Abstract
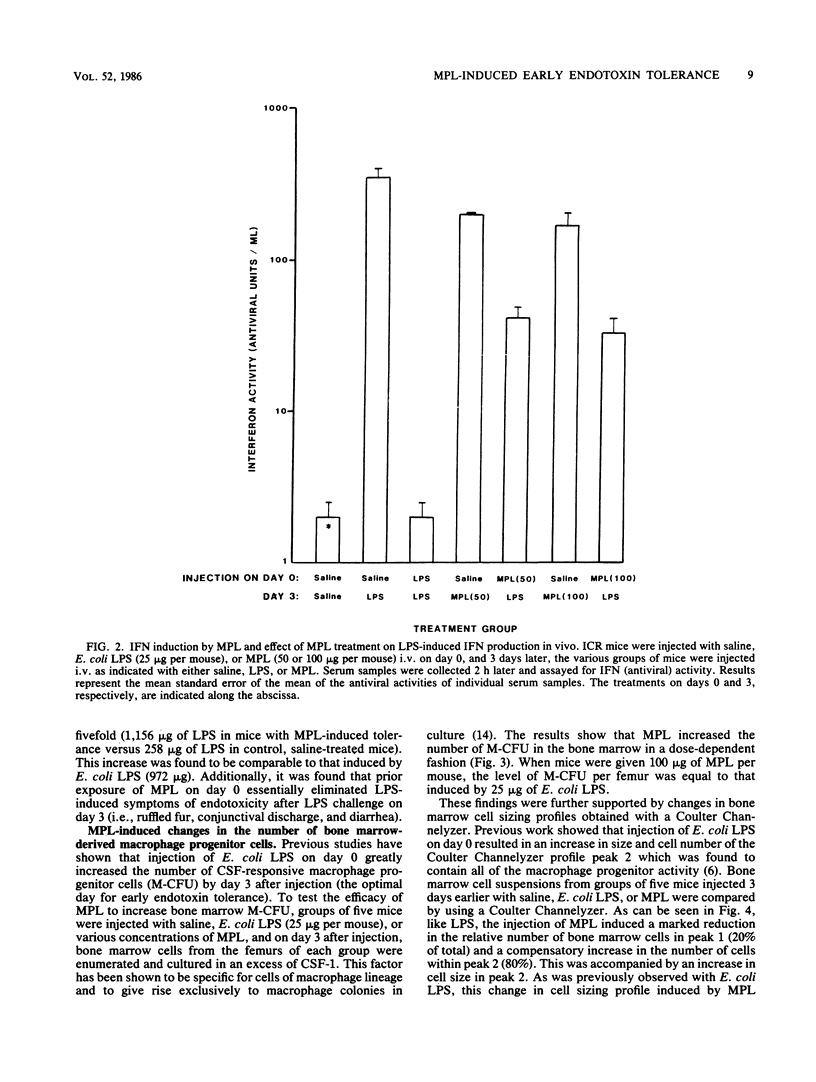
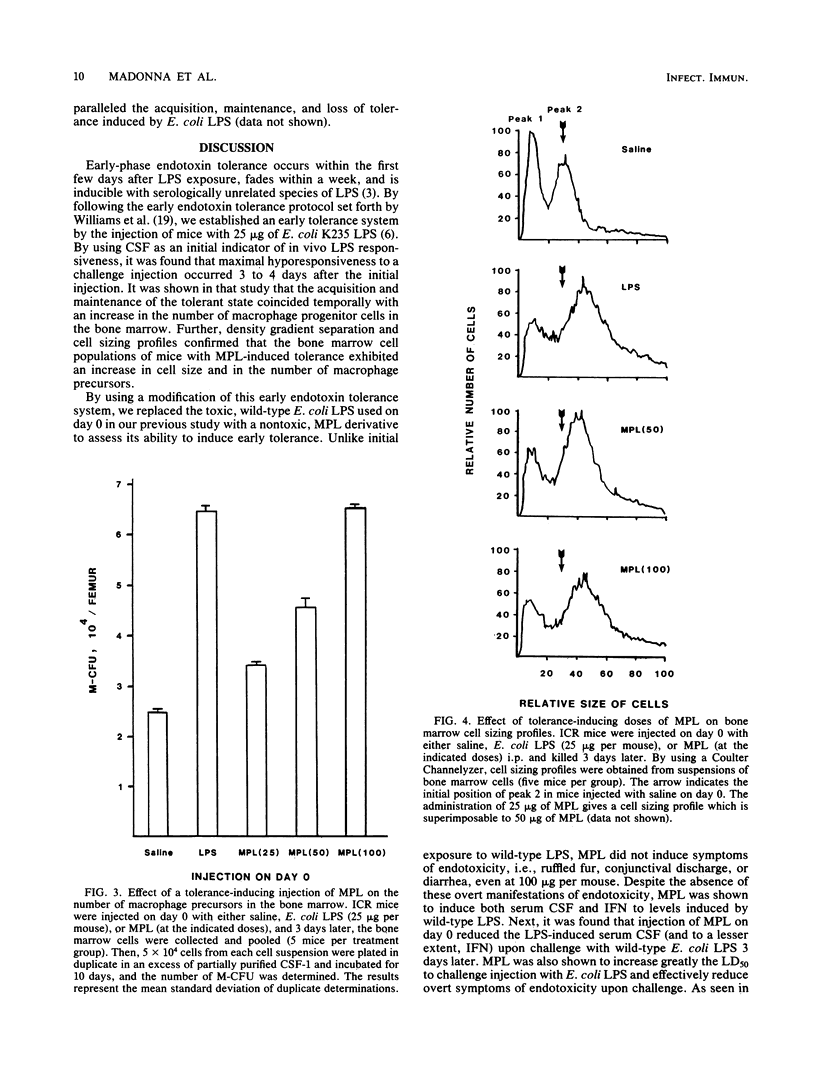
After a sublethal exposure to lipopolysaccharide (LPS) or to lipid A, which is that portion of the LPS molecule associated with endotoxicity, a transient period ensues during which a normally responsive individual is rendered hyporesponsive to LPS-induced toxicity. This period has been defined as early-phase endotoxin tolerance. Recently, a nontoxic derivative of lipid A from Salmonella typhimurium, monophosphoryl lipid A (MPL), was isolated and purified. In this study, we assessed the ability of MPL to induce early endotoxin tolerance. Initial injection of MPL resulted in a dose-dependent stimulation of both serum colony-stimulating factor and serum interferon, indicators of in vivo LPS responsiveness. In contrast, MPL failed to induce the symptoms of endotoxicity which are normally seen after injection of even sublethal amounts of intact endotoxin or lipid A preparations. Injection of MPL on day 0 reduced significantly the amount of LPS-induced serum colony-stimulating factor and interferon produced upon challenge with Escherichia coli LPS 3 days later and also mitigated toxic manifestations, as evidenced by a marked increase in the 50% lethal dose. Like the early tolerance induced by wild-type (toxic) LPS, MPL-induced tolerance was characterized by an accompanying elevation in the number of bone marrow-derived macrophage progenitor cells and by an alteration in bone marrow cell sizing profiles. These results indicate that MPL is effective in inducing a state of LPS-hyporesponsiveness without the toxic side effects of endotoxin and that the structural component(s) necessary for induction of early-phase endotoxin tolerance is contained within MPL.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry L. J. Bacterial toxins. CRC Crit Rev Toxicol. 1977 Nov;5(3):239–318. doi: 10.3109/10408447709082601. [DOI] [PubMed] [Google Scholar]
- MacVittie T. J. Alterations induced in macrophage and granulocyte-macrophage colony-forming cells by a single injection of mice with Corynebacterium parvum. J Reticuloendothel Soc. 1979 Nov;26(5):479–490. [PubMed] [Google Scholar]
- Madonna G. S., Vogel S. N. Early endotoxin tolerance is associated with alterations in bone marrow-derived macrophage precursor pools. J Immunol. 1985 Dec;135(6):3763–3771. [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CFS) levels. Immunology. 1971 Sep;21(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982 Oct 10;257(19):11808–11815. [PubMed] [Google Scholar]
- Ribi E., Cantrell J. L., Takayama K., Qureshi N., Peterson J., Ribi H. O. Lipid A and immunotherapy. Rev Infect Dis. 1984 Jul-Aug;6(4):567–572. doi: 10.1093/clinids/6.4.567. [DOI] [PubMed] [Google Scholar]
- Rubinstein S., Familletti P. C., Pestka S. Convenient assay for interferons. J Virol. 1981 Feb;37(2):755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Raetz C. R., Ribi E., Peterson J., Cantrell J. L., Pearson F. C., Wiggins J., Johnson A. G. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infect Immun. 1984 Aug;45(2):350–355. doi: 10.1128/iai.45.2.350-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Ribi E., Cantrell J. L. Separation and characterization of toxic and nontoxic forms of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):439–443. doi: 10.1093/clinids/6.4.439. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., English K. E., O'Brien A. D. Silica enhancement of murine endotoxin sensitivity. Infect Immun. 1982 Nov;38(2):681–685. doi: 10.1128/iai.38.2.681-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams Z., Hertogs C. F., Pluznik D. H. Use of mice tolerant to lipopolysaccharide to demonstrate requirement of cooperation between macrophages and lymphocytes to generate lipopolysaccharide-induced colony-stimulating factor in vivo. Infect Immun. 1983 Jul;41(1):1–5. doi: 10.1128/iai.41.1.1-5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Stinebring W. R. Interferon appearance stimulated by endotoxin, bacteria, or viruses in mice pre-treated with Escherichia coli endotoxin or infected with Mycobacterium tuberculosis. Nature. 1965 Oct 30;208(5009):456–458. doi: 10.1038/208456a0. [DOI] [PubMed] [Google Scholar]



