Abstract
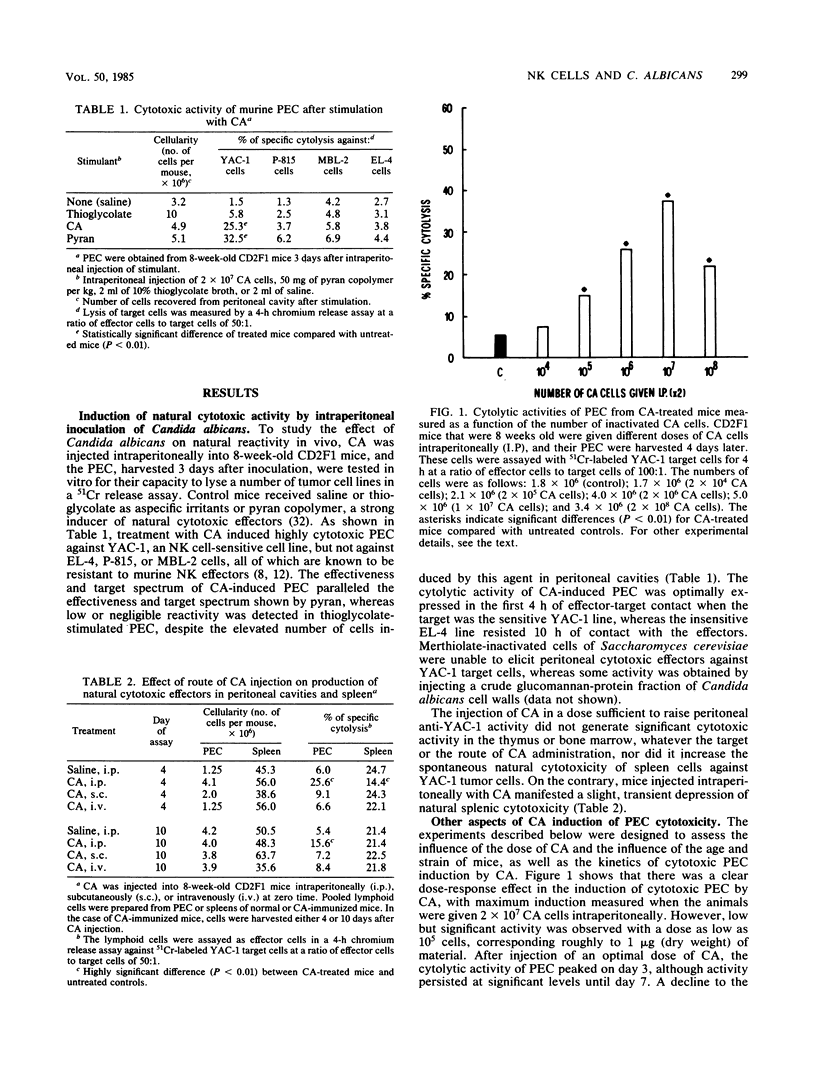
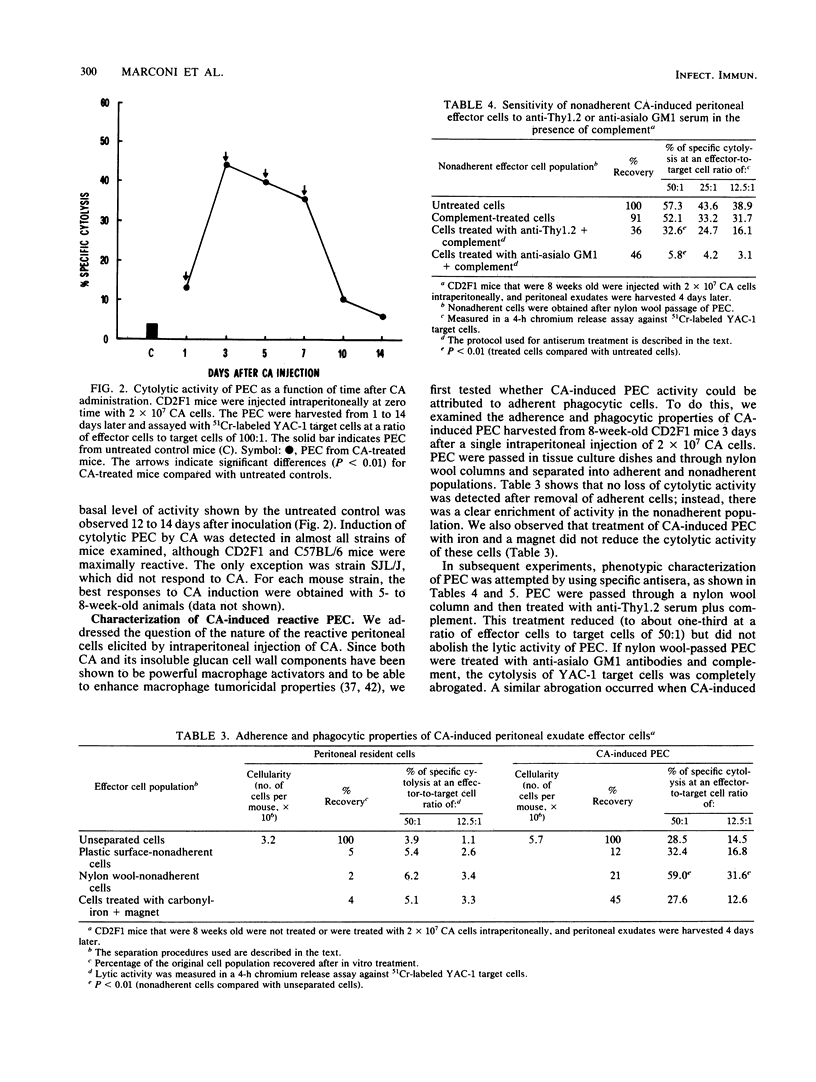
Injection of merthiolate-inactivated yeast form cells of Candida albicans into the peritoneal cavities of mice induced the appearance of a cytolytic effector population against YAC-1 tumor cell lines. This induction was maximally manifested in 5- to 8-week-old animals 3 to 4 days after injection of 2 X 10(7)C. albicans cells, and the peritoneal lytic population exerted its optimum cytotoxic effect after 4 h of incubation. No significant natural cytotoxic activity was generated by C. albicans in the bone marrow or thymus, whereas there was a slight, transient, but significant depression of natural splenic cytotoxicity. Experiments performed to characterize the natural cytotoxic population elicited by the inactivated yeast showed that the effectors were nonadherent, nonphagocytic cells. Moreover, the anti-YAC-1 lytic activity was partially sensitive to anti-Thy1.2 serum and was completely abrogated by treatment of peritoneal nonadherent cells with monoclonal anti-asialo GM1 antibodies. Finally, the peritoneal population of cytotoxic cells induced by C. albicans was fully susceptible to Ly5.1 plus anti-immunoglobulin G2a and complement lysis. Although different cell populations could be induced by inactivated C. albicans, all of our data support the view that the anti-YAC-1 activity was entirely attributable to natural killer lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccarini M., Bistoni F., Puccetti P., Garaci E. Natural cell-mediated cytotoxicity against Candida albicans induced by cyclophosphamide: nature of the in vitro cytotoxic effector. Infect Immun. 1983 Oct;42(1):1–9. doi: 10.1128/iai.42.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A., Bistoni F., Cenci E., Pesce C. D., Tissi L., Marconi P. Immunopotentiation of anticancer chemotherapy by Candida albicans, other yeasts and insoluble glucan in an experimental lymphoma model. Sabouraudia. 1982 Jun;20(2):115–125. doi: 10.1080/00362178285380191. [DOI] [PubMed] [Google Scholar]
- Cikes M., Friberg S., Jr, Klein G. Progressive loss of H-2 antigens with concomitant increase of cell-surface antigen(s) determined by Moloney leukemia virus in cultured murine lymphomas. J Natl Cancer Inst. 1973 Feb;50(2):347–362. doi: 10.1093/jnci/50.2.347. [DOI] [PubMed] [Google Scholar]
- Cutler J. E., Lloyd R. K. Enhanced antibody responses induced by Candida albicans in mice. Infect Immun. 1982 Dec;38(3):1102–1108. doi: 10.1128/iai.38.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Ballet J. J., Griscelli C. Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharidic antigens present in the serum of patients with chronic mucocutaneous candidiasis. J Clin Invest. 1978 Nov;62(5):1005–1013. doi: 10.1172/JCI109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn J. P., McCoy J. L., Fefer A. Cross-resistance to the transplantation of syngeneic Friend, Moloney, and Rauscher virus-induced tumors. Cancer Res. 1968 Mar;28(3):434–439. [PubMed] [Google Scholar]
- Herberman R. B., Aoki T., Nunn M., Lavrin D. H., Soares N., Gazdar A., Holden H., Chang K. S. Specificity of 51Cr-release cytotoxicity of lymphocytes immune to murine sarcoma virus. J Natl Cancer Inst. 1974 Oct;53(4):1103–1111. doi: 10.1093/jnci/53.4.1103. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T. Low density of Thy 1 antigen on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1978 Jul;121(1):304–309. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Staal S., Djeu J. Y. Augmentation of natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic target cells. Int J Cancer. 1977 Apr 15;19(4):555–564. doi: 10.1002/ijc.2910190417. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Jimenez B. E., Murphy J. W. In vitro effects of natural killer cells against Paracoccidioides brasiliensis yeast phase. Infect Immun. 1984 Nov;46(2):552–558. doi: 10.1128/iai.46.2.552-558.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Kokoshis P. L., Williams D. L., Cook J. A., Di Luzio N. R. Increased resistance to Staphylococcus aureus infection and enhancement in serum lysozyme activity by glucan. Science. 1978 Mar 24;199(4335):1340–1342. doi: 10.1126/science.628841. [DOI] [PubMed] [Google Scholar]
- Landolfo S., Herberman R. B., Holden H. T. Macrophage-lymphocyte interaction in migration inhibition factor (MIF) production against soluble or cellular tumor-associated antigens. I. Characteristics and genetic control of two different mechanisms of stimulating MIF production. J Immunol. 1978 Aug;121(2):695–701. [PubMed] [Google Scholar]
- Lohmann-Matthes M. L., Domzig W., Roder J. Promonocytes have the functional characteristics of natural killer cells. J Immunol. 1979 Oct;123(4):1883–1886. [PubMed] [Google Scholar]
- Marconi P., Bistoni F., Cassone A., Frati L., Baccarini M., Garaci E., Bonmassar E. The role of the peritoneal cavity in successful treatment of a murine lymphoma with chemotherapy and non-specific immunostimulation. Cancer Immunol Immunother. 1982;13(2):128–133. doi: 10.1007/BF00205313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi P., Cassone A., Baccarini M., Tissi L., Garaci E., Bonmassar E., Frati L., Bistoni F. Relationships among tumor load, route of tumor inoculation, and response to immunochemotherapy in a murine lymphoma model. J Natl Cancer Inst. 1983 Aug;71(2):299–307. [PubMed] [Google Scholar]
- Marconi P., Cassone A., Tissi L., Baccarini M., Puccetti P., Garaci E., Bonmassar E., Bistoni F. Cellular mechanisms underlying the adjuvant activity of Candida albicans in a mouse lymphoma model. Int J Cancer. 1982 Apr 15;29(4):483–488. doi: 10.1002/ijc.2910290420. [DOI] [PubMed] [Google Scholar]
- Mattes M. J., Sharrow S. O., Herberman R. B., Holden H. T. Identification and separation of Thy-1 positive mouse spleen cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Immunol. 1979 Dec;123(6):2851–2860. [PubMed] [Google Scholar]
- Mattia E., Cassone A. Inducibility of germ-tube formation in Candida albicans at different phases of yeast growth. J Gen Microbiol. 1979 Aug;113(2):439–442. doi: 10.1099/00221287-113-2-439. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., McCormack R. T., Gehrz R. C. Two mechanisms of inhibition of human lymphocyte proliferation by soluble yeast mannan polysaccharide. Infect Immun. 1984 Mar;43(3):1041–1046. doi: 10.1128/iai.43.3.1041-1046.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo E., Haller O., Kimura A., Wigzell H. An analysis of conditions allowing Corynebacterium parvum to cause either augmentation or inhibition of natural killer cell activity against tumor cells in mice. Int J Cancer. 1978 Apr 15;21(4):444–452. doi: 10.1002/ijc.2910210408. [DOI] [PubMed] [Google Scholar]
- Ojo E., Haller O., Wigzell H. Corynebacterium parvum-induced peritoneal exudate cells with rapid cytolytic activity against tumour cells are non-phagocytic cells with characteristics of natural killer cells. Scand J Immunol. 1978;8(3):215–222. doi: 10.1111/j.1365-3083.1978.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Oldham R. K., Ortaldo J. R., Holden H. T., Herberman R. B. Direct comparison of three isotopic release microtoxicity assays as measures of cell-mediated immunity to Gross virus-induced lymphomas in rats. J Natl Cancer Inst. 1977 Apr;58(4):1061–1067. doi: 10.1093/jnci/58.4.1061. [DOI] [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Generation of suppressor cells in the response of human lymphocytes to a polysaccharide from Candida albicans. J Immunol. 1981 Jun;126(6):2151–2155. [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Human lymphocyte-activating properties of a purified polysaccharide from Candida albicans: B and T cell cooperation in the mitogenic response. J Immunol. 1980 Nov;125(5):2082–2088. [PubMed] [Google Scholar]
- Puccetti P., Santoni A., Riccardi C., Holden H. T., Herberman R. B. Activation of mouse macrophages by pyran copolymer and role in augmentation of natural killer activity. Int J Cancer. 1979 Dec 15;24(6):819–825. doi: 10.1002/ijc.2910240621. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Kastello M. D., Harrington D. G., Crabbs C. L., Peters C. J., Jemski J. V., Scott G. H., Di Luzio N. R. Glucan-induced enhancement of host resistance to selected infectious diseases. Infect Immun. 1980 Oct;30(1):51–57. doi: 10.1128/iai.30.1.51-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas V., Rogers T. J. Studies on the cellular nature of Candida albicans-induced suppression. J Immunol. 1983 Jan;130(1):376–379. [PubMed] [Google Scholar]
- Roder J. C., Karre K., Kiessling R. Natural killer cells. Prog Allergy. 1981;28:66–159. [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Suppression of lymphocyte blastogenesis by Candida albicans. Clin Immunol Immunopathol. 1978 Jul;10(3):298–305. doi: 10.1016/0090-1229(78)90185-x. [DOI] [PubMed] [Google Scholar]
- Seijelid R., Bögwald J., Lundwall A. Glycan stimulation of macrophages in vitro. Exp Cell Res. 1981 Jan;131(1):121–129. doi: 10.1016/0014-4827(81)90413-4. [DOI] [PubMed] [Google Scholar]
- Shimamura K., Habu S., Fukui H., Akatsuka A., Okumura K., Tamaoki N. Morphology and function of ganglio-N-tetraosylceramide-positive lymphocyte mediators of natural killer activity. J Natl Cancer Inst. 1982 Mar;68(3):449–455. [PubMed] [Google Scholar]
- Sihvola M., Hurme M. The development of NK cell activity in thymectomized bone marrow chimaeras. Immunology. 1984 Sep;53(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- Tracey D. E., Wolfe S. A., Durdik J. M., Henney C. S. BCG-induced murine effector cells. I. Cytolytic activity in peritoneal exudates: an early response to BCG. J Immunol. 1977 Sep;119(3):1145–1151. [PubMed] [Google Scholar]
- Uchida A., Micksche M. In vitro augmentation of natural killing activity by OK-432. Int J Immunopharmacol. 1981;3(4):365–375. doi: 10.1016/0192-0561(81)90032-1. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Hibbs J. B., Jr Enhanced macrophage tumoricidal activity and tumor suppression or regression caused by heat-killed Candida albicans. J Natl Cancer Inst. 1979 Nov;63(5):1273–1278. [PubMed] [Google Scholar]
- Williams D. L., Cook J. A., Hoffmann E. O., Di Luzio N. R. Protective effect of glucan in experimentally induced candidiasis. J Reticuloendothel Soc. 1978 Jun;23(6):479–490. [PubMed] [Google Scholar]
- Wolfe S. A., Tracey D. E., Henney C. S. BCG-induced murine effector cells. II. Characterization of natural killer cells in peritoneal exudates. J Immunol. 1977 Sep;119(3):1152–1158. [PubMed] [Google Scholar]
- Yanagawa E., Uchida A., Kokoschka E. M., Micksche M. Natural cytotoxicity of lymphocytes and monocytes and its augmentation by OK432 in melanoma patients. Cancer Immunol Immunother. 1984;16(3):131–136. doi: 10.1007/BF00205418. [DOI] [PMC free article] [PubMed] [Google Scholar]


