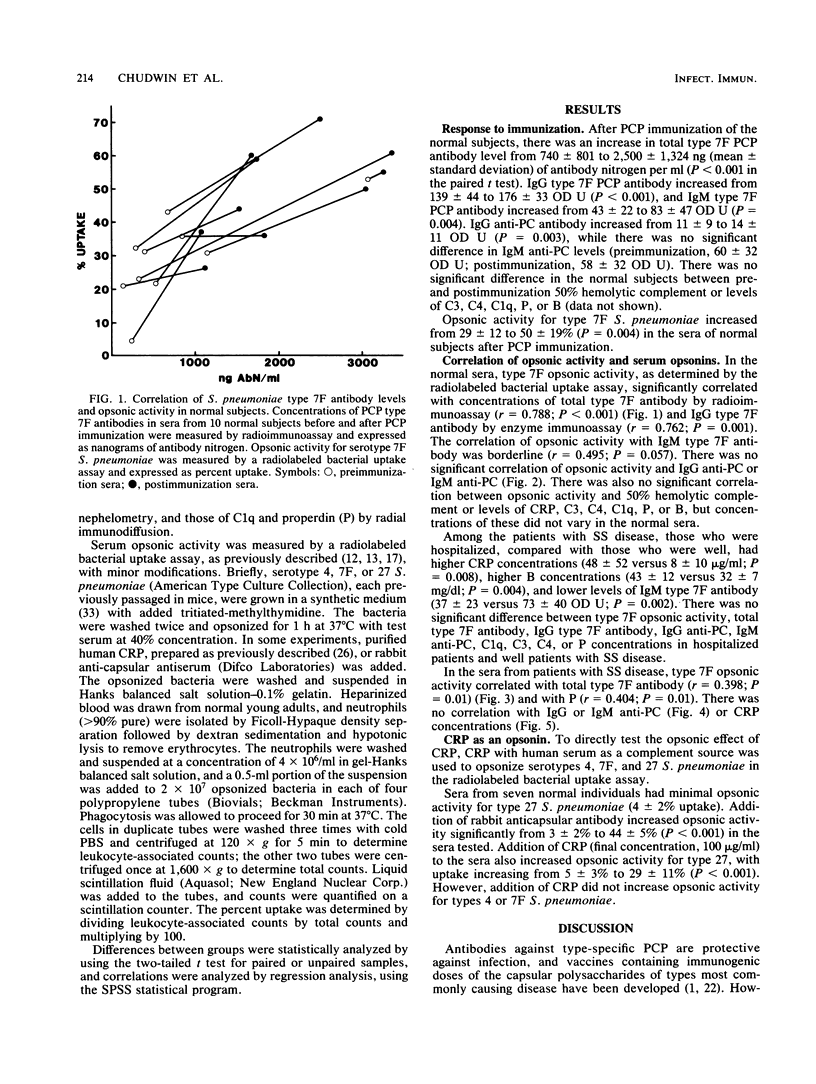
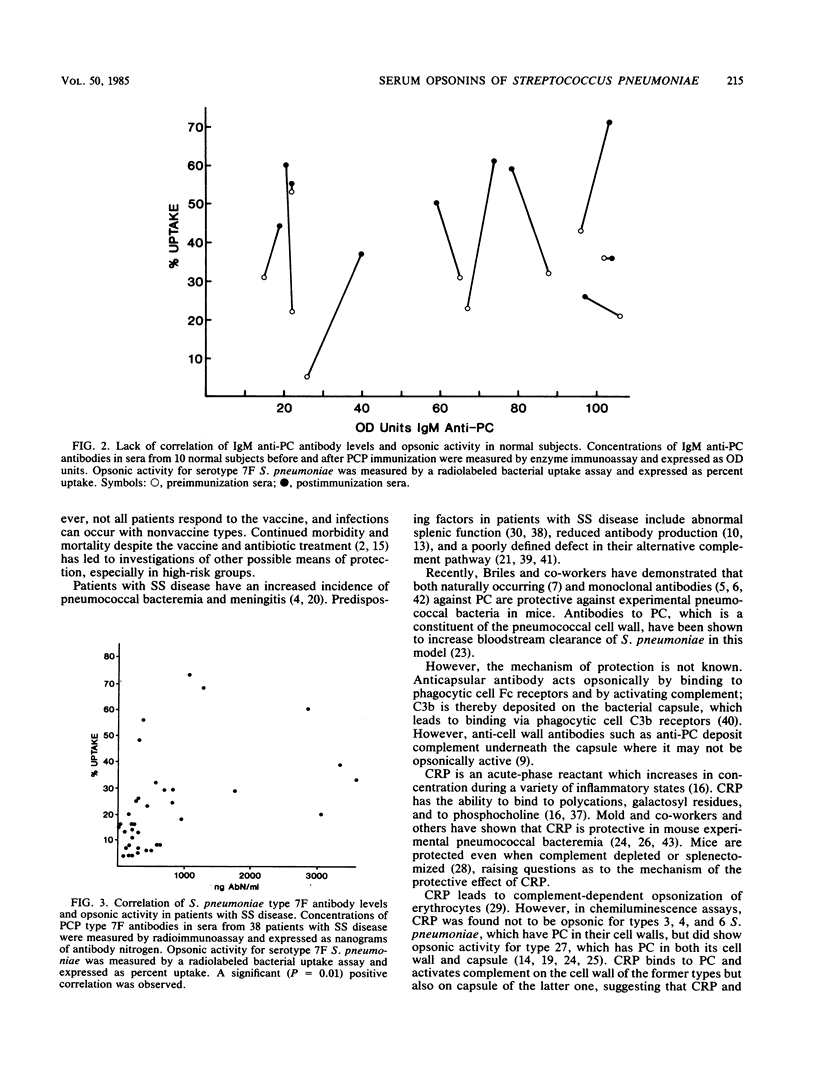
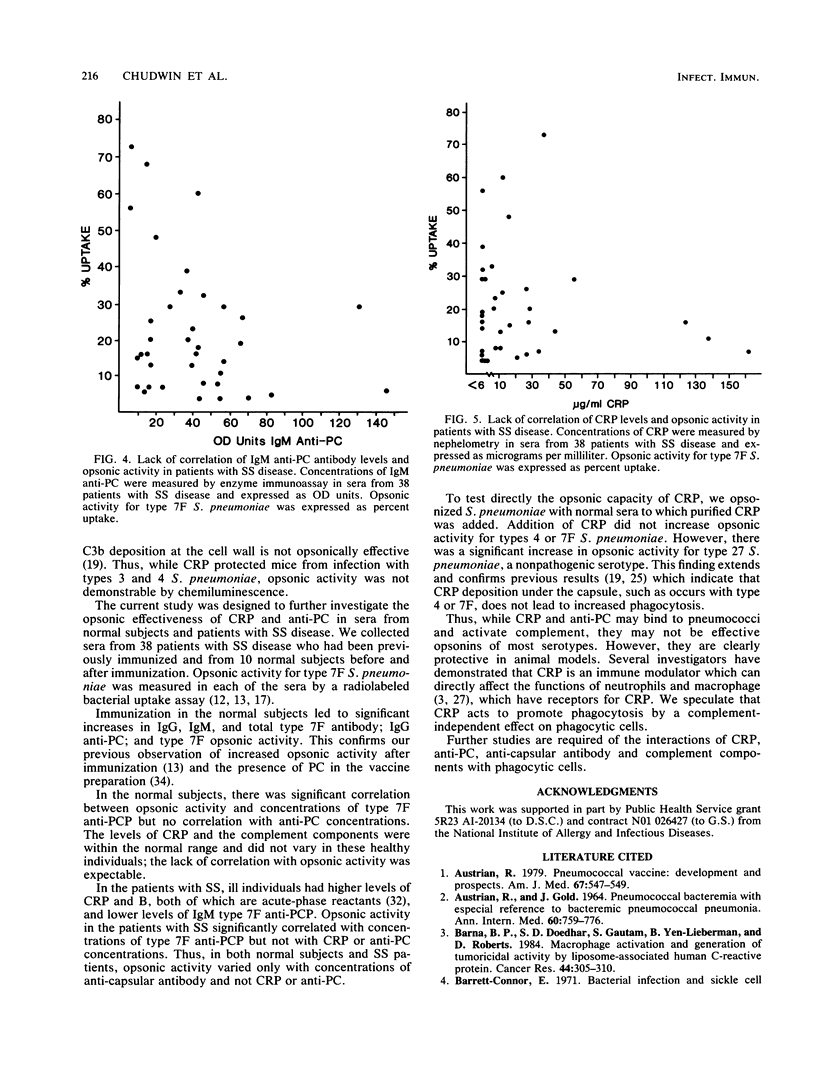
Abstract
C-reactive protein (CRP), an acute-phase reactant which binds to phosphocholine (PC) in the pneumococcal cell wall, and anti-PC antibodies are protective against experimental pneumococcal bacteremia in mice. To determine the relative opsonic capacities of CRP and anti-PC compared with those of antibodies against pneumococcal capsular polysaccharides (anti-PCP), we correlated in vitro opsonic activity for serotype 7F Streptococcus pneumoniae with concentrations of CRP, anti-PC, and anti-type 7F PCP in human sera from 10 normal subjects and 38 patients with sickle cell (SS) disease, a high-risk group for pneumococcal infection. Opsonic activity, measured by a radiolabeled bacterial uptake assay, correlated with anti-PCP levels but not with CRP or anti-PC in both the normal subjects and patients with SS disease. Addition of CRP to normal sera did not increase opsonic activity for serotypes 4 and 7F S. pneumoniae, although it did so for serotype 27, a nonpathogenic strain unique for having PC in its capsule. CRP and anti-PC were not effective opsonins when they bound to the pneumococcal cell wall rather than the capsule. The protective effects of CRP or anti-PC against these serotypes may be produced by means other than complement-dependent opsonization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., GOLD J. PNEUMOCOCCAL BACTEREMIA WITH ESPECIAL REFERENCE TO BACTEREMIC PNEUMOCOCCAL PNEUMONIA. Ann Intern Med. 1964 May;60:759–776. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- Austrian R. Pneumococcal vaccine: development and prospects. Am J Med. 1979 Oct;67(4):547–549. doi: 10.1016/0002-9343(79)90222-5. [DOI] [PubMed] [Google Scholar]
- Barna B. P., Deodhar S. D., Gautam S., Yen-Lieberman B., Roberts D. Macrophage activation and generation of tumoricidal activity by liposome-associated human C-reactive protein. Cancer Res. 1984 Jan;44(1):305–310. [PubMed] [Google Scholar]
- Barrett-Connor E. Bacterial infection and sickle cell anemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine (Baltimore) 1971 Mar;50(2):97–112. [PubMed] [Google Scholar]
- Briles D. E., Claflin J. L., Schroer K., Forman C. Mouse Igg3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature. 1981 Nov 5;294(5836):88–90. doi: 10.1038/294088a0. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982 Oct 1;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Hammer C. H., Burch C. G., Frank M. M. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J Clin Invest. 1982 Jan;69(1):85–98. doi: 10.1172/JCI110444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Joiner K. A., Cole R. M., Berger M. Localization of complement component 3 on Streptococcus pneumoniae: anti-capsular antibody causes complement deposition on the pneumococcal capsule. Infect Immun. 1983 Jan;39(1):403–409. doi: 10.1128/iai.39.1.403-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan G. R., Schiffman G. Antibody responses to polyvalent pneumococcal vaccine in infants with sickle cell anemia. J Pediatr. 1980 Feb;96(2):264–266. doi: 10.1016/s0022-3476(80)80820-1. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Chudwin D. S., Wara D. W., Lameris-Martin N. B., Ammann A. J. Effect of antibody concentration on opsonic requirements for phagocytosis in vitro of Streptococcus pneumoniae types 7 and 19. Proc Soc Exp Biol Med. 1983 Feb;172(2):178–186. doi: 10.3181/00379727-172-41543. [DOI] [PubMed] [Google Scholar]
- Chudwin D. S., Wara D. W., Matthay K. K., Caulfield M. H., Schiffmann G., Mentzer W. C., Ammann A. J. Increased serum opsonic activity and antibody concentration in patients with sickle cell disease after pneumococcal polysaccharide immunization. J Pediatr. 1983 Jan;102(1):51–54. doi: 10.1016/s0022-3476(83)80285-6. [DOI] [PubMed] [Google Scholar]
- Edwards K. M., Gewurz H., Lint T. F., Mold C. A role for C-reactive protein in the complement-mediated stimulation of human neutrophils by type 27 Streptococcus pneumoniae. J Immunol. 1982 Jun;128(6):2493–2496. [PubMed] [Google Scholar]
- Finland M. Pneumonia and pneumococcal infections, with special reference to pneumococcal pneumonia. The 1979 J. Burns Amberson lecture. Am Rev Respir Dis. 1979 Sep;120(3):481–502. doi: 10.1164/arrd.1979.120.3.481. [DOI] [PubMed] [Google Scholar]
- Gewurz H., Mold C., Siegel J., Fiedel B. C-reactive protein and the acute phase response. Adv Intern Med. 1982;27:345–372. [PubMed] [Google Scholar]
- Giebink G. S., Verhoef J., Peterson P. K., Quie P. G. Opsonic requirements for phagocytosis of Streptococcus pneumoniae types VI, XVIII, XXIII, and XXV. Infect Immun. 1977 Nov;18(2):291–297. doi: 10.1128/iai.18.2.291-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B. M. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol Methods. 1979;28(1-2):187–192. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- Holzer T. J., Edwards K. M., Gewurz H., Mold C. Binding of C-reactive protein to the pneumococcal capsule or cell wall results in differential localization of C3 and stimulation of phagocytosis. J Immunol. 1984 Sep;133(3):1424–1430. [PubMed] [Google Scholar]
- Johnston R. B., Jr Increased susceptibility to infection in sickle cell disease: review of its occurrence and possible causes. South Med J. 1974 Nov;67(11):1342–1348. doi: 10.1097/00007611-197411000-00018. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Newman S. L., Struth A. G. An abnormality of the alternate pathway of complement activation in sickle-cell disease. N Engl J Med. 1973 Apr 19;288(16):803–808. doi: 10.1056/NEJM197304192881601. [DOI] [PubMed] [Google Scholar]
- McDaniel L. S., Benjamin W. H., Jr, Forman C., Briles D. E. Blood clearance by anti-phosphocholine antibodies as a mechanism of protection in experimental pneumococcal bacteremia. J Immunol. 1984 Dec;133(6):3308–3312. [PubMed] [Google Scholar]
- Mold C., Du Clos T. W., Nakayama S., Edwards K. M., Gewurz H. C-reactive protein reactivity with complement and effects on phagocytosis. Ann N Y Acad Sci. 1982;389:251–262. doi: 10.1111/j.1749-6632.1982.tb22141.x. [DOI] [PubMed] [Google Scholar]
- Mold C., Edwards K. M., Gewurz H. Effect of C-reactive protein on the complement-mediated stimulated of human neutrophils by Streptococcus pneumoniae serotypes 3 and 6. Infect Immun. 1982 Sep;37(3):987–992. doi: 10.1128/iai.37.3.987-992.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold C., Nakayama S., Holzer T. J., Gewurz H., Du Clos T. W. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J Exp Med. 1981 Nov 1;154(5):1703–1708. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen R. F., Osmand A. P., Lint T. F., Gewurz H. Interaction of C-reactive protein with lymphocytes and monocytes: complement-dependent adherence and phagocytosis. J Immunol. 1976 Sep;117(3):774–781. [PubMed] [Google Scholar]
- Nakayama S., Gewurz H., Holzer T., Du Clos T. W., Mold C. The role of the spleen in the protective effect of C-reactive protein in Streptococcus pneumoniae infection. Clin Exp Immunol. 1983 Nov;54(2):319–326. [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Mold C., Gewurz H., du Clos T. W. Opsonic properties of C-reactive protein in vivo. J Immunol. 1982 Jun;128(6):2435–2438. [PubMed] [Google Scholar]
- Pearson H. A., Cornelius E. A., Schwartz A. D., Zelson J. H., Wolfson S. L., Spencer R. P. Transfusion-reversible functional asplenia in young children with sickle-cell anemia. N Engl J Med. 1970 Aug 13;283(7):334–337. doi: 10.1056/NEJM197008132830703. [DOI] [PubMed] [Google Scholar]
- SICARD A. M. A NEW SYNTHETIC MEDIUM FOR DIPLOCOCCUS PNEUMONIAE, AND ITS USE FOR THE STUDY OF RECIPROCAL TRANSFORMATIONS AT THE AMIA LOCUS. Genetics. 1964 Jul;50:31–44. doi: 10.1093/genetics/50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman G., Douglas R. M., Bonner M. J., Robbins M., Austrian R. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody response to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J Immunol Methods. 1980;33(2):133–144. doi: 10.1016/s0022-1759(80)80004-4. [DOI] [PubMed] [Google Scholar]
- Schutte M., DiCamelli R., Murphy P., Sadove M., Gewurz H. C3 proactivator (C3PA) as an acute phase reactant. Clin Exp Immunol. 1974 Oct;18(2):251–256. [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Clarke S., Robbins J. B. Protection against pneumococcal infection in mice conferred by phosphocholine-binding antibodies: specificity of the phosphocholine binding and relation to several types. Infect Immun. 1983 Feb;39(2):993–999. doi: 10.1128/iai.39.2.993-999.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen U. B., Henrichsen J. C-polysaccharide in a pneumococcal vaccine. Acta Pathol Microbiol Immunol Scand C. 1984 Dec;92(6):351–356. doi: 10.1111/j.1699-0463.1984.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Volanakis J. E., Kaplan M. H. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971 Feb;136(2):612–614. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- Wara D. W. Host defense against Streptococcus pneumoniae: the role of the spleen. Rev Infect Dis. 1981 Mar-Apr;3(2):299–309. doi: 10.1093/clinids/3.2.299. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Thomas E. J., Sissons J. G. Complement activation in asymptomatic patients with sickle cell anaemia. Clin Exp Immunol. 1979 Apr;36(1):130–139. [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A. Opsonins: their function, identity, and clinical significance. J Pediatr. 1973 May;82(5):747–753. doi: 10.1016/s0022-3476(73)80062-9. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A. The role of complement in the host's defense against Streptococcus pneumoniae. Rev Infect Dis. 1981 Mar-Apr;3(2):289–298. doi: 10.1093/clinids/3.2.289. [DOI] [PubMed] [Google Scholar]
- Yother J., Forman C., Gray B. M., Briles D. E. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infect Immun. 1982 Apr;36(1):184–188. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Volanakis J. E., Briles D. E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in mice. J Immunol. 1982 May;128(5):2374–2376. [PubMed] [Google Scholar]



