Abstract
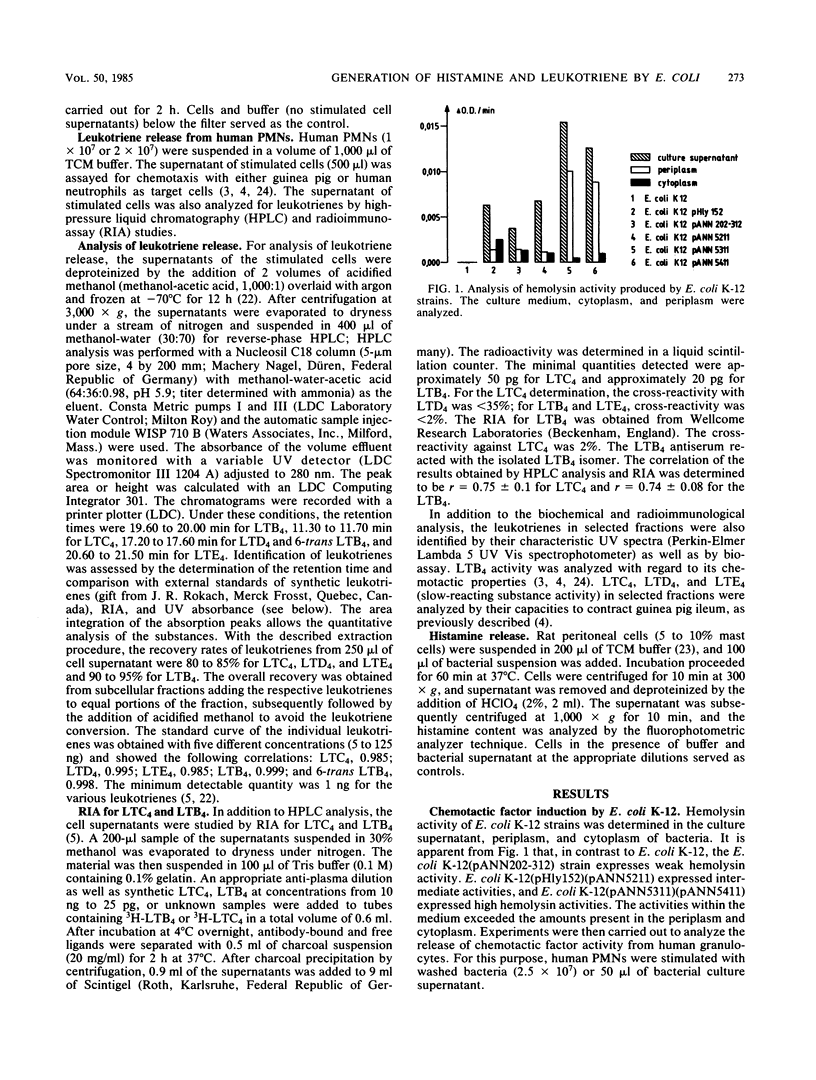
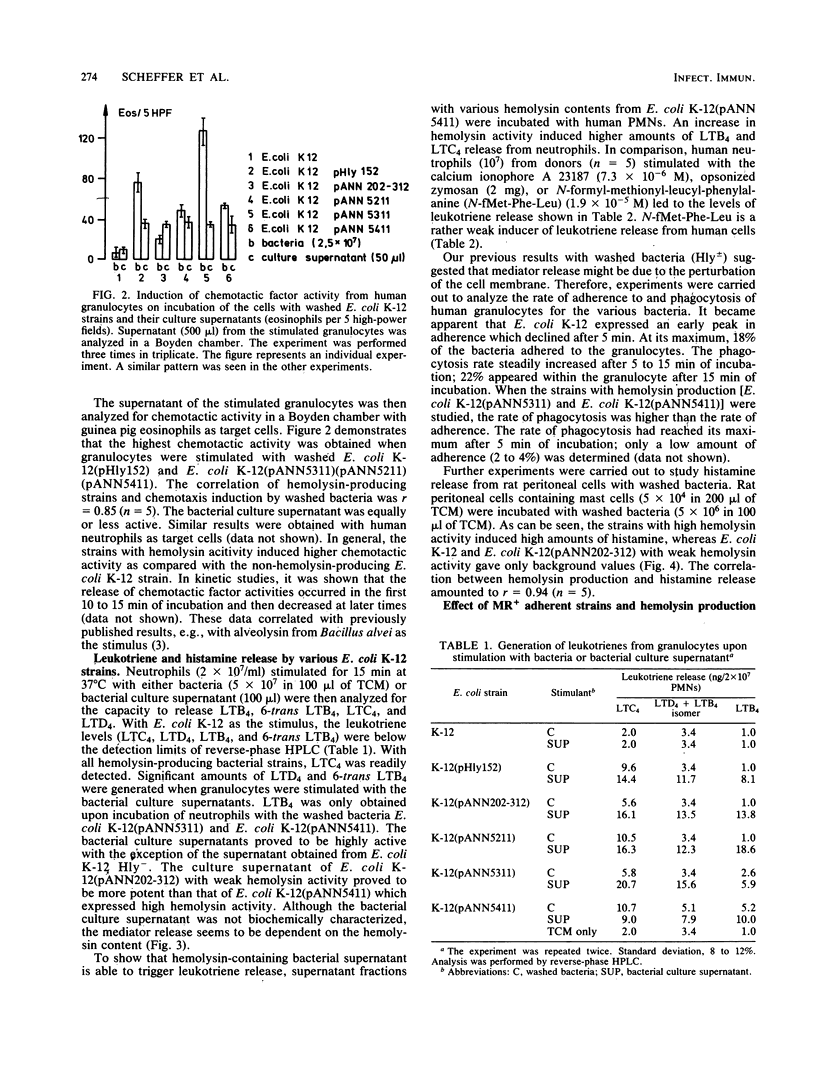
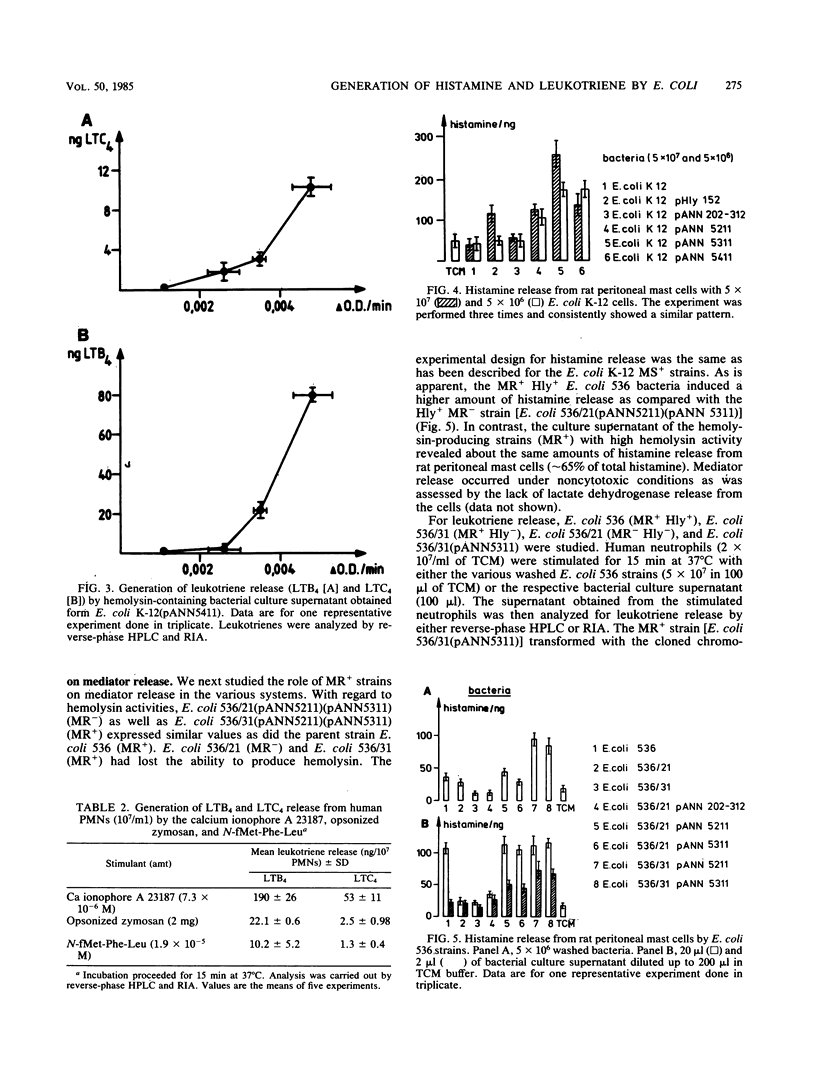
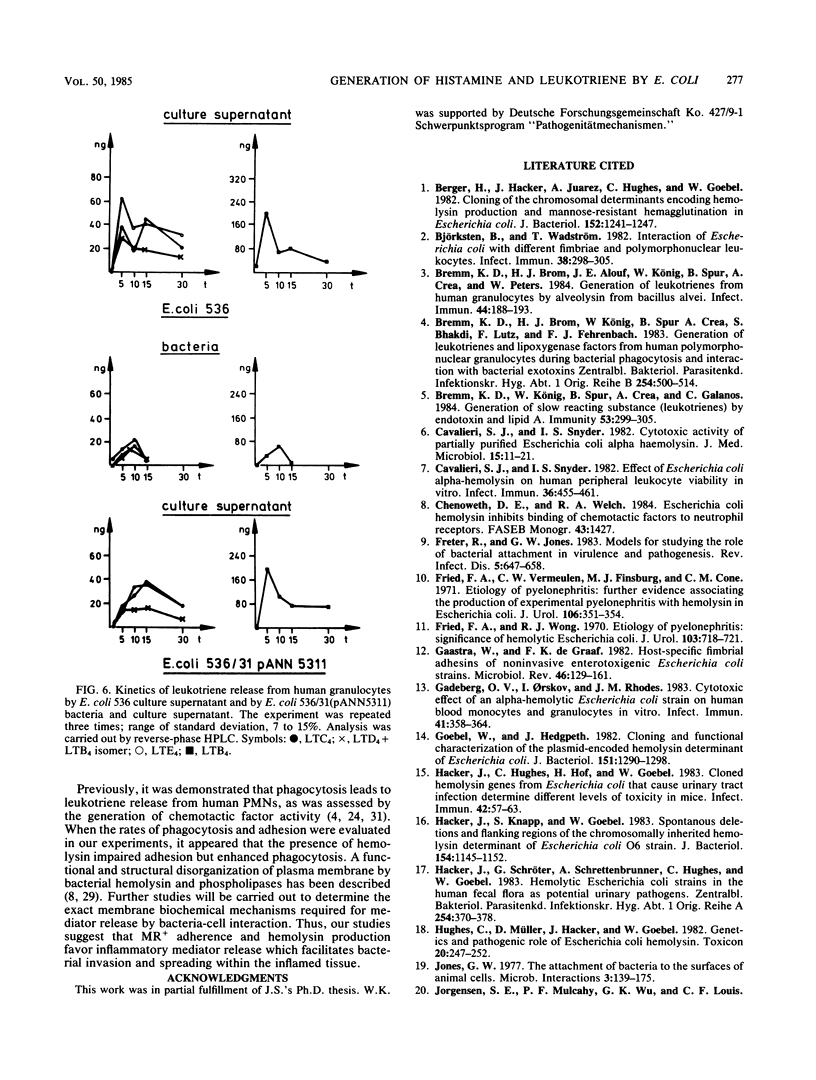
We investigated the role of bacterial adherence and hemolysin production from Escherichia coli parent and genetically cloned strains as to their effects on histamine release from rat mast cells and leukotriene generation from human polymorphonuclear granulocytes. These mediators were involved in the induction of inflammatory disease processes and led, for example, to enhancement of vascular permeability, chemotaxis (leukotriene B4 [LTB4]), chemoaggregation, lysosomal enzyme release, and smooth muscle contraction, (LTC4, LTD4, and LTE4). Washed bacteria (E. coli K-12 MS+ Hly +/-; E. coli 536 MS+ MR +/-) as well as their culture supernatants were analyzed. Washed E. coli K-12 (Hly+), unlike Hly- strains, induced high amounts of histamine release from rat mast cells and chemotactic activity from human polymorphonuclear granulocytes. Significant leukotriene release was obtained with washed E. coli K-12 Hly+ strains and their bacterial culture supernatants. Leukotriene induction was dependent on the amount of hemolysin activity present in the supernatant. However, additional soluble factors should also be considered. The presence of hemolysin appeared to accelerate and enhance the rate of phagocytosis of bacteria by neutrophils. When E. coli 536 (MS+ MR +/- Hly +/-) strains were analyzed, the simultaneous presence of MR+ pili and hemolysin production led to an increase in histamine release as compared with MR- Hly+ strains. The genetically cloned MR+ Hly+ E. coli 536 strain induced higher amounts of leukotrienes as compared with the wild-type strain. Our data suggest a potent role for adhesins and hemolysin as virulence factors in inducing the release of inflammatory mediators.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger H., Hacker J., Juarez A., Hughes C., Goebel W. Cloning of the chromosomal determinants encoding hemolysin production and mannose-resistant hemagglutination in Escherichia coli. J Bacteriol. 1982 Dec;152(3):1241–1247. doi: 10.1128/jb.152.3.1241-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstén B., Wadström T. Interaction of Escherichia coli with different fimbriae and polymorphonuclear leukocytes. Infect Immun. 1982 Oct;38(1):298–305. doi: 10.1128/iai.38.1.298-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., Brom H. J., Alouf J. E., König W., Spur B., Crea A., Peters W. Generation of leukotrienes from human granulocytes by alveolysin from Bacillus alvei. Infect Immun. 1984 Apr;44(1):188–193. doi: 10.1128/iai.44.1.188-193.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., Brom J., König W., Spur B., Crea A., Bhakdi S., Lutz F., Fehrenbach F. J. Generation of leukotrienes and lipoxygenase factors from human polymorphonuclear granulocytes during bacterial phagocytosis and interaction with bacterial exotoxins. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Jul;254(4):500–514. [PubMed] [Google Scholar]
- Bremm K. D., König W., Spur B., Crea A., Galanos C. Generation of slow-reacting substance (leukotrienes) by endotoxin and lipid A from human polymorphonuclear granulocytes. Immunology. 1984 Oct;53(2):299–305. [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Cytotoxic activity of partially purified Escherichia coli alpha haemolysin. J Med Microbiol. 1982 Feb;15(1):11–21. doi: 10.1099/00222615-15-1-11. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte viability in vitro. Infect Immun. 1982 May;36(2):455–461. doi: 10.1128/iai.36.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried F. A., Vermeulen C. W., Ginsburg M. J., Cone C. M. Etiology of pyelonephritis: further evidence associating the production of experimental pyelonephritis with hemolysis in Escherichia coli. J Urol. 1971 Sep;106(3):351–354. doi: 10.1016/s0022-5347(17)61286-2. [DOI] [PubMed] [Google Scholar]
- Fried F. A., Wong R. J. Etiology of pyelonephritis: significance of hemolytic Escherichia coli. J Urol. 1970 Jun;103(6):718–721. doi: 10.1016/s0022-5347(17)62033-0. [DOI] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeberg O. V., Orskov I., Rhodes J. M. Cytotoxic effect of an alpha-hemolytic Escherichia coli strain on human blood monocytes and granulocytes in vitro. Infect Immun. 1983 Jul;41(1):358–364. doi: 10.1128/iai.41.1.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Hedgpeth J. Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1290–1298. doi: 10.1128/jb.151.3.1290-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Hughes C., Hof H., Goebel W. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect Immun. 1983 Oct;42(1):57–63. doi: 10.1128/iai.42.1.57-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Knapp S., Goebel W. Spontaneous deletions and flanking regions of the chromosomally inherited hemolysin determinant of an Escherichia coli O6 strain. J Bacteriol. 1983 Jun;154(3):1145–1152. doi: 10.1128/jb.154.3.1145-1152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schröter G., Schrettenbrunner A., Hughes C., Goebel W. Hemolytic Escherichia coli strains in the human fecal flora as potential urinary pathogens. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 May;254(3):370–378. [PubMed] [Google Scholar]
- Hughes C., Müller D., Hacker J., Goebel W. Genetics and pathogenic role of Escherichia coli haemolysin. Toxicon. 1982;20(1):247–252. doi: 10.1016/0041-0101(82)90210-0. [DOI] [PubMed] [Google Scholar]
- Jorgensen S. E., Mulcahy P. F., Wu G. K., Louis C. F. Calcium accumulation in human and sheep erythrocytes that is induced by Escherichia coli hemolysin. Toxicon. 1983;21(5):717–727. doi: 10.1016/0041-0101(83)90277-5. [DOI] [PubMed] [Google Scholar]
- Kaijser B. Immunology of Escherichia coli: K antigen and its relation to urinary-tract infection. J Infect Dis. 1973 Jun;127(6):670–677. doi: 10.1093/infdis/127.6.670. [DOI] [PubMed] [Google Scholar]
- Kusecek B., Wloch H., Mercer A., Vaisänen V., Pluschke G., Korhonen T., Achtman M. Lipopolysaccharide, capsule, and fimbriae as virulence factors among O1, O7, O16, O18, or O75 and K1, K5, or K100 Escherichia coli. Infect Immun. 1984 Jan;43(1):368–379. doi: 10.1128/iai.43.1.368-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köller M., Schönfeld W., Knöller J., Bremm K. D., König W., Spur B., Crea A., Peters W. The metabolism of leukotrienes in blood plasma studied by high-performance liquid chromatography. Biochim Biophys Acta. 1985 Jan 9;833(1):128–134. doi: 10.1016/0005-2760(85)90260-7. [DOI] [PubMed] [Google Scholar]
- König W., Ishizaka K. Binding of rat IgE with the subcellular components of normal rat mast cells. Immunochemistry. 1976 Apr;13(4):345–353. doi: 10.1016/0019-2791(76)90346-3. [DOI] [PubMed] [Google Scholar]
- König W., Kunau H. W., Borgeat P. Induction and comparison of the eosinophil chemotactic factor with endogenous hydroxyeicosatetraenoic acids: its inhibition by arachidonic acid analogs. Adv Prostaglandin Thromboxane Leukot Res. 1982;9:301–314. [PubMed] [Google Scholar]
- Linggood M. A., Ingram P. L. The role of alpha haemolysin in the virulence of Escherichia coli for mice. J Med Microbiol. 1982 Feb;15(1):23–30. doi: 10.1099/00222615-15-1-23. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Phan S. H., Krutzsch H., Showell H. J., Feltner D. E., Nairn R., Becker E. L., Ward P. A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984 May 10;259(9):5430–5439. [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möllby R., Thelestam M., Geoffroy C., Alouf J. E. Two different modes of membrane damaging action by bacterial thiol-activated haemolysins. Toxicon. 1982;20(1):229–232. doi: 10.1016/0041-0101(82)90206-9. [DOI] [PubMed] [Google Scholar]
- Müller D., Hughes C., Goebel W. Relationship between plasmid and chromosomal hemolysin determinants of Escherichia coli. J Bacteriol. 1983 Feb;153(2):846–851. doi: 10.1128/jb.153.2.846-851.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Salmon J. A. Release of leukotriene B4 from human neutrophils and its relationship to degranulation induced by N-formyl-methionyl-leucyl-phenylalanine, serum-treated zymosan and the ionophore A23187. Immunology. 1983 Sep;50(1):65–73. [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Robinson M. K. Determinants that increase the serum resistance of Escherichia coli. Infect Immun. 1980 Jul;29(1):278–280. doi: 10.1128/iai.29.1.278-280.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch H., König W. Phospholipase A2 and arachidonic acid: a common link in the generation of the eosinophil chemotactic factor (ECF) from human PMN by various stimuli. Scand J Immunol. 1980;11(4):409–418. doi: 10.1111/j.1365-3083.1980.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Survival of cultured cells after functional and structural disorganization of plasma membrane by bacterial haemolysins and phospholipases. Toxicon. 1983;21(6):805–815. doi: 10.1016/0041-0101(83)90069-7. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Hull R., Falkow S. Molecular cloning and physical characterization of a chromosomal hemolysin from Escherichia coli. Infect Immun. 1983 Oct;42(1):178–186. doi: 10.1128/iai.42.1.178-186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch J. F., Postma P., Koopman P. A., de Graaff J., MacLaren D. M., van Brenk D. G., Guinée P. A. Virulence of urinary and faecal Escherichia coli in relation to serotype, haemolysis and haemagglutination. J Hyg (Lond) 1982 Jun;88(3):567–577. doi: 10.1017/s002217240007042x. [DOI] [PMC free article] [PubMed] [Google Scholar]


