Abstract
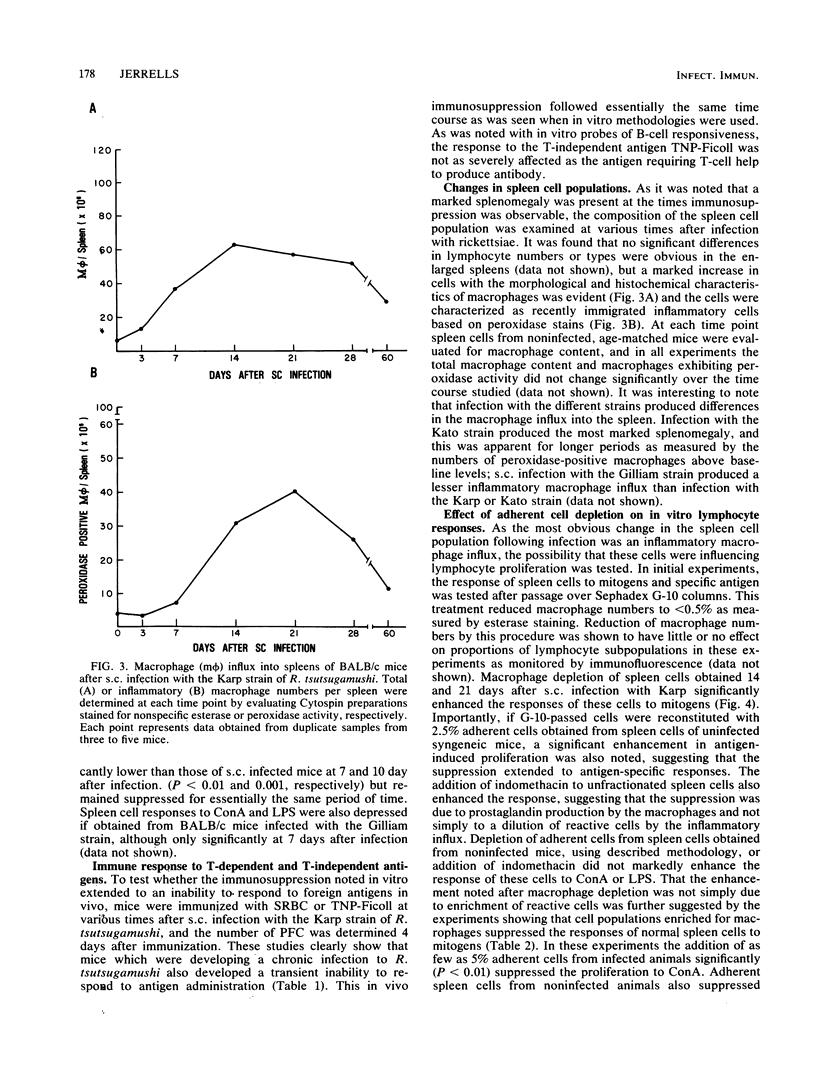
Measures of general immunocompetency such as lymphocyte responses to mitogens and alloantigens and the ability to produce antibody to T-dependent and T-independent antigens were evaluated during the development of chronic infections with Rickettsia tsutsugamushi resulting from subcutaneous infection of BALB/c mice. It was found that a transient immunosuppression was demonstrable regardless of the infecting strain of rickettsiae; however, the immunosuppression produced by the Karp and Kato strains was more pronounced and longer lived. As a marked splenomegaly resulting from inflammatory macrophage influx accompanied this immunosuppression, mitogen- and antigen-induced lymphocyte proliferation was also evaluated after adherent cell depletion or in the presence of indomethacin, and both treatments significantly improved the responses. Isolated splenic macrophages were shown to suppress the responses of lymphocytes from naive mice as well as to exhibit parameters of activation including tumor cell cytolysis and cytostasis and the ability to inhibit the replication of R. tsutsugamushi in vitro. These data suggest an association between macrophage activation involved in rickettsial clearance and a transient immunosuppression.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boraschi D., Niederhuber J. E. Regulation of macrophage suppression and cytotoxicity by interferon role of Ia-bearing macrophages. J Immunol. 1982 Nov;129(5):1854–1858. [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Cheers C., Pavlov H., Riglar C., Madraso E. Macrophage activation during experimental murine brucellosis. III. Do macrophages exert feedback control during brucellosis? Cell Immunol. 1980 Jan;49(1):168–177. doi: 10.1016/0008-8749(80)90066-0. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Eisemann C. S., Osterman J. V. Proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1976 Jul;14(1):155–162. doi: 10.1128/iai.14.1.155-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Osterman J. V. Host defenses in experimental scrub typhus: genetics of natural resistance to infection. Infect Immun. 1978 Feb;19(2):583–588. doi: 10.1128/iai.19.2.583-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Rosenstreich D. L., Taylor B. A., Osterman J. V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980 Sep;125(3):1395–1399. [PubMed] [Google Scholar]
- Jerrells T. R. Association of an inflammatory I region-associated antigen-positive macrophage influx and genetic resistance of inbred mice to Rickettsia tsutsugamushi. Infect Immun. 1983 Nov;42(2):549–557. doi: 10.1128/iai.42.2.549-557.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Dean J. H., Richardson G., Cannon G. B., Herberman R. B. Increased monocyte-mediated cytostasis of lymphoid cell lines in breast and lung cancer patients. Int J Cancer. 1979 Jun 15;23(6):768–776. doi: 10.1002/ijc.2910230606. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Eisemann C. S. Role of T-lymphocytes in production of antibody to antigens of Rickettsia tsutsugamushi and other Rickettsia species. Infect Immun. 1983 Aug;41(2):666–674. doi: 10.1128/iai.41.2.666-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Development of specific and cross-reactive lymphocyte proliferative responses during chronic immunizing infections with Rickettsia tsutsugamushi. Infect Immun. 1983 Apr;40(1):147–156. doi: 10.1128/iai.40.1.147-156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: delayed-type hypersensitivity responses of inbred mice. Infect Immun. 1982 Jan;35(1):117–123. doi: 10.1128/iai.35.1.117-123.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: inflammatory response of congenic C3H mice differing at the Ric gene. Infect Immun. 1981 Mar;31(3):1014–1022. doi: 10.1128/iai.31.3.1014-1022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Mongini P. K., Sieckmann D., Paul W. E. Role of T lymphocytes in the response to TNP-AECM-Ficoll. J Immunol. 1980 Sep;125(3):1066–1070. [PubMed] [Google Scholar]
- Mongini P. K., Stein K. E., Paul W. E. T cell regulation of IgG subclass antibody production in response to T-independent antigens. J Exp Med. 1981 Jan 1;153(1):1–12. doi: 10.1084/jem.153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infections: protection against lethal Rickettsia tsutsugamushi infections by treatment of mice with macrophage-activating agents. J Leukoc Biol. 1984 Apr;35(4):385–396. doi: 10.1002/jlb.35.4.385. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Tokunaga T. Induction of suppressor T cells in delayed-type hypersensitivity to Mycobacterium bovis BCG in low-responder mice. Infect Immun. 1980 May;28(2):331–335. doi: 10.1128/iai.28.2.331-335.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks S. C., Jr, Osterman J. V., Hetrick F. M. Plaque assay and cloning of scrub typhus rickettsiae in irradiated L-929 cells. J Clin Microbiol. 1977 Jul;6(1):76–80. doi: 10.1128/jcm.6.1.76-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer B. A., Hetrick F. M., Jerrells T. J. Production of gamma interferon in mice immune to Rickettsia tsutsugamushi. Infect Immun. 1984 Jan;43(1):59–65. doi: 10.1128/iai.43.1.59-65.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer B. A., Hetrick F. M., Jerrells T. R. Gamma interferon production in response to homologous and heterologous strain antigens in mice chronically infected with Rickettsia tsutsugamushi. Infect Immun. 1984 Oct;46(1):237–244. doi: 10.1128/iai.46.1.237-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riglar C., Cheers C. Macrophage activation during experimental murine brucellosis. II. Inhibition of in vitro lymphocyte proliferation by brucella-activated macrophages. Cell Immunol. 1980 Jan;49(1):154–167. doi: 10.1016/0008-8749(80)90065-9. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Eisenberg G. H., Jr, Osterman J. V. Host defenses in experimental scrub typhus: effect of chloramphenicol. Infect Immun. 1977 Nov;18(2):324–329. doi: 10.1128/iai.18.2.324-329.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



