Abstract
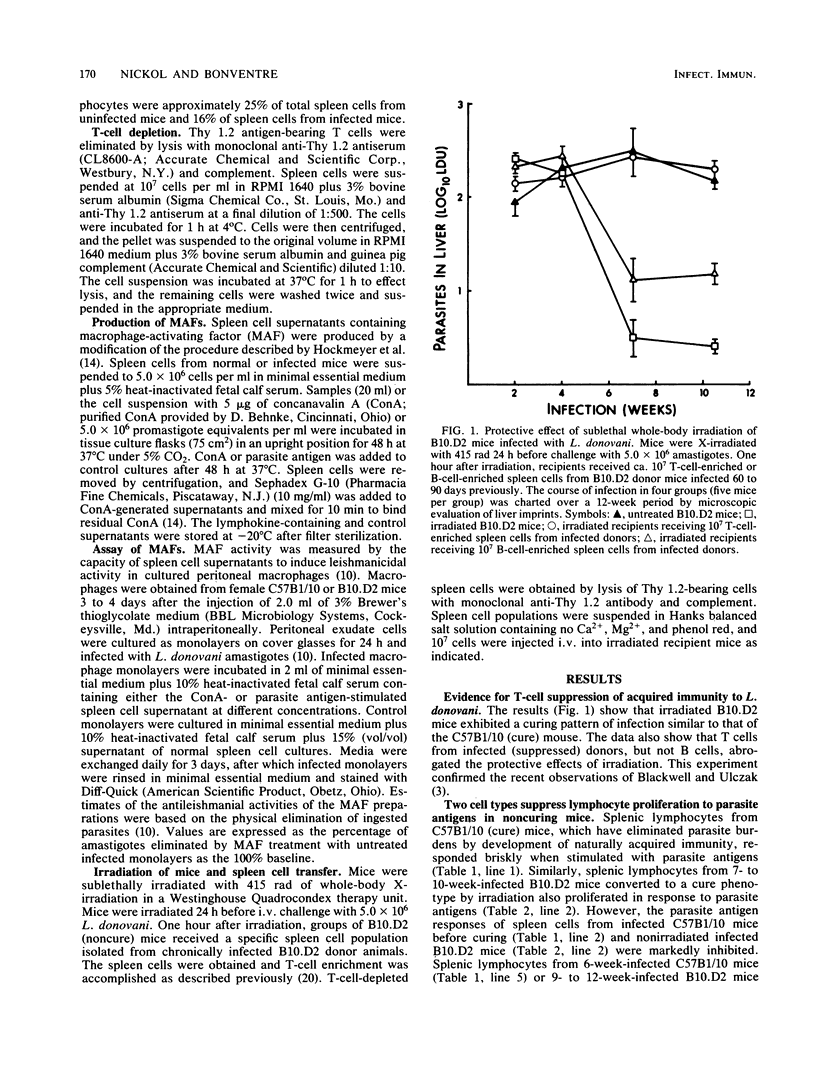
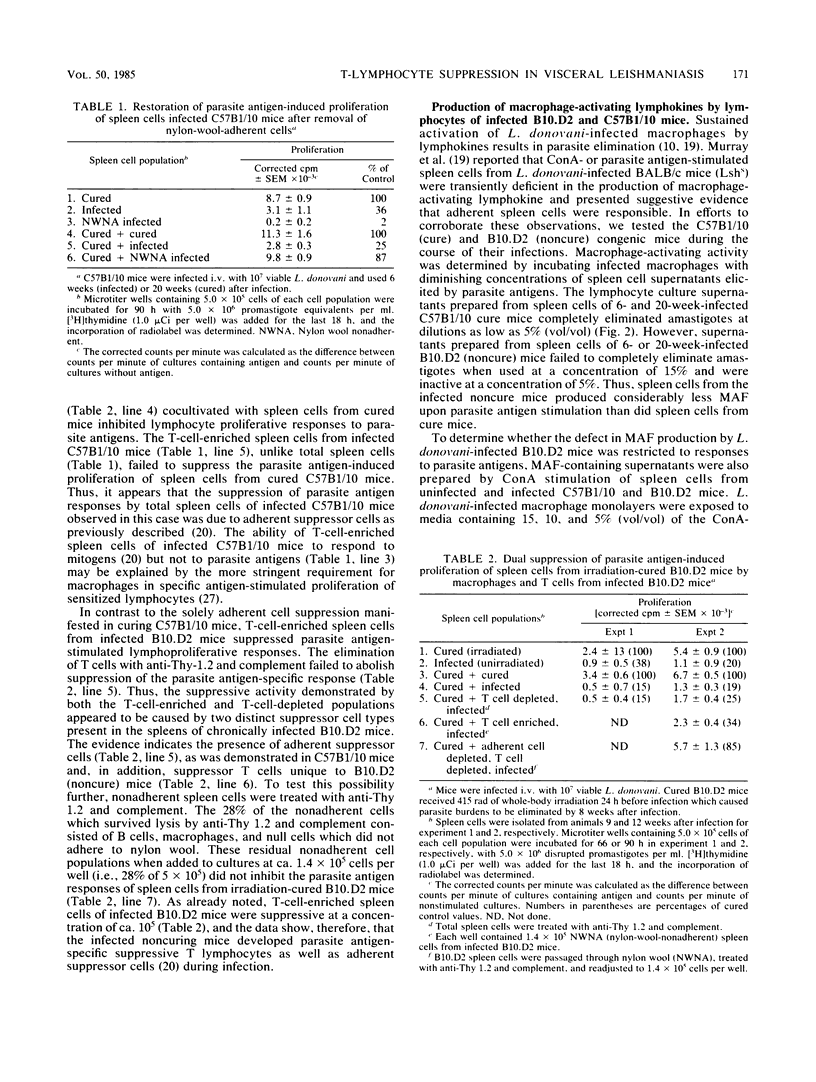
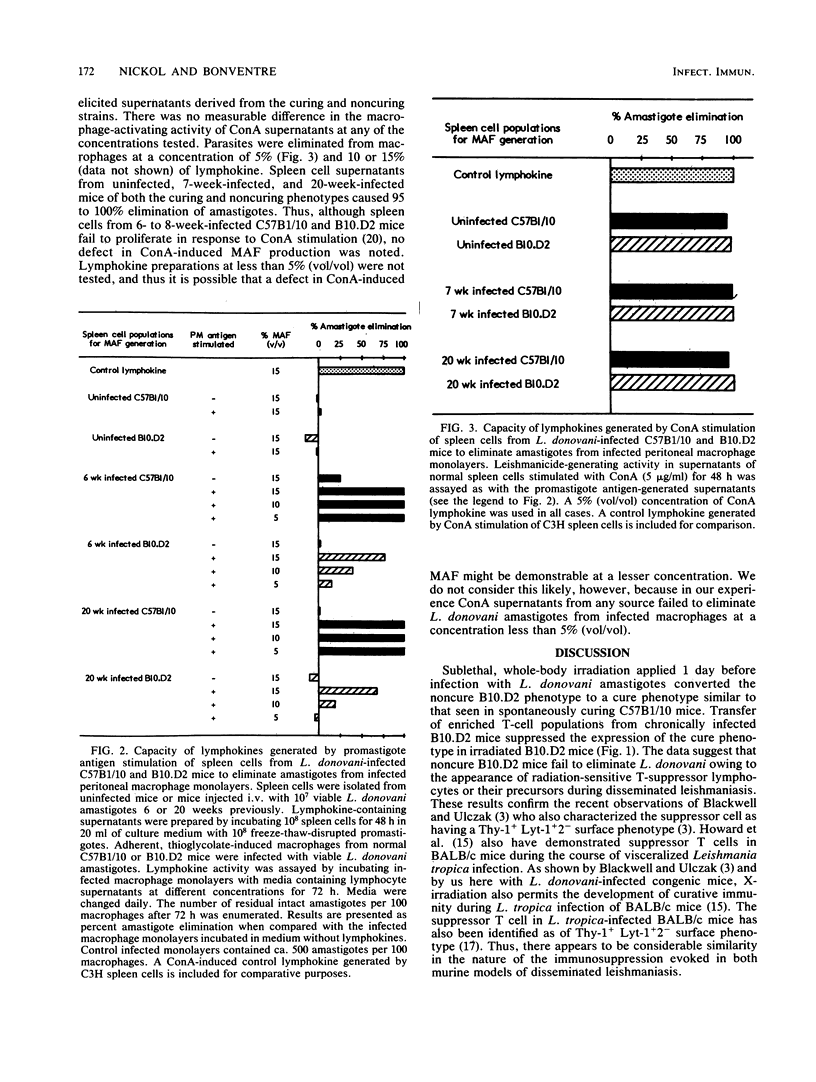
This paper continues a comparative study (A. D. Nickol and P. F. Bonventre, Infect. Immun. 50:160-168, 1985) describing immune responses exhibited by congenic, Lshs mouse strains C57B1/10 (cure) and B10.D2 (noncure) during the course of disseminated leishmaniasis. We report that sublethal whole-body irradiation of B10.D2 mice before challenge with Leishmania donovani converted the noncuring mice to a curing phenotype. Splenic lymphocytes from L. donovani-infected B10.D2 mice failed to proliferate in response to parasite antigen stimulation in vitro. Splenic lymphocytes from irradiated, cured B10.D2 mice regained the capacity to respond to the parasite antigen stimulus. Transfer of T cells but not B cells from L. donovani-infected B10.D2 mice prevented the acquisition of immunity and recovery from infection in X-irradiated mice. In addition, a splenic T-cell population from L. donovani-infected B10.D2 mice suppressed the proliferation in vitro of parasite antigen-stimulated lymphocytes of irradiation-cured B10.D2 mice. Suppressor T cells were not demonstrable in the spleens of spontaneously cured C57B1/10 mice. Splenic lymphocytes from infected B10.D2 mice were deficient in the production of macrophage-activating factor (MAF) upon stimulation by L. donovani antigens in vitro. Deficient MAF production was specific for parasite antigen stimulation, because MAF production subsequent to concanavalin A stimulation of splenic lymphocytes from infected B10.D2 mice was not suppressed. The data suggest that a genetically based immunological defect in B10.D2 mice prevents the acquisition of effective cell-mediated immunity and subsequent elimination of L. donovani from tissue macrophages. The immunological deficit, not apparent in the curing C57B1/10, appears to be caused by the development of parasite antigen-specific suppressor T cells during the course of the disseminated leishmaniasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berendt M. J., North R. J. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med. 1980 Jan 1;151(1):69–80. doi: 10.1084/jem.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M. Genetic control of recovery from visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1982;76(2):147–151. doi: 10.1016/0035-9203(82)90262-0. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M., Ulczak O. M. Immunoregulation of genetically controlled acquired responses to Leishmania donovani infection in mice: demonstration and characterization of suppressor T cells in noncure mice. Infect Immun. 1984 Apr;44(1):97–102. doi: 10.1128/iai.44.1.97-102.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimos C., Lazanakis S., Thomaidis T. Antibody response after immunisation with typhoid-paratyphoid A and B vaccine in kala-azar. Acta Paediatr Scand. 1966 May;55(3):301–304. doi: 10.1111/j.1651-2227.1966.tb17658.x. [DOI] [PubMed] [Google Scholar]
- Dye E. S., North R. J. T cell-mediated immunosuppression as an obstacle to adoptive immunotherapy of the P815 mastocytoma and its metastases. J Exp Med. 1981 Oct 1;154(4):1033–1042. doi: 10.1084/jem.154.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Greene M. I., Sehon A. H. Regulation of the immune response to tumor antigens. II. The nature of immunosuppressor cells in tumor-bearing hosts. J Immunol. 1976 Mar;116(3):800–806. [PubMed] [Google Scholar]
- Greene M. I., Perry L. L. Regulation of the immune response to tumor antigen. VI. Differential specificities of suppressor T cells or their products and effector T cells. J Immunol. 1978 Dec;121(6):2363–2366. [PubMed] [Google Scholar]
- Górski A. J., Dupont B., Hansen J. A., Good R. A. Leukocyte migration inhibitory factor (LMIF) induced by concanavalin A: standardized microassay for production in vitro. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3197–3200. doi: 10.1073/pnas.72.8.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidaris C. G., Bonventre P. F. Elimination of Leishmania donovani amastigotes by activated macrophages. Infect Immun. 1981 Sep;33(3):918–926. doi: 10.1128/iai.33.3.918-926.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Longo D. L., Matis L. A. The relationship between immune interferon production and proliferation in antigen-specific, MHC-restricted T cell lines and clones. J Immunol. 1983 Sep;131(3):1049–1055. [PubMed] [Google Scholar]
- Hellström K. E., Hellström I., Kant J. A., Tamerius J. D. Regression and inhibition of sarcoma growth by interference with a radiosensitive T-cell population. J Exp Med. 1978 Sep 1;148(3):799–804. doi: 10.1084/jem.148.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepner Levy L., Mendes E. Impaired cell-mediated immunity in patients with kala-azar. Allergol Immunopathol (Madr) 1981 Mar-Apr;9(2):109–112. [PubMed] [Google Scholar]
- Ho M., Koech D. K., Iha D. W., Bryceson A. D. Immunosuppression in Kenyan visceral leishmaniasis. Clin Exp Immunol. 1983 Feb;51(2):207–214. [PMC free article] [PubMed] [Google Scholar]
- Hockmeyer W. T., Walters D., Gore R. W., Williams J. S., Fortier A. H., Nacy C. A. Intracellular destruction of Leishmania donovani and Leishmania tropica amastigotes by activated macrophages: dissociation of these microbicidal effector activities in vitro. J Immunol. 1984 Jun;132(6):3120–3125. [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. IV. Prophylactic effect of sublethal irradiation as a result of abrogation of suppressor T cell generation in mice genetically susceptible to Leishmania tropica. J Exp Med. 1981 Mar 1;153(3):557–568. doi: 10.1084/jem.153.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Hale C., Howard J. G. Immunologic regulation of experimental cutaneous leishmaniasis. V. Characterization of effector and specific suppressor T cells. J Immunol. 1982 Apr;128(4):1917–1922. [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Visceral leishmaniasis in congenic mice of susceptible and resistant phenotypes: immunosuppression by adherent spleen cells. Infect Immun. 1985 Oct;50(1):160–168. doi: 10.1128/iai.50.1.160-168.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Ellis J., Chaplan S., Cohn Z. Trypanosoma cruzi: in vivo and in vitro correlation between T-cell activation and susceptibility in inbred strains of mice. Exp Parasitol. 1981 Jun;51(3):325–334. doi: 10.1016/0014-4894(81)90120-x. [DOI] [PubMed] [Google Scholar]
- Perry L. L., Benacerraf B., Greene M. I. Regulation of the immune response to tumor antigen. IV. Tumor antigen-specific suppressor factor(s) bear I-J determinants and induce suppressor T cells in vivo. J Immunol. 1978 Dec;121(6):2144–2147. [PubMed] [Google Scholar]
- Rocklin R. E. Production of migration inhibitory factor by non-dividing lymphocytes. J Immunol. 1973 Mar;110(3):674–678. [PubMed] [Google Scholar]
- Rotter V., Trainin N. Inhibition of tumor growth in syngeneic chimeric mice mediated by a depletion of suppressor T cells. Transplantation. 1975 Jul;20(1):68–74. doi: 10.1097/00007890-197507000-00011. [DOI] [PubMed] [Google Scholar]
- Treves A. J., Carnaud C., Trainin N., Feldman M., Cohen I. R. Enhancing T lymphocytes from tumor-bearing mice suppress host resistance to a syngeneic tumor. Eur J Immunol. 1974 Nov;4(11):722–727. doi: 10.1002/eji.1830041104. [DOI] [PubMed] [Google Scholar]
- Ulczak O. M., Blackwell J. M. Immunoregulation of genetically controlled acquired responses to Leishmania donovani infection in mice: the effects of parasite dose, cyclophosphamide and sublethal irradiation. Parasite Immunol. 1983 Sep;5(5):449–463. doi: 10.1111/j.1365-3024.1983.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]


