Abstract
Sustained hyperleptinemia of 8 ng/ml was induced for 28 days in normal Wistar rats by infusing a recombinant adenovirus containing the rat leptin cDNA (AdCMV-leptin). Hyperleptinemic rats exhibited a 30–50% reduction in food intake and gained only 22 g over the experimental period versus 115–132 g in control animals that received saline infusions or a recombinant virus containing the β-galactosidase gene (AdCMV-βGal). Body fat was absent in hyperleptinemic rats, whereas control rats pair-fed to the hyperleptinemic rats retained ≈50% body fat. Further, plasma triglycerides and insulin levels were significantly lower in hyperleptinemic versus pair-fed controls, while fatty acid and glucose levels were similar in the two groups, suggestive of enhanced insulin sensitivity in the hyperleptinemic animals. Thus, despite equivalent reductions in food intake and weight gain in hyperleptinemic and pair-fed animals, identifiable fat tissue was completely ablated only in the former group, raising the possibility of a specific lipoatrophic activity for leptin.
The obesity of ob/ob mice is the result of leptin deficiency caused by a mutation in the ob gene (1), and it responds dramatically to injection therapy with recombinant leptin administered intraperitoneally (2–4). In normal lean rats the effect of high levels of leptin is less clear; large doses of the hormone were reported to reduce food intake and body weight, but the effect was less striking than in leptin-deficient ob/ob mice (2, 4).
To obtain a more complete assessment of the effects of chronic hyperleptinemia in normal animals, we have examined food intake and body weight in normal rats in which sustained 6- to 10-fold increases in plasma leptin levels were induced by recombinant adenovirus-mediated gene delivery. In hyperleptinemic rats studied for 28 days, we observed a 30% reduction in food intake and a virtual cessation of body weight gain, accompanied by disappearance of grossly identifiable adipose tissue. In marked contrast, only partial reduction of fat depots occurred in pair-fed controls despite a similar degree of weight loss.
MATERIALS AND METHODS
Cloning of Rat Leptin and Measurement of Its Expression by Reverse Transcriptase–PCR.
Total RNA was prepared from 1 g of epididymal adipose tissue (for cDNA cloning) or from 0.5 g of liver (for measurement of leptin expression) of rats by extraction with TRIzol (Life Technologies, Gaithersburg, MD) following the protocol of the manufacturer. Oligo-dT was used to prime first-strand cDNA synthesis, using a cDNA synthesis kit (CLONTECH). After treatment of the first strand cDNA with DNase-free RNase, the leptin gene product was amplified by PCR using upstream sense primer 5′-GGAGGAATCCCTGCTCCAGC-3′ and downstream antisense primer 5′-CTTCTCCTGAGGATACCTGG-3′, based on the rat leptin gene sequence (5). For both cDNA cloning and leptin mRNA measurement, amplification was performed using one cycle at 94°C for 3 min, followed by 35 cycles at 92°C for 45 sec, 54°C for 45 sec, and 72°C for 1 min, and then final extension at 70°C for 10 min. As a control for RNA quality, we also measured β-actin expression, using the same amplification conditions as for leptin and a previously described oligonucleotide pair (6).
Preparation of Recombinant Adenovirus.
A 640-bp PCR fragment containing the entire leptin coding region was ligated to pCR TM 2.1 (Invitrogen) according to the manufacturer’s protocol. Sequence analysis confirmed that several clones contained the intact leptin cDNA. A BamHI- and XbaI-restricted leptin cDNA fragment that included 60 bp of 5′-untranslated region and 76 bp of 3′-untranslated region was ligated to similarly treated pACCMVpLpA (7). The resulting plasmid was cotransfected with pJM17 (8) into 293 cells by calcium phosphate/DNA coprecipitation to generate the new recombinant virus termed AdCMV-leptin, using previously described methods (9). Virus DNA was isolated and the presence of the leptin gene insert was confirmed by PCR using the primers described above and by Southern blotting with an oligonucleotide (5′-CGGATACCGACTGCGTGTGTGAAATGTCAT-3′) complementary to the rat leptin cDNA. Stocks of AdCMV-leptin were amplified and purified as described (9) and stored at −70°C in PBS with 0.2% BSA and 10% glycerol at 1–3 × 1012 plaque-forming units/ml. A virus containing the bacterial β-galactosidase gene under control of the cytomegalovirus promoter (AdCMV-βGal) was prepared and used as described previously (10).
Cell Culture.
Human embryonic kidney 293 cells were propagated in 60-mm (for cotransfection) or 150-mm (for amplification of viral stocks) culture dishes in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 units of penicillin per ml, and 10 μg of streptomycin per ml at 37°C/5% CO2. All medium components were from GIBCO/BRL.
Animals.
Male Wistar rats were obtained from Charles River Breeding Laboratories. Before adenovirus infusion studies, all rats received standard rat chow (Teklad F6 8664; Teklad, Madison, WI) ad libitum and had free access to water. “Pair-fed” animals were provided with the same amount of food as ingested by AdCMV-leptin-infused animals on a daily basis in the experiments shown in Fig. 2A.
Figure 2.
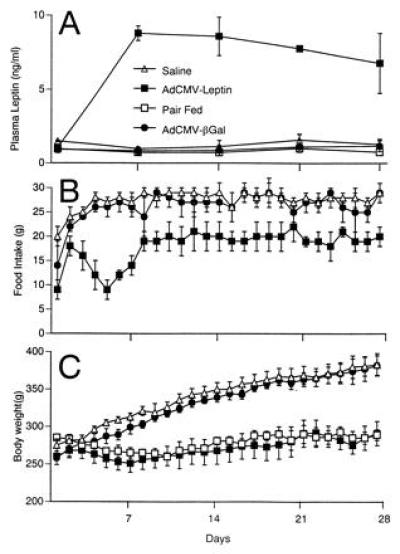
Plasma leptin, food intake and body weight. Plasma leptin levels (A), food intake (B), and body weight (C) were measured in Wistar rats that received infusions of AdCMV-leptin, AdCMV-βGal, or saline, and in Wistar rats pair-fed to AdCMV-leptin-infused rats. Values represent mean ± SEM for four animals in each group.
Virus Infusion in Normal Wistar Rats.
Polyethylene tubing (PE-50; Becton Dickinson) was anchored in the left carotid artery of 9-week-old Wistar rats of ≈250-300 g under sodium pentobarbital anesthesia (50 mg/kg; Abbott) and exteriorized via a subcutaneous tunnel. Animals were placed in a jacket and tubing was connected to a tether and swivel (Harvard Bioscience, South Natick, MA). Tubing was filled with heparinized saline (1000 units/dl) until virus infusion was begun 3 days after surgery. Before infusion, adenovirus samples were suspended in saline and filtered through a 0.2-μm filter. Two milliliters of AdCMV-leptin or AdCMV-βGal containing a total of 1 × 1012 plaque-forming units, or as a second control, 2 ml of saline alone, were infused into conscious animals over a 10-min period. Animals were studied in individual metabolic cages (Nalgene), and food intake and body weight were measured daily.
Plasma Measurements.
Blood samples were collected from the tail vein in capillary tubes coated with EDTA at weekly intervals beginning 72 hr after adenovirus infusion. Plasma was stored at −20°C until the time of leptin assay. Plasma leptin was assayed using the Linco leptin assay kit (Linco Research Immunoassay, St. Charles, MO). Plasma insulin was assayed by standard methods. Free fatty acids (FFA) were measured with a kit according to the recommendations of the manufacturer (Boehringer Mannheim).
Tissue Preparation.
All animals were sacrificed under sodium pentobarbital anesthesia. Tissues were dissected immediately, washed with sterilized and ice-chilled 10 mM phosphate buffer saline, and frozen in liquid nitrogen. Tissues were kept at −70°C until RNA extraction.
Morphology.
Bouin’s-fixed paraffin-embedded serial sections of perfused pancreata were stained with hematoxylin/eosin and examined with light microscopy.
RESULTS
Leptin mRNA Levels in Liver.
Previous studies with recombinant adenoviruses administered to intact rodents have demonstrated that >99% of reporter gene expression is found in the liver (10). Based on this finding, we examined leptin gene expression in liver samples from normal 9-week-old Wistar rats that received via an intravenous catheter an infusion of either AdCMV-leptin virus, or, as a control, the AdCMV-βGal virus containing the gene for bacterial β-galactosidase. Twenty-eight days after virus infusion, leptin mRNA was undetectable in AdCMV-βGal-treated animals but was strongly expressed in samples from AdCMV-leptin-treated rats (Fig. 1). Thus, the leptin transgene was efficiently expressed in liver, and this tissue was the likely site of leptin production in the studies that follow.
Figure 1.
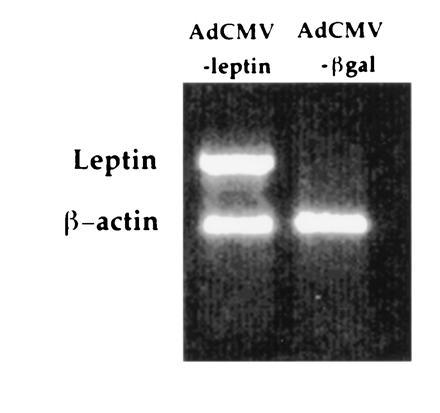
Expression of leptin mRNA in liver. Rats were infused with recombinant adenoviruses containing the rat leptin cDNA (AdCMV-leptin) or the β-galactosidase gene (AdCMV-βGal). Liver samples were collected at 28 days after viral infusion and subjected to reverse transcriptase-PCR analysis of leptin and β-actin mRNA expression. Leptin mRNA was detected only in liver samples from AdCMV-leptin-treated animals.
Plasma Leptin Levels.
The mean plasma leptin level in AdCMV-βGal-treated animals and saline-infused animals averaged 0.8 ± 0.2 and 1.5 ± 0.2 ng/ml, respectively. In AdCMV-leptin-treated rats, by contrast, the mean plasma leptin level rose within 3 days to 8 ± 0.4 ng/ml and remained at or near this level throughout the 28 days of observation (Fig. 2A).
Effects of Primary Hyperleptinemia on Food Intake and Body Weight.
Beginning immediately after the infusion, rats treated with either AdCMV-βGal or AdCMV-leptin exhibited a 24-hr period of reduced food intake compared with the preinfusion level. This effect, which was not observed in saline-infused controls, was associated with a slight increase in plasma aspartate aminotransferase, measured 3 days after viral infusion (107 units/liter and 180 units/liter in the AdCMV-leptin- and AdCMV-βGal-treated animals, respectively, compared with 81 units/liter in the controls). In the rats treated with AdCMV-βGal, the pattern of food intake and weight gain became identical to that of saline-infused controls by the second postoperative day (Fig. 2 B and C). Hyperleptinemic rats, by contrast, exhibited a 30–50% reduction in food intake and failed to gain body weight over the 28-day period of observation (Fig. 2B). At the end of 28 days, their body weight averaged 285 ± 16 g, while that of AdCMV-βGal-treated controls averaged 384 ± 16 g (Fig. 2C). The weight gain in saline- and AdCMV-βGal-infused controls averaged 132 ± 13 and 115 ± 10 g per 28 days, respectively, while in AdCMV-leptin-infused animals it averaged only 22 ± 17 g per 28 days (P < 0.05).
Comparison of Effects of Body Weight of Caloric Restriction With and Without Hyperleptinemia.
To determine if the changes in body weight in the hyperleptinemic rats were the result of the reduction in food intake, four age-matched Wistar rats were pair-fed to the hyperleptinemic rats for an identical period of time. They gained 1 ± 15 g per 28 days and the pattern of their body weight resembled that of the hyperleptinemic rats (Fig. 2C), suggesting that the effect of the hyperleptinemia on body weight could be accounted for entirely by the reduced food intake. Plasma leptin levels in the pair-fed rats averaged 0.9 ± 0.15 ng/ml, similar to the AdCMV-βGal- and saline-infused controls (Fig. 2A).
Comparison of Fat Depots in Calorically Restricted Rats With and Without Hyperleptinemia.
Postmortem examination revealed a virtual absence of all body fat in hyperleptinemic rats. Fat could not be identified in either subcutaneous, visceral, retroperitoneal, or epididymal fat depots (Fig. 3). This was in sharp contrast, not only to the AdCMV-βGal-treated animals and saline-infused controls, but also to the pair-fed animals in which fat depots were preserved in all sites, although below normal control levels (Fig. 3).
Figure 3.
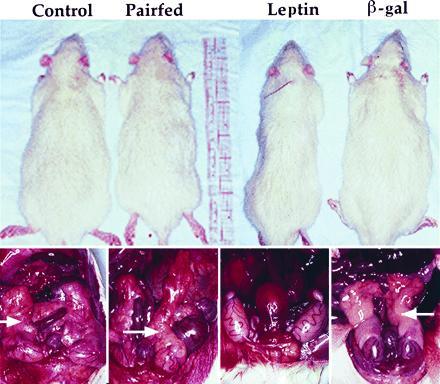
Body appearance and absence of fat depots in hyperleptinemic rats. Overall body appearance (Upper) and epididymal fat in dissected animals (Lower) are compared in saline- (control), AdCMV-leptin-, and AdCMV-βGaL-infused rats, and in rats pair-fed to AdCMV-leptin-treated animals. Epididymal fat depots are identified by arrows (Lower). The photographs are representative of results in four animals per group.
Plasma Glucose, Insulin, and FFA Levels.
Elevated FFA levels are known to cause insulin resistance (11–14) and to stimulate insulin secretion (15). Since the disappearance of fatty tissue in hyperleptinemic rats eliminates the principal source of plasma FFA, we compared plasma FFA, glucose, and insulin levels in the various groups in blood specimens obtained from the tail vein between 1200 and 1400 hours. Twenty-eight days after AdCMV-βGal or saline infusions, FFA levels averaged 0.75 ± 0.1 and 0.80 ± 0.1 mM, respectively. Plasma triglycerides (TG) in these groups averaged 111 ± 21 and 101 ± 9 mg/dl, respectively. In hyperleptinemic rats, after body fat had disappeared, FFA averaged 0.34 ± 0.03 mM and TG averaged 29 ± 4 mg/dl. In pair-fed hypoleptinemic rats, FFA averaged 0.38 ± 0.03 mM and TG averaged 58 ± 12 mg/dl. Blood glucose levels were normal in all groups. Plasma insulin, which averaged 28 ± 4 microunits/ml before the hyperleptinemia, measured 8.4 ± 1 microunits/ml at 28 days, presumably reflecting a greater sensitivity of target tissues to insulin action. Insulin levels were reduced to a lesser degree in the pair-fed controls (18.4 ± 6.0 microunits/ml). These results are summarized in Table 1.
Table 1.
Plasma TG, FFA, glucose, and insulin levels in AdCMV-leptin- and AdCMV-βGal-infused Wistar rats before and after virus infusion, in saline-infused controls, and in normal rats pair-fed to AdCMV-leptin-infused rats
| TG,
mg/dl
|
FFA, mM
|
Glucose,
mg/dl
|
Insulin, microunits/ml
|
|||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Controls | 93 ± 10 | 101 ± 9 | 0.71 ± 0.09 | 0.80 ± 0.13 | 102 ± 4 | 105 ± 3 | 28 ± 2 | 29 ± 3 |
| AdCMV-leptin | 96 ± 8 | 29 ± 4* | 0.73 ± 0.07 | 0.34 ± 0.03† | 103 ± 3 | 91 ± 10 | 28 ± 4 | 8 ± 1† |
| Pair-fed | 95 ± 4 | 58 ± 12‡ | 0.66 ± 0.13 | 0.38 ± 0.07‡ | 105 ± 5 | 96 ± 5 | 29 ± 5 | 18 ± 6 |
| AdCMV-βGal | 105 ± 28 | 111 ± 21 | 0.81 ± 0.04 | 0.75 ± 0.06 | 100 ± 4 | 101 ± 3 | 26 ± 2 | 29 ± 5 |
Values represent mean ± SEM; n = 4.
P < 0.005 vs. before;
P < 0.02 vs. before; and
P < 0.05 vs. before.
Pancreatic Morphology.
The reduction in plasma insulin in hyperleptinemic rats could reflect either improved insulin action because of the low levels of FFA or β-cell damage caused by the marked hyperleptinemia. Inspection of islets in pancreatic sections stained with hematoxylin/eosin taken from AdCMV-leptin-infused or control rats revealed no morphologic differences.
DISCUSSION
In the current study, we have used recombinant adenovirus technology to demonstrate the effects of hyperleptinemia in normal animals. While the finding that increased circulating leptin levels result in reduced food intake and body weight gain compared with normoleptinemic controls is consistent with previous work (2–4), the magnitude of the effect was unanticipated and could not be ascribed to the effects of lowered food intake alone. There was complete disappearance of visible body fat in AdCMV-leptin animals, a finding not approached in animals that were pair-fed with the hyperleptinemic group.
This difference is presumed to be the result of the thermogenic effect of the hyperleptinemia (3), but it raises the possibility of a specific effect of unregulated hyperleptinemia on fat storage in adipocytes. In addition, while FFA levels were similarly reduced in hyperleptinemic and pair-fed animals relative to the two control groups (AdCMV-βGal- or saline-infused animals) and glucose levels remained normal in all animals, hyperleptinemic animals exhibited markedly lower plasma insulin and TG levels than pair-fed animals or controls. These results provide support for the view that the relative resistance of fat depots to therapeutic restriction of food intake in human obesity may be ameliorated by leptin replacement, as has been previously suggested (1).
The low TG and FFA in hyperleptinemic rats was associated with evidence of increased insulin sensitivity, manifested by the normal blood glucose levels despite a 60% reduction in plasma insulin levels. The more modest decrease in TG in pair-fed controls and their retention of ≈50% of normal fat depots was associated with a much smaller decrease in insulin levels than observed in hyperleptinemic rats. These data suggest that insulin sensitivity is linked to the level of tissue fat and are consistent with the now widely accepted view that increased tissue FFA interferes with glucose metabolism (11–14) and increases insulin secretion (15, 16). Not explained in this study is the mechanism by which plasma FFA in the “lipoatrophic” hyperleptinemic rats are maintained at the same level as in pair-fed controls. Perhaps this reflects a high rate of lipolysis in residual adipocytes that have not yet been entirely emptied of fat.
Finally, these studies provide fresh support for the use of recombinant adenovirus as a tool for conducting physiologic studies. Earlier work has demonstrated high efficiency delivery of genes encoding enzymes or receptors to liver of intact rodents (10, 17, 18). In those studies, however, expression of the transgenes was generally found to wane dramatically within 2 weeks of viral infusion. In the current work, leptin gene expression in liver and hyperleptinemia were maintained at relatively constant levels for 28 days. It has been suggested that the short duration of expression of adenovirally transferred genes in whole animals may be due to an immunologic reaction against virally-encoded genes, and that longer expression requires engineering of vectors that do not express these genes (19, 20). Our study was conducted with adenoviruses lacking only E1a among early viral genes, which would have been presumed to be highly immunogenic. The relatively long duration of expression achieved in our studies may suggest that the identity of the transgene is relevant to duration of expression. Different from previous studies with genes encoding the enzyme ornithine transcarbamoylase (17) or the LDL receptor (10, 18), the leptin transgene product was probably secreted from liver cells as rapidly as it was synthesized, suggesting that accumulation of expressed proteins within cells may be a factor in eliciting immune responses or in shortening cell survival.
Acknowledgments
We thank Drs. Daniel Foster, Scott Grundy, and J. Denis McGarry for critical reading of the manuscript and Kay McCorkle, Donna Lehman, and Falguni Trieu for outstanding technical support. Doris Himelrick, Kay Naughton, and Susan Kennedy provided secretarial assistance. This work was supported by National Institutes of Health Grants DK02700-37 and P50H2598801, a National Institutes of Health/Juvenile Diabetes Foundation Diabetes Interdisciplinary Research Program grant, and Department of Veterans Affairs Institutional Research Support Grant SMI 821–109. R.O. is supported by an American Physiological Society/Genentech Inc. fellowship.
Footnotes
Abbreviations: FFA, free fatty acid(s); TG, triglyceride(s).
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–431. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 3.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 4.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 5.Murakami T, Shima K. Biochem Biophys Res Commun. 1995;209:944–952. doi: 10.1006/bbrc.1995.1589. [DOI] [PubMed] [Google Scholar]
- 6.Wang M-Y, Zhou Y-T, Newgard C B, Unger R H. FEBS Lett. 1996;392:87–90. doi: 10.1016/0014-5793(96)00790-9. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Foix A M, Coats W S, Baque S, Alam T, Gerard R D, Newgard C B. J Biol Chem. 1992;276:25129–25134. [PubMed] [Google Scholar]
- 8.McCrory W J, Bautista D S, Graham F L. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 9.Becker T C, Noel R J, Coats W S, Gomez-Foix A M, Alam T, Gerard R D, Newgard C B. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 10.Herz J, Gerard R D. Proc Natl Acad Sci USA. 1993;90:2812–2817. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randle P J, Garland P B, Hales C N, Newsholme E A. Lancet. 1963;i:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 12.Ferranini E, Barrett E J, Bevilequas S, DeFronzo R A. J Clin Invest. 1983;72:1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groop L C, Saluranta C, Shank M, Bonnadonna R C, Ferranini E, DeFronzo R A. J Clin Endocrinol Metab. 1991;72:96–107. doi: 10.1210/jcem-72-1-96. [DOI] [PubMed] [Google Scholar]
- 14.McGarry J D. Science. 1992;258:766–774. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 15.Stein D T, Esser V, Stevenson B E, Jane K E, Whiteside J H, Daniels M B, Chen S, McGarry J D. J Clin Invest. 1996;97:2728–2735. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milburn J L, Hirose H, Lee Y H, Nagasawa Y, Ogawa A, Ohneda M, Beltrandel Rio H, Newgard C B, Johnson J H, Unger R H. J Biol Chem. 1995;270:1295–1299. doi: 10.1074/jbc.270.3.1295. [DOI] [PubMed] [Google Scholar]
- 17.Stratford-Perricaudet L D, Levrero M, Chasse J-F, Pericaudet M, Briand P. Hum Gene Ther. 1990;1:241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi S, Brown M S, Goldstein J L, Gerard R D, Hammer R E, Herz J. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelhardt J F, Ye X, Doranz B, Wilson J M. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]


