: t (6) = 3.08, P<0.05; 180 min: t (6) = 3.85, P<0.01; 360 min: t (6) = 2.76; P<0.05; NAc: 90 min: t (6) = 11.91, P<0.001; 180 min: t (6) = 2.68, P<0.05; 360 min: t (6) = 3.16; P<0.05; Fig. 2C and D respectively]. Cocaine also induced p-MKP-1 protein levels in both the CPu and NAc [CPu: 45 min: t (6) = 2.97, P<0.05; 90 min: t (6) = 3.17, P<0.05; NAc: 45 min: t (6) = 2.49, P<0.05; 90 min: t (6) = 3.91, P<0.01; Fig. 3C and D, respectively]. The pattern of cocaine induction in the CPu and NAc was similar for all four proteins [Fig 1.-3]. In addition, acute cocaine administration did not alter total protein levels of ERK and MKP-1 in both brain regions [Table 1A and B].
Fig. 1. The time course of cocaine effects on p-ERK.
in (A) CPu and (B) NAc. Results represent as protein levels over α-tubulin expressed as percentage of saline control (4 animals per group). 5, 15, 30, or 60 min after rats were given injections. M is the molecular marker in kDa. *p <0.05 as compared with respective saline group.
Fig. 2. The time course of cocaine effects on Fos-like protein expression.
c-Fos in (A) CPu and (B) NAc; FosB in (C) CPu and (D) NAc. Results represent as protein levels over α-tubulin expressed as percentage of saline control (4 animals per group). 45, 90, 180, or 360 min after rats were given injections. M is the molecular marker in kDa. *p <0.05 as compared with respective saline group.
Fig. 3. The time course of cocaine effects on p-MKP-1.
in (A) CPu and (B) NAc. Results represent as protein levels over α-tubulin in the CPu expressed as percentage of saline control (4 animals per group). 45, 90, 180, or 360 min after rats were given injections. M is the molecular marker in kDa. *p <0.05 as compared with respective saline group.
Table 1.
Total protein levels after cocaine/antagonists treatments in the rat CPu and NAc.
| A. Cocaine effect on ERK | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time | 5min | 15min | 30min | 60min | ||||
| Treatment | saline | cocaine | saline | cocaine | saline | cocaine | saline | cocaine |
| CPu | 100.00±4.62 | 94.99±10.24 | 100.00±11.50 | 82.11±8.73 | 100.00±20.41 | 92.48±26.17 | 100.00±31.50 | 101.86±24.81 |
| NAc | 100.00±8.18 | 88.16±5.85 | 100.00±1.78 | 94.94±6.10 | 100.00±1.94 | 97.65±10.45 | 100.00±9.72 | 111.89±2.73 |
| B. Cocaine effect on MKP-1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time | 45min | 90min | 180min | 360min | ||||
| Treatment | saline | cocaine | saline | cocaine | saline | cocaine | saline | cocaine |
| CPu | 100.00±5.35 | 133.10±15.12 | 100.00±9.06 | 100.22±11.90 | 100.00±14.46 | 120.56±19.35 | 100.00±26.65 | 85.47±9.59 |
| NAc | 100.00±6.50 | 103.72±17.51 | 100.00±5.57 | 99.94±13.64 | 100.00±11.25 | 75.04±9.15 | 100.00±7.97 | 92.41±7.54 |
| C. Cocaine/antagonists effects on MKP-1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | S/S | S/C | MK/S | MK/C | SCH/S | SCH/C | ||
| CPu | 100.00±11.44 | 110.00±13.29 | 114.37±23.36 | 100.36±9.04 | 124.23±13.75 | 98.21±15.74 | ||
| NAc | 100.00±12.21 | 99.53±5.53 | 99.47±9.30 | 109.39±9.06 | 107.22±10.92 | 110.45±14.90 | ||
The ratio of total proteins over α-tubulin is expressed relative to saline controls which are set at 100%. Data are represented as mean ±SEM. (A) Time course of ERK protein levels after acute cocaine in CPu and NAc. (B) Time course of MKP-1 protein levels after acute cocaine in CPu and NAc. (C) MKP-1 levels after antagonist (MK801 or SCH23390) pretreatment followed by cocaine or saline injections. [S/S= saline/saline; S/C=saline/cocaine; MK/S=MK801/saline; MK/C=MK801/cocaine; SCH/S=SCH23390/saline; SCH/C=SCH23390/cocaine].
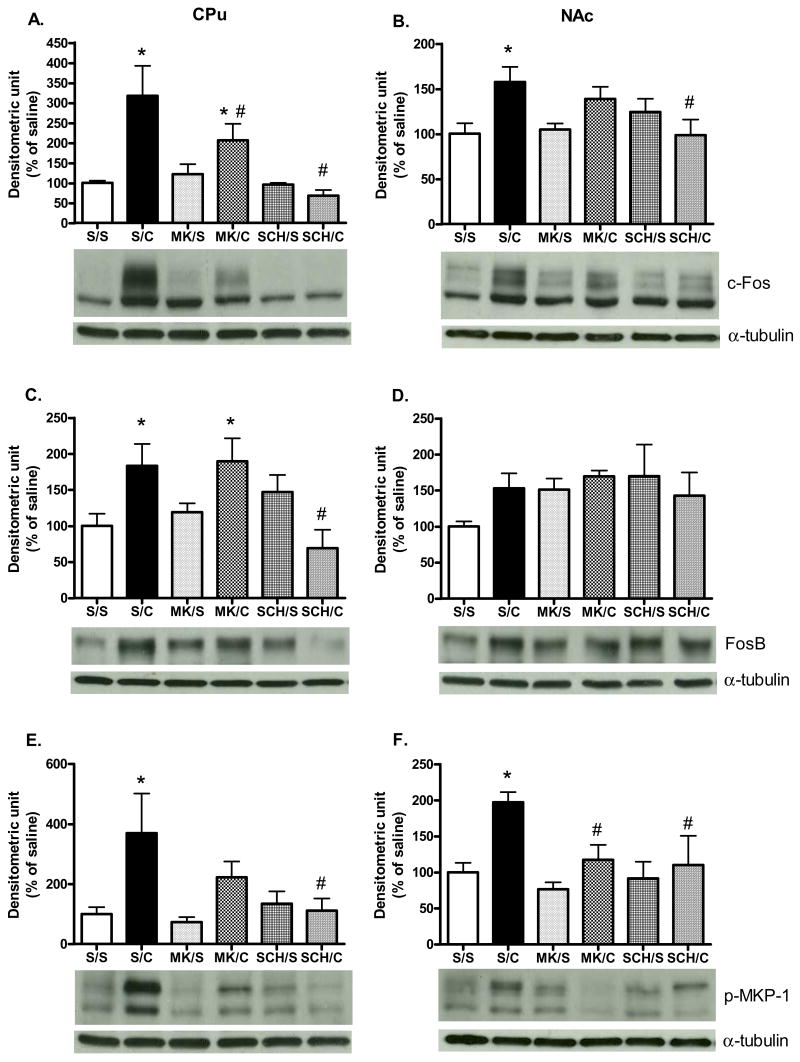
Effects of MK801 and SCH23390 pre-treatments on cocaine-induced Fos protein expression and p-MKP-1
In the CPu, a significant antagonist treatment main effect was found [F (5, 29) = 3.06, P<0.05; Fig. 4A]. Both MK801 and SCH23390 pre-treatments blocked acute cocaine-induced c-Fos protein induction [P<0.05]. However, in the NAc, although a main effect of antagonist pretreatment was observed in c-Fos protein expression [F (5, 29) = 2.92, P<0.05; Fig. 4B], only SCH23390 attenuated cocaine-induced c-Fos expression when compared with saline/cocaine-treated rats [P<0.05].
Fig. 4. Effects of MK801 and SCH23390 on cocaine-induced Fos-like protein expression and p-MKP-1.
c-Fos in (A) CPu and (B) NAc; FosB in (C) CPu and (D) NAc; p-MKP-1 in (E) CPu and (F) NAc. Rats were pre-administrated saline, MK801, or SCH23390 30 min before a single saline or cocaine injection. Results represent as protein levels over α-tubulin expressed as percentage of saline/saline control (5 animals per group). *p <0.05 as compared with saline/saline group and #p<0.05 as compared with saline/cocaine group. [S/S= saline/saline; S/C=saline/cocaine; MK/S=MK801/saline; MK/C=MK801/cocaine; SCH/S=SCH23390/saline; SCH/C=SCH23390/cocaine].
In the CPu, a significant main effect of antagonist treatment on cocaine-induced FosB protein expression was found [F (5, 29) = 3.77, P<0.05; Fig. 4C]. SCH23390 blocked cocaine induction of FosB protein levels [P<0.05]. However, neither MK801 nor SCH23390 altered cocaine effects on FosB in the NAc. Similar to Fig. 3, cocaine administration significantly increased p-MKP-1 protein levels in the CPu. A significant main effect of antagonist treatment in p-MKP-1 protein levels was obtained [F (5, 29) = 3.06, P<0.05; Fig. 4E]. SCH23390 blocked the increase in p-MKP-1 after cocaine injection [P<0.05]. Although MK801 pre-treatment decreased cocaine-induced p-MKP-1 protein levels by approximately 40%, it failed to reach the significant level [P>0.05]. In the NAc, a significant main effect of antagonist treatment on cocaine-induced p-MKP-1 was observed [F (5, 29) = 3.54, P<0.05; Fig. 4F]; where both MK801 and SCH23390 pre-treatments reduced cocaine-induced p-MKP-1 activation [P<0.05]. As shown in Table 1C, antagonists pre-treatment and/or acute cocaine injections had no effect on non-phosphorylated MKP-1 protein levels in both CPu and NAc.
3. Discussion
In the present study, after a single cocaine injection, a rapid and transient increase of p-ERK protein levels was observed in the CPu and NAc. This is consistent with studies demonstrating that acute cocaine induced ERK activation in drug reward associated areas (Corbille et al., 2007; Jenab et al., 2005; Sun et al., 2007; Valjent, et al., 2000; 2005; Zhang et al., 2004). In addition, we further showed the delayed elevation of IEG protein expression and phosphorylation in response to acute cocaine administration. Two transcription factors, Elk-1 and CREB, have been characterized as nuclear targets of ERK activation (Adams and Sweatt, 2002; Davis et al., 2000; Hill et al., 1993). Previous studies have reported that acute cocaine administration increases Elk-1 phosphorylation in the striatum (Jenab et al., 2005; Sun et al., 2007; Valjent et al., 2000). Pharmacological inhibition of ERK pathway signaling also attenuates acute cocaine-induced c-Fos and FosB protein rexprssion in the striatum (Radwanska et al., 2006; Guan et al., 2008). In mice CPu and NAc, acute cocaine injection augments CREB phosphorylation in ERK-dependent manner (Brami-Cherrier et al., 2005; Kano et al., 1995; Karasinska et al., 2005; Walters et al., 2003). To the best of our knowledge there is no evidence showing that acute cocaine increases CREB phosphorylation in the CPu of rats. However, in rats, we have observed an elevation of CREB phosphorylation in the NAc after acute cocaine injection (Nazarian et al., unpublished observation). Furthermore, ERK-regulated phosphorylation of pp90 ribosomal S6 kinase, a kinase mediating CREB phosphorylation, was increased in response to acute cocaine administration in the CPu of Fischer rats (Sun et al., 2007). Thus, ERK-activated Elk-1 and/or CREB may underlie cocaine-induced gene expression in both CPu and NAc.
Numerous studies have demonstrated that acute cocaine induces c-fos, FosB mRNA and Fos-like protein expression (Brown et al., 1992; Chen et al., 1995; Graybiel et al., 1990; Hope et al., 1992; Jenab et al., 2003; Steiner and Gerfen, 1993; Sun et al., 2007; Young et al., 1991). Herein, similar to previous studies suggesting distinct cocaine effects on Fos-like protein induction (Nestler et al., 2001;Ranwanska et al., 2006; Young et al., 1991), we also found that acute cocaine administration induces differential activation profiles in immediate early gene proteins expression in both areas examined: a rapid and transient c-Fos protein expression (45–180 min) and delayed activation of FosB (90–360 min). A recent study has indicated that acute cocaine induced robust and moderate histone H4 acetylation in c-fos and FosB promoters, respectively (Kumar et al., 2005). In addition, after chronic cocaine injection, the histone H3 acetylation is evident in FosB but not in c-fos. Therefore, the distinct protein expression and histone modification may further promote different transcriptional regulation after cocaine exposure.
Accumulating evidence has established that the interaction between DA-D1 and NMDA receptors in the postsynaptic region is necessary for Fos-like proteins induction in the striatum (Berretta et al., 1992; Das et al., 1997). Previous studies have shown that, in the CPu, the acute cocaine-induced c-fos mRNA and protein expression is dependent on both DA-D1 and NMDA receptor activation (Jenab et al., 2003; Torres and Rivier, 1993; Young et al., 1991). We also demonstrated that DA D1 antagonist, SCH23390, abolished cocaine-mediated c-Fos protein expression in the CPu and NAc. However, only in the CPu, the NMDA receptor antagonist, MK801, partially inhibited c-Fos expression. Three plausible explanations may underlie the regional discrepancy in response to NMDA receptor antagonism. First, microdialysis studies in freely moving rats have indicated that systematic MK801 administration (0.2–0.5 mg/kg) significantly increases extracellular DA levels in the prefrontal cortex and the NAc but not in the CPu (Mathe et al., 1996; Wedzony et al., 1993; Wolf et al., 1993). In addition, previous studies indicated that moderate to high doses of MK801 (0.5–8.0 mg/kg) may induce Fos-like protein expression in various brain regions including the striatum (Draunow and Faull, 1990; Liu et al., 1994; Storvik et al., 2006). However, in both CPu and NAc, low dose of MK801 (0.1–0.3 mg/kg) itself had no effect on c-fos mRNA induction (De Leonibus et al., 2002; Dalia and Wallace, 1995). Similarly, although MK801 (0.25 mg/kg) itself did not change basal c-Fos protein expression in present study, it is possible that both MK801 and cocaine administration may elevate on dopamine outflow in the NAc by a synergistic action and result in inefficiency of MK801 blockade of c-Fos protein expression. Nevertheless, the relationship between MK801-induced extracellular DA level and postsynaptic IEG protein expression should be further elucidated. Secondly, as postulated by Zhai et al (2008), glutamate signaling may play a subsidiary role while DA signaling is dominant. In our CPu extract, NMDA antagonism partially blocked cocaine-induced c-Fos protein expression similar to a previous study using higher dose of MK801 or CPP (Torres and River, 1993). On the other hand, administration of SCH23390 abolished cocaine-induced c-Fos protein expression in both brain regions. In the CPu, DA-D1 but not NMDA receptor blockade also prevents the early development of FosB expression after acute cocaine injection. Since high dose of cocaine was used in present study, it is tentative to suggest that dopaminergic signaling cascade is the major component mediating IEG expression. Finally, CPu and NAc are two neuronal substrates in mediating distinct cocaine-induced behavioral responses (Hyman et al., 2006). With detail examination, we have demonstrated that acute cocaine induced high and moderate magnitude IEG proteins expression in the CPu and NAc, respectively (Fig. 2). A different activation time course was also observed in both brain regions. One hour after cocaine administration, a fully developed c-Fos protein expression may occur in the CPu depending on both DA and glutamate signaling. However, DA-D1 receptors activation is necessary for a premature/initial c-Fos development in the NAc. Thus, the different activation profile and sensitivity in response to receptor antagonism may reflect a shift in neuronal activity between both regions underlying cocaine’s psychomotor responses.
MKP-1 protein, an IEG product, is the phosphatase involved in the inactivation of ERK in several transfected cells (Alessi et al., 1993; Sun et al., 1993). P-ERK has been reported to induce and stabilize p-MKP-1 without altering its intrinsic capability to downregulate ERK activation (Brondello et al., 1999). Previous studies have shown that acute methamphetamine administration induces MKP-1 mRNA in the striatum (Ujike et al., 2002; Takaki et al., 2001). In the CPu and NAc, we further demonstrate an elevation of p-MKP-1 protein levels in DA-D1 and NMDA receptor-dependent manner. After acute cocaine, the increasing p-MKP-1 protein levels is followed by the downregulation of p-ERK suggesting that p-MKP-1 may regulate the time-limited activation of ERK. Interestingly, acute cocaine administration coincidentally induces c-Fos protein expression and p-MKP-1. Although a different experimental paradigm, Sgambato et al (1998) showed a spatially coincident distribution of c-fos and MKP-1 mRNAs in the striatum via electrical stimulation of the glutamatergic corticostriatal pathway. In addition, blocking Elk-1 and CREB activation using intrastriatal infusion of the ERK inhibitor, PD 98059, completely abolished c-fos and MKP-1 induction. Since the dose of antagonists used in present study has been shown to attenuate acute cocaine-induced p-ERK in the CPu (Jenab et al., 2005), it is reasonable to suggest that the decrease in cocaine-activated p-MKP-1 after DA D1 and NMDA receptor antagonism is due to the inactivation of ERK signaling. Taken together, in both CPu and NAc, we demonstrated that p-MKP-1 protein levels were significantly increased after single cocaine injection. Perhaps, via the ERK-mediated intracellular cascade, DA-D1 and NMDA receptor antagonist pretreatment abolished cocaine-induced p-MKP-1. In addition, administration of abused drug and/or striatal stimulation may regulate MKP-1 gene induction and its protein phosphorylation underlying the synaptic plasticity in the striatum. However, the contribution of total MKP-1 and p-MKP-1 protein to p-ERK inactivation after cocaine administration should be further examined.
In summary, several drugs, including cocaine, stimulate the expression of IEG in specific regions resulting in the modulation of downstream genes expression. Our data demonstrate that acute cocaine administration induces the IEG protein expression (c-Fos and FosB) and phosphorylation (p-MKP-1). However, such protein activation profiles vary in the CPu and NAc, indicating that cocaine exerts differential influence on the two brain regions. In addition, the activation of DA-D1 and/or NMDA receptors seems to converge on ERK-mediated signaling and control alternation of IEG underlying the cocaine-induced neuronal plasticity and psychomotor effects.
4. Method
4.1. Animals
60-day-old male Fischer rats (Charles River, Raleigh, NC) were individually housed in Plexiglas chambers (20 × 20 × 41 cm). Rats were maintained on a 12-hour light/dark cycle (lights on at 9:00 a.m.) with free access to food and water. Animal care and use was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85–23, Bethesda, MD) and approved by the Institutional Animal Care and Use Committee of Hunter College.
4.2. Drug and antibodies
Cocaine hydrochloride, MK-801, and SCH23390 hydrochloride were purchased from Sigma Chemical Co. (St. Louis, MO). Primary antibodies for ERK (#9102), p-ERK (#9101), p-MKP-1 (#2857), c-Fos (#4384) and FosB (#2251) were purchased from Cell Signaling Technologies (Beverly, MA). Antibodies against MKP-1 (sc-370) and α-tubulin (sc-8035) were purchased from Santa Cruz Technologies (Santa Cruz, CA). Both horseradish peroxidase-conjugated anti-rabbit IgG (NA-934) and anti-mouse IgG (NA-931) were from Amersham Pharmacia (Piscataway, NJ).
4.3. Drug administration
Cocaine solutions were prepared by dissolution in physiological saline (0.9%) and injected intra-peritoneally (i.p.). One week after arrival, rats received an injection of saline (1 ml/kg) or cocaine (30 mg/kg). For evaluating p-ERK protein levels, rats were sacrificed 5, 15, 30, or 60 min after drug treatment. For the measurement of p-MKP-1, c-Fos and FosB protein expression, rats received same doses of saline or cocaine administration and sacrificed 45, 90, 180 or 360 min later. For experimental paradigm involving antagonists, rats were administered MK801 (0.25 mg/kg in 0.9% saline, i.p.) or SCH23390 (0.25 mg/kg in 0.9% saline, i.p.) 30 min before single cocaine (30 mg/kg) or saline injections and sacrificed 60 min after the last injection. The doses of MK801 and SCH23390 were previously reported to inhibit the acute cocaine-induced pERK in the striatum (Jenab et al., 2005).
4.4. Protein preparation and measurement
After decapitation (following a brief 20 s exposure to CO2), rat brains were removed, flash frozen in 2-methylbutane (−40° C), and stored at −80° C until used. The coronal slices (1mm thick) were cut out in a matrix (ASI instruments, Warren, MI) and areas of CPu and NAc were simultaneously dissected out on a cold glass plate. Tissue samples were homogenized by using a Polytron handheld homogenizer (Kinematica, Luzern, Switzerland) in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 10% Glycerol, 1% Triton X-100, 1% Igepal CA-630, 1% sodium dexycholic acid) containing a mixture of phosphatase inhibitors. After 30 min incubation, homogenates were centrifuged at 13,000 rpm for 15 min at 4° C. Supernatants were then collected and stored at −80° C until used. Total protein content was determined using a Bradford kit from Bio-Rad Laboratories (Hercules, CA).
4.5. Western blot analysis
Protein samples were analyzed by using Western blot analysis as previously described (Jenab et al., 2005). Briefly, equal amount of protein extracts were boiled in Lammeli buffer containing 1% β-mercaptoethanol for 5 min and ran on SDS-PAGE gels, then transferred to PVDF membranes. Membranes were blocked with 5% nonfat dry milk for 1hr at room temperature and then incubated with primary antibodies of ERK (1:1000), p-ERK (1:1000), MKP-1 (1:500), p-MKP-1 (1:1000), c-Fos (1:1000), and FosB (1:1000), individually, overnight at 4° C. After three washes with Tris-Tween-20 Buffer (TBST; pH = 7.4), membranes were incubated with their appropriate secondary antibodies (1:1000) for 1hr at room temperature followed by three more washes with TBST. Antibody binding was detected by using an enhanced chemiluminescence kit (ECL; Amersham Pharmacia, Piscataway, NJ). Intensity of protein bands was quantified with a computer densitometer and Image Quant Program (Molecular Dynamics). For normalization of protein levels, all membranes were re-probed with α-tubulin antibody (1:1000).
4.6. Statistical analysis
Protein levels were expressed as a ratio to α-tubulin levels. Data was expressed as mean %± SEM relative to respective saline controls, which were arbitrarily set at 100%. Student’s t-tests were used to determine differences between cocaine- and saline-treated animals at each time point. To determine the effect of antagonist treatment on cocaine-induced activation, a one-way ANOVA was used followed by with LSD post hoc analysis when appropriate. Determination of statistically significant differences was considered at 0.05 level [P<0.05].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- Adams JP, Sweatt JD. Molecular psychology: Role for ERK MAP kinase cascade in memory. Annu Rev Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Smythe C, Keyse SM. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993;8:2015–2020. [PubMed] [Google Scholar]
- Berretta S, Robertson HA, Graybiel AM. Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. J Neurophysiol. 1992;68:767–777. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Hervé D, Darragh J, Corvol JC, Pages C, Arthur SJ, Girault JA, Caboche J. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondello JM, Brunet A, Pouysségur J, McKenzie FR. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J Biol Chem. 1997;272:1368–1376. doi: 10.1074/jbc.272.2.1368. [DOI] [PubMed] [Google Scholar]
- Brondello JM, Pouysségur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2414–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Chen J, Nye HE, Kelz MB, Hiroi N, Nakabeppu Y, Hope BT, Nestler EJ. Regulation of delta FosB and FosB-like proteins by electroconvulsive seizure and cocaine treatments. Mol Pharmacol. 1995;48:880–889. [PubMed] [Google Scholar]
- Corbillé AG, Valjent E, Marsicano G, Ledent C, Lutz B, Hervé D, Girault JA. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci. 2007;27:6937–6947. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalia A, Wallace LJ. Amphetamine induction of c-fos in the nucleus accumbens is not inhibited by glutamate antagonists. Brain Res. 1995;694:299–307. doi: 10.1016/0006-8993(95)00794-q. [DOI] [PubMed] [Google Scholar]
- Das S, Grunert M, Williams L, Vincent SR. NMDA and D1 receptors regulate the phosphorylation of CREB and the induction of c-fos in striatal neurons in primary culture. Synapse. 1997;25:227–233. doi: 10.1002/(SICI)1098-2396(199703)25:3<227::AID-SYN1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element binding protein to control long term potentiation dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leonibus E, Mele A, Oliverio A, Pert A. Distinct pattern of c-fos mRNA expression after systemic and intra-accumbens amphetamine and MK-801. Neuroscience. 2002;115:67–78. doi: 10.1016/s0306-4522(02)00415-3. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull RL. MK801 induces c-fos protein in the thalamic and neocortical neurons of rat brain. Neurosci Lett. 1990;113:144–150. doi: 10.1016/0304-3940(90)90294-j. [DOI] [PubMed] [Google Scholar]
- Duff JL, Monia BP, Berk BC. Mitogen-activated protein (MAP) kinase is regulated by the MAP kinase phosphatase (MKP-1) in vascular smooth muscle cells. Effect of actinomycin D and antisense oligonucleotides. J Biol Chem. 1995;270:7161–7166. doi: 10.1074/jbc.270.13.7161. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Hu J, Li S. Involvement of extracellular signal-regulated protein kinase in acute cocaine-induced c-fos in nucleus accumbens. Neurosci Lett. 2008;438:155–158. doi: 10.1016/j.neulet.2008.04.035. [DOI] [PubMed] [Google Scholar]
- Hill CS, Marais R, John S, Wynne J, Dalton S, Treisman R. Functional analysis of a growth factor responsive transcription factor complex. Cell. 1993;73:395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Acad Sci USA. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jenab S, Festa ED, Nazarian A, Wu HB, Sun WL, Hazim R, Russo SJ, Quinones-Jenab V. Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Brain Res Mol Brain Res. 2005;142:134–138. doi: 10.1016/j.molbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Jenab S, Festa ED, Russo SJ, Wu HB, Inturrisi CE, Quinones-Jenab V. MK-801 attenuates cocaine induction of c-fos and preprodynorphin mRNA levels in Fischer rats. Brain Res Mol Brain Res. 2003;117:237–239. doi: 10.1016/s0169-328x(03)00319-x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effects of daily cocaine and morphine treatment on somatodendritic and terminal field dopamine release. J Neurochem. 1988;50:1498–1504. doi: 10.1111/j.1471-4159.1988.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Kano T, Suzuki Y, Shibuya M, Kiuchi K, Hagiwara M. Cocaine-induced CREB phosphorylation and c-Fos expression are suppressed in Parkinsonism model mice. Neuroreport. 1995;6:2197–2200. doi: 10.1097/00001756-199511000-00023. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, Cheng R, O’Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci. 2005;22:1741–1750. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. 2005 doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Liu J, Nickolenko J, Sharp FR. Morphine induces c-fos and junB in striatum and nucleus accumbens via D1 and N-methyl-D-aspartate receptors. Proc Acad Sci USA. 1994;91:8537–8541. doi: 10.1073/pnas.91.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Kreek MJ. Acute tolerance to the dopamine response induced by a binge pattern of cocaine administration in male rats: An in vivo microdialysis study. J Pharmacol Exp Ther. 1994;268:916–921. [PubMed] [Google Scholar]
- Mathe JM, Nomikos GG, Hildebrand BE, Hertel P, Svensson TH. Prazosin inhibits MK-801-induced hyperlocomotion and dopamine release in the nucleus accumbens. Eur J Pharmacol. 1996;309:1–11. doi: 10.1016/0014-2999(96)00315-9. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Acad Sci USA. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K, Valjent E, Trzaskos J, Caboche J, Kaczmarek L. Regulation of cocaine–induced activator protein 1 transcription factors by the extracellular signal-regulated kinase pathway. Neuroscience. 2006;137:253–264. doi: 10.1016/j.neuroscience.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha A, Husson I, Satpute SS, Küppenbender KD, Ren JQ, Guerriero RM, Standaert DG, Kosofsky BE. L-type Ca2+ channels mediate adaptation of extracellular signal-regulated kinase 1/2 phosphorylation in the ventral tegmental area after chronic amphetamine treatment. J Neurosci. 2004;24:7464–7476. doi: 10.1523/JNEUROSCI.0612-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology. 1997;134:309–17. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Sgambato V, Pagès C, Rogard M, Besson MJ, Caboche J. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci. 1998;18:8814–8825. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Mo Q, Guo H, Kunko PM, Robinson S. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storvik M, Tiikkainen P, van Iersel M, Wong G. Distinct gene expression profiles in adult rat brain after acute MK-801 and cocaine treatments. Eur Neuropsychopharmacol. 2006;16:211–219. doi: 10.1016/j.euroneuro.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Sun WL, Zhou L, Hazim R, Quinones-Jenab V, Jenab S. Effects of acute cocaine on ERK and DARPP-32 phosphorylation pathways in the caudate-putamen of Fischer rats. Brain Res. 2007;1178:12–19. doi: 10.1016/j.brainres.2007.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki M, Ujike H, Kodama M, Takehisa Y, Nakata K, Kuroda S. Two kinds of mitogen-activated protein kinase phosphatases, MKP-1 and MKP-3, are differently activated by acute and chronic methamphetamine treatment in the rat brain. J Neurochem. 2001;79:679–688. doi: 10.1046/j.1471-4159.2001.00615.x. [DOI] [PubMed] [Google Scholar]
- Torres G, Rivier C. Cocaine-induced expression of striatal c-fos in the rat is inhibited by NMDA receptor antagonists. Brain Res Bull. 1993;30:173–176. doi: 10.1016/0361-9230(93)90055-g. [DOI] [PubMed] [Google Scholar]
- Ujike H, Takaki M, Kodama M, Kuroda S. Gene expression related to synaptogenesis, neuritogenesis, and MAP kinase in behavioral sensitization to psychostimulants. Ann NY Acad Sci. 2002;965:55–67. doi: 10.1111/j.1749-6632.2002.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson JM, Maldanado R, Caboche J. Involvement of the extracellular signal regulated kinase cascade for cocaine rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Herve E, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Kuo YC, Blendy JA. Differential distribution of CREB in the mesolimbic dopamine reward pathway. J Neurochem. 2003;87:1237–1244. doi: 10.1046/j.1471-4159.2003.02090.x. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Klimek V, Golembiowska K. MK-801 elevates the extracellular concentration of dopamine in the rat prefrontal cortex and increases the density of striatal dopamine D1 receptors. Brain Res. 1993;622:325–329. doi: 10.1016/0006-8993(93)90839-f. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Hu XT. Behavioral sensitization to MK-801 (dizocilpine): neurochemical and electrophysiological correlates in the mesoaccumbens dopamine system. Behav Pharmacol. 1993;4:429–442. [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Acad Sci USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H, Li Y, Wang X, Lu L. Drug-induced Alterations in the Extracellular Signal-regulated Kinase (ERK) Signalling Pathway: Implications for Reinforcement and Reinstatement. Cell Mol Neurobiol. 2008;28:157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine induced intracellular signaling and gene expression are oppositly regulated by dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann NY Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]