Abstract
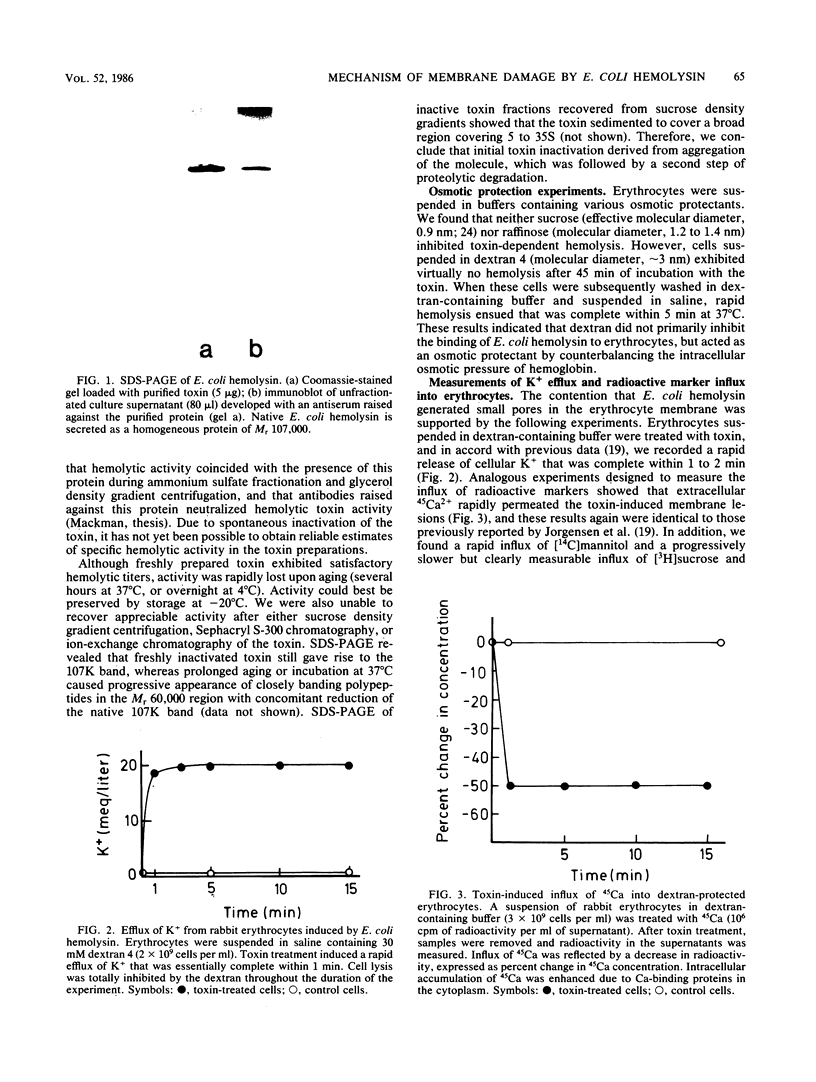
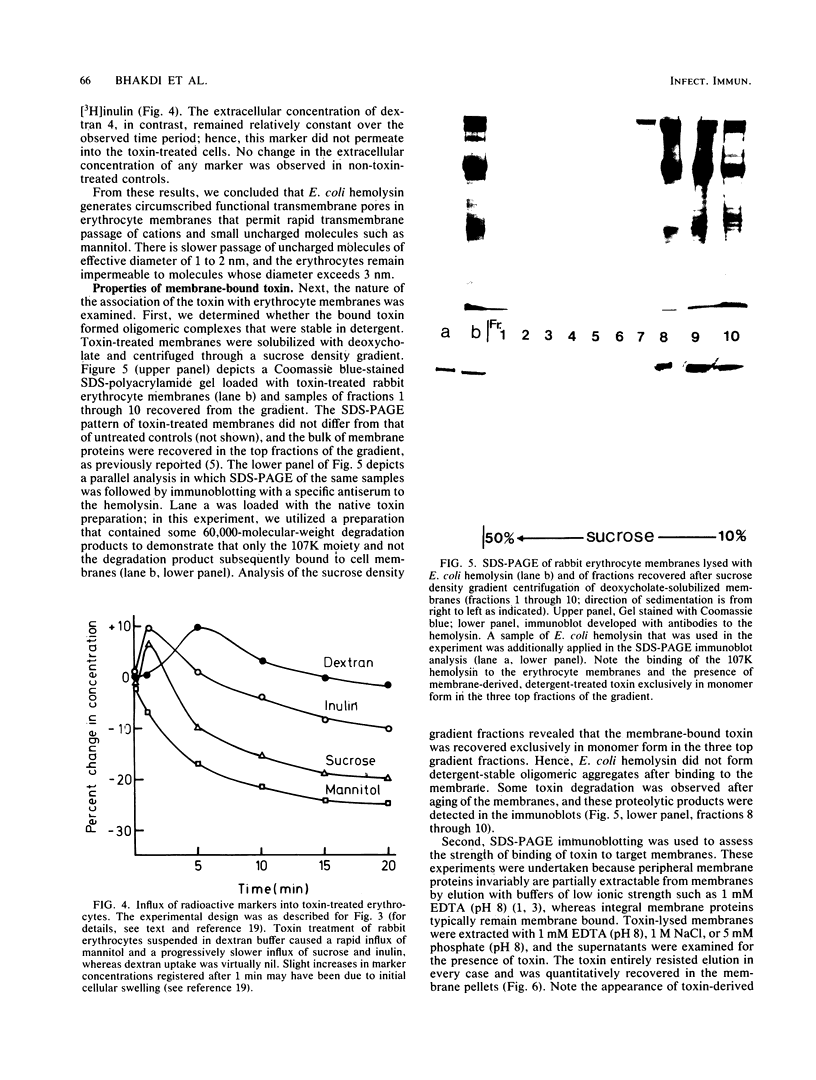
Escherichia coli hemolysin is secreted as a water-soluble polypeptide of Mr 107,000. After binding to target erythrocytes, the membrane-bound toxin resembled an integral membrane protein in that it was refractory towards extraction with salt solutions of low ionic strength. Toxin-induced hemolysis could be totally inhibited by addition of 30 mM dextran 4 (mean Mr, 4,000; molecular diameter approximately 3 nm) to the extracellular medium. Uncharged molecules of smaller size (e.g., sucrose, with a molecular diameter of 0.9 nm, or raffinose, with a molecular diameter of 1.2 to 1.3 nm) did not afford such protection. Treatment of erythrocytes suspended in dextran-containing buffer with the toxin induced rapid efflux of cellular K+ and influx of 45Ca2+, as well as influx of [14C]mannitol and [3H]sucrose. [3H]inulin only slowly permeated into toxin-treated cells, and [3H]dextran uptake was virtually nil. Membranes lysed with high doses of E. coli hemolysin exhibited no recognizable ultrastructural lesions when examined by negative-staining electron microscopy. Sucrose density gradient centrifugation of deoxycholate-solubilized target membranes led to recovery of the toxin exclusively in monomer form. Incubation of toxin-treated cells with trypsin caused limited proteolysis with the generation of membrane-bound, toxin-derived polypeptides of Mr approximately 80,000 without destroying the functional pore. We suggest that E. coli hemolysin may damage cell membranes by partial insertion into the lipid bilayer and generation of a discrete, hydrophilic transmembrane pore with an effective diameter of approximately 3 nm. In contrast to the structured pores generated by cytolysins of gram-positive bacteria such as staphylococcal alpha-toxin and streptolysin O, pore formation by E. coli hemolysin may be caused by the insertion of toxin monomers into the target lipid bilayers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Knüfermann H., Wallach D. F. Two-dimensional separation of erythrocyte membrane proteins. Biochim Biophys Acta. 1975 Jul 18;394(4):550–557. doi: 10.1016/0005-2736(75)90140-6. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Mechanism of complement cytolysis and the concept of channel-forming proteins. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):311–324. doi: 10.1098/rstb.1984.0092. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by complement. Biochim Biophys Acta. 1983 Aug 11;737(3-4):343–372. doi: 10.1016/0304-4157(83)90006-0. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke E. M., Ewins S. P. Properties of strains of Escherichia coli isolated from a variety of sources. J Med Microbiol. 1975 Feb;8(1):107–111. doi: 10.1099/00222615-8-1-107. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried F. A., Vermeulen C. W., Ginsburg M. J., Cone C. M. Etiology of pyelonephritis: further evidence associating the production of experimental pyelonephritis with hemolysis in Escherichia coli. J Urol. 1971 Sep;106(3):351–354. doi: 10.1016/s0022-5347(17)61286-2. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN H., BARROW P., GOLDBERG B. Effect of antibody and complement on permeability control in ascites tumor cells and erythrocytes. J Exp Med. 1959 Nov 1;110:699–713. doi: 10.1084/jem.110.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Hedgpeth J. Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1290–1298. doi: 10.1128/jb.151.3.1290-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Carreró M. I., Zabala J. C., de la Cruz F., Ortiz J. M. Purification of alpha-hemolysin from an overproducing E. coli strain. Mol Gen Genet. 1985;199(1):106–110. doi: 10.1007/BF00327518. [DOI] [PubMed] [Google Scholar]
- Hacker J., Hughes C., Hof H., Goebel W. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect Immun. 1983 Oct;42(1):57–63. doi: 10.1128/iai.42.1.57-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Membrane glycoprotein receptor and hole-forming properties of a cytolytic protein toxin. Biochemistry. 1982 Mar 30;21(7):1662–1667. doi: 10.1021/bi00536a029. [DOI] [PubMed] [Google Scholar]
- Hughes C., Hacker J., Roberts A., Goebel W. Hemolysin production as a virulence marker in symptomatic and asymptomatic urinary tract infections caused by Escherichia coli. Infect Immun. 1983 Feb;39(2):546–551. doi: 10.1128/iai.39.2.546-551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtlein M., Schiessl S., Wagner W., Rdest U., Kreft J., Goebel W. Transport of hemolysin by Escherichia coli. J Cell Biochem. 1983;22(2):87–97. doi: 10.1002/jcb.240220203. [DOI] [PubMed] [Google Scholar]
- Jorgensen S. E., Mulcahy P. F., Wu G. K., Louis C. F. Calcium accumulation in human and sheep erythrocytes that is induced by Escherichia coli hemolysin. Toxicon. 1983;21(5):717–727. doi: 10.1016/0041-0101(83)90277-5. [DOI] [PubMed] [Google Scholar]
- Lutz F., Grieshaber S., Schmidt K. Permeability changes of Ehrlich mouse ascites tumor cells induced by a cytotoxin from Pseudomonas aeruginosa. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jul;320(1):78–80. doi: 10.1007/BF00499077. [DOI] [PubMed] [Google Scholar]
- Mackman N., Holland I. B. Functional characterization of a cloned haemolysin determinant from E. coli of human origin, encoding information for the secretion of a 107K polypeptide. Mol Gen Genet. 1984;196(1):129–134. doi: 10.1007/BF00334104. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Regulation of haemolysin synthesis in E. coli determined by HLY genes of human origin. Mol Gen Genet. 1985;199(1):111–116. doi: 10.1007/BF00327519. [DOI] [PubMed] [Google Scholar]
- Rennie R. P., Freer J. H., Arbuthnott J. P. The kinetics of erythrocyte lysis by Escherichia coli haemolysin. J Med Microbiol. 1974 May;7(2):189–195. doi: 10.1099/00222615-7-2-189. [DOI] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W., Goebel W. Synthesis and secretion of hemolysin by Escherichia coli. J Bacteriol. 1980 Oct;144(1):53–59. doi: 10.1128/jb.144.1.53-59.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Falkow S. Characterization of Escherichia coli hemolysins conferring quantitative differences in virulence. Infect Immun. 1984 Jan;43(1):156–160. doi: 10.1128/iai.43.1.156-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch J. F., Postma P., Koopman P. A., de Graaff J., MacLaren D. M., van Brenk D. G., Guinée P. A. Virulence of urinary and faecal Escherichia coli in relation to serotype, haemolysis and haemagglutination. J Hyg (Lond) 1982 Jun;88(3):567–577. doi: 10.1017/s002217240007042x. [DOI] [PMC free article] [PubMed] [Google Scholar]