Abstract
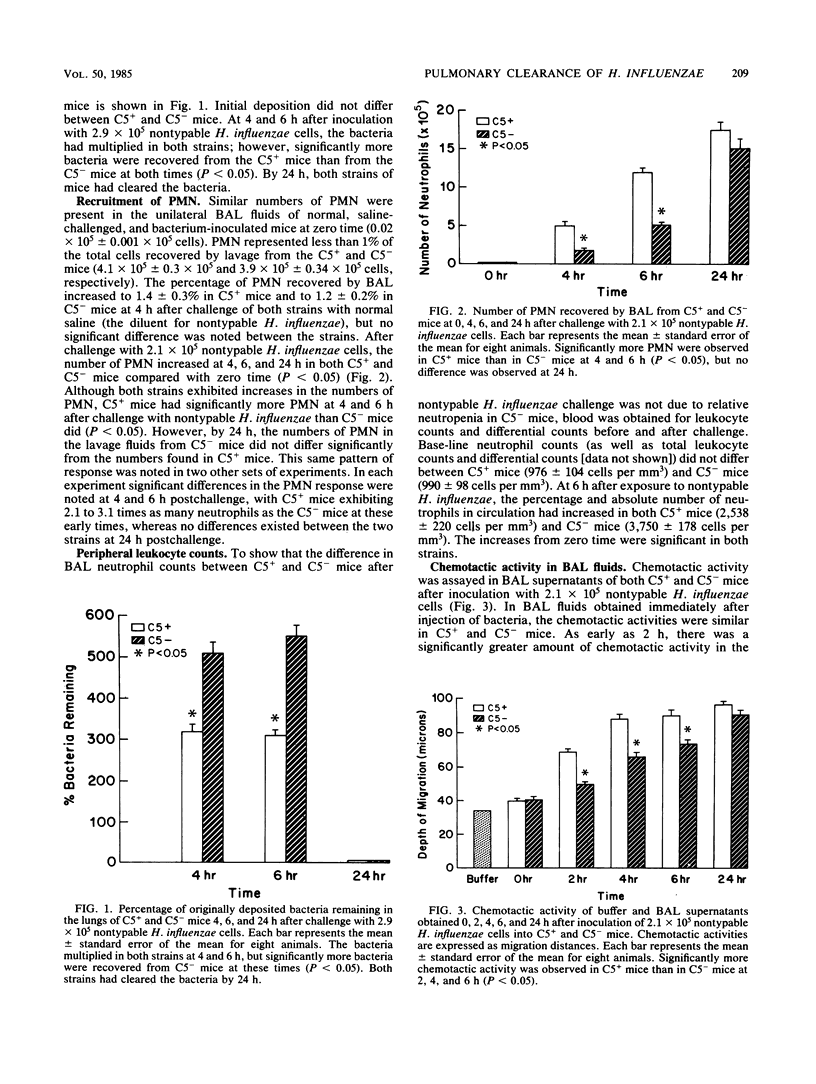
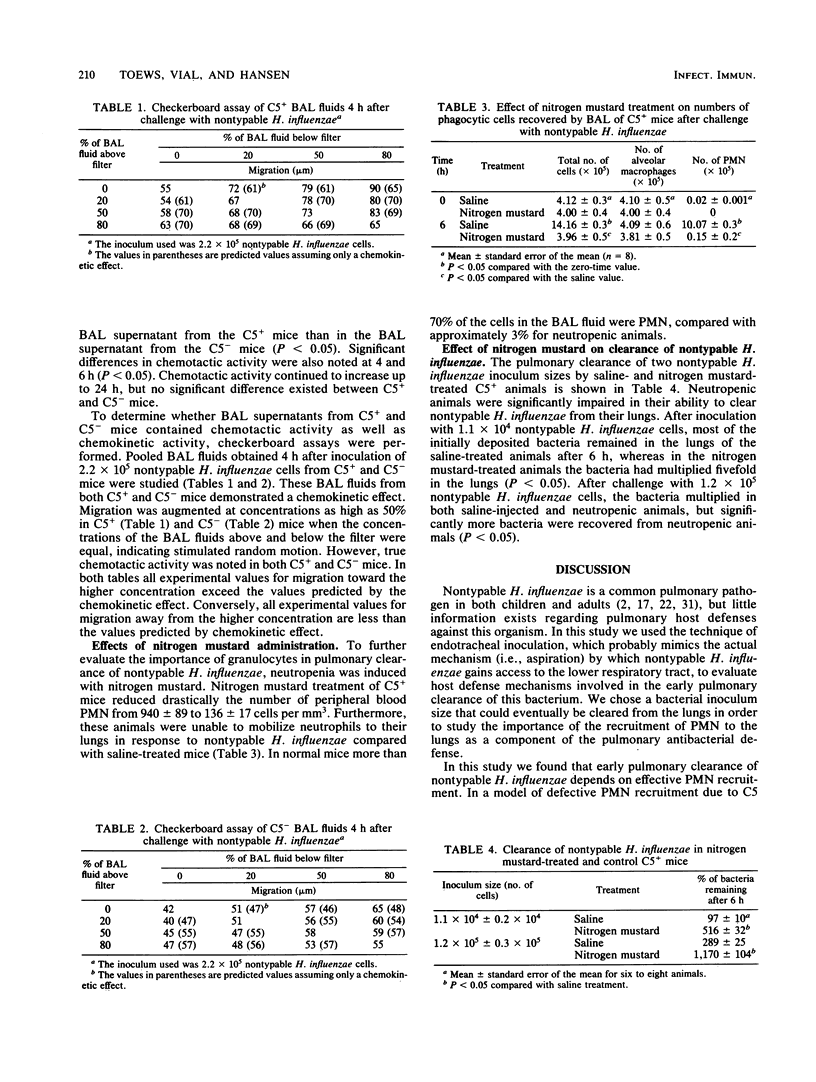
We used a mouse model system to investigate the pulmonary defense mechanisms involved in clearance of nontypable Haemophilus influenzae from the lower respiratory tract. The importance of the C5 complement protein molecule in polymorphonuclear leukocyte (PMN) recruitment was studied by using congenic C5-sufficient B10.D2/nSn (C5+) and C5-deficient B10.D2/oSn (C5-) mice. The C5+ and C5- mice were inoculated with saline or nontypable H. influenzae via an endobronchial catheter. Clearance of bacteria was studied by using quantitative lung cultures. Bronchoalveolar lavage was performed at several time intervals. The number of cells in the lavage fluid were counted, and chemotactic activity was assayed in lavage fluid by the leading front technique, using human PMN in modified Boyden chambers. Pulmonary clearance of bacteria was significantly impaired in the absence of C5 (P less than 0.05). The C5+ mice recruited significantly more PMN after challenge with nontypable H. influenzae than C5- mice did (P less than 0.05), but significant PMN recruitment occurred in C5- mice. Similarly, although chemotactic activity was present in both C5+ and C5- mice, significantly more intraalveolar chemotactic activity was noted in C5+ mice than in C5- mice (P less than 0.05). The C5 molecule yields important chemotaxins during this early time period, but other chemotaxins are also present within the alveoli, demonstrating the redundancy of the inflammatory response after pulmonary challenge with nontypable H. influenzae. Nitrogen mustard-induced neutropenic animals were studied to evaluate the functional importance of PMN in pulmonary clearance of nontypable H. influenzae. Pulmonary clearance of nontypable H. influenzae was significantly impaired in neutropenic animals (P less than 0.05). Our results indicate that the prompt appearance of PMN in lungs is crucial for early clearance of nontypable H. influenzae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk S. L., Holtsclaw S. A., Wiener S. L., Smith J. K. Nontypeable Haemophilus influenzae in the elderly. Arch Intern Med. 1982 Mar;142(3):537–539. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Desai U., Kreutzer D. L., Showell H., Arroyave C. V., Ward P. A. Acute inflammatory pulmonary reactions induced by chemotactic factors. Am J Pathol. 1979 Jul;96(1):71–83. [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Fales H. M., Crystal R. G. Human alveolar macrophage-derived chemotactic factor for neutrophils. Stimuli and partial characterization. J Clin Invest. 1980 Sep;66(3):473–483. doi: 10.1172/JCI109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gallin J. I., Fauci A. S. Immunologic reactivity of the lung: the in vivo and in vitro generation of a neutrophil chemotactic factor by alveolar macrophages. Am Rev Respir Dis. 1978 Jan;117(1):15–23. doi: 10.1164/arrd.1978.117.1.15. [DOI] [PubMed] [Google Scholar]
- Jay S. J., Johanson W. G., Jr, Pierce A. K., Reisch J. S. Determinants of lung bacterial clearance in normal mice. J Clin Invest. 1976 Apr;57(4):811–817. doi: 10.1172/JCI108356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974 Aug;54(2):349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierowski J. A., Gallin J. I., Reynolds H. Y. Mechanism for the inflammatory response in primate lungs. Demonstration and partial characterization of an alveolar macrophage-derived chemotactic factor with preferential activity for polymorphonuclear leukocytes. J Clin Invest. 1977 Feb;59(2):273–281. doi: 10.1172/JCI108638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen G. L., McCarthy K., Webster R. O., Henson J., Henson P. M. A differential effect of C5a and C5a des Arg in the induction of pulmonary inflammation. Am J Pathol. 1980 Jul;100(1):179–192. [PMC free article] [PubMed] [Google Scholar]
- Larsen G. L., Mitchell B. C., Henson P. M. The pulmonary response of C5 sufficient and deficient mice to immune complexes. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):434–439. doi: 10.1164/arrd.1981.123.4.434. [DOI] [PubMed] [Google Scholar]
- Merrill W. W., Naegel G. P., Matthay R. A., Reynolds H. Y. Alveolar macrophage-derived chemotactic factor: kinetics of in vitro production and partial characterization. J Clin Invest. 1980 Feb;65(2):268–276. doi: 10.1172/JCI109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Kubitschek K. R., Crennan J., Baughn R. E. Pneumonia and acute febrile tracheobronchitis due to haemophilus influenzae. Ann Intern Med. 1983 Oct;99(4):444–450. doi: 10.7326/0003-4819-99-4-444. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Müller-Eberhard H. J. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967 Jan 1;125(1):1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrio J. M., Toews G. B., Lipscomb M. F., Pierce A. K. Granulocyte-alveolar-macrophage interaction in the pulmonary clearance of Staphylococcus aureus. Am Rev Respir Dis. 1983 Mar;127(3):335–341. doi: 10.1164/arrd.1983.127.3.335. [DOI] [PubMed] [Google Scholar]
- Rehm S. R., Gross G. N., Pierce A. K. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest. 1980 Aug;66(2):194–199. doi: 10.1172/JCI109844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982 Mar;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shann F., Gratten M., Germer S., Linnemann V., Hazlett D., Payne R. Aetiology of pneumonia in children in Goroka Hospital, Papua New Guinea. Lancet. 1984 Sep 8;2(8402):537–541. doi: 10.1016/s0140-6736(84)90764-5. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Shin H., Dannenberg A. M., Jr Macrophage proteinase and inflammation: the production of chemotactic activity from the fifth complement by macrophage proteinase. J Immunol. 1972 Oct;109(4):896–898. [PubMed] [Google Scholar]
- Tarr P. I., Hosea S. W., Brown E. J., Schneerson R., Sutton A., Frank M. M. The requirement of specific anticapsular IgG for killing of Haemophilus influenzae by the alternative pathway of complement activation. J Immunol. 1982 Apr;128(4):1772–1775. [PubMed] [Google Scholar]
- Toews G. B., Pierce A. K. The fifth component of complement is not required for the clearance of Staphylococcus aureus. Am Rev Respir Dis. 1984 Apr;129(4):597–601. [PubMed] [Google Scholar]
- Toews G. B., Vial W. C. The role of C5 in polymorphonuclear leukocyte recruitment in response to Streptococcus pneumoniae. Am Rev Respir Dis. 1984 Jan;129(1):82–86. doi: 10.1164/arrd.1984.129.1.82. [DOI] [PubMed] [Google Scholar]
- Toews G. B., Viroslav S., Hart D. A., Hansen E. J. Pulmonary clearance of encapsulated and unencapsulated Haemophilus influenzae strains. Infect Immun. 1984 Aug;45(2):437–442. doi: 10.1128/iai.45.2.437-442.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone F. H., Franklin M., Sun F. F., Goetzl E. J. Alveolar macrophage lipoxygenase products of arachidonic acid: isolation and recognition as the predominant constituents of the neutrophil chemotactic activity elaborated by alveolar macrophages. Cell Immunol. 1980 Sep 1;54(2):390–401. doi: 10.1016/0008-8749(80)90219-1. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Garcia M. L. Enhancement of neutrophils function as a result of prior exposure to chemotactic factor. J Clin Invest. 1980 Aug;66(2):167–175. doi: 10.1172/JCI109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial W. C., Toews G. B., Pierce A. K. Early pulmonary granulocyte recruitment in response to Streptococcus pneumoniae. Am Rev Respir Dis. 1984 Jan;129(1):87–91. doi: 10.1164/arrd.1984.129.1.87. [DOI] [PubMed] [Google Scholar]
- Wald E. R., Milmoe G. J., Bowen A., Ledesma-Medina J., Salamon N., Bluestone C. D. Acute maxillary sinusitis in children. N Engl J Med. 1981 Mar 26;304(13):749–754. doi: 10.1056/NEJM198103263041302. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970 Mar;104(3):535–543. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



