Abstract
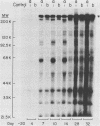
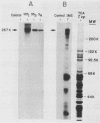
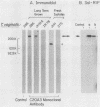
To produce monoclonal antibodies (MAbs) to highly immunogenic membrane proteins of Trichomonas vaginalis NYH286, the sera of subcutaneously infected BALB/c mice were first monitored for antibody to trichomonad surface proteins. The sera possessed antibody to one major surface protein by 7 days and antibody to numerous other trichomonad membrane proteins by 4 weeks postinfection. A hybridoma was then generated that synthesized an MAb, designated C20A3, which reacted to a parasite-derived glycoprotein possessing a molecular weight of 267,000 (267K glycoprotein). The immunogen corresponded to the single high-molecular-weight immunogenic surface protein recognized by 7-day mouse antisera. The MAb differentiated T. vaginalis isolates by a whole-cell enzyme-linked immunosorbent assay and by indirect immunofluorescence, using either fixed or live organisms. All isolates, however, possessed C20A3-reactive material when tested by enzyme-linked immunosorbent assay, using detergent extracts of the isolates incubated with MAb-coated microtiter well plates. The epitope was accessible to antibody binding on live T. vaginalis organisms expressing the major immunogen, and the 267K glycoprotein was readily removed from the parasite membranes by trypsinizing the intact trichomonads. The antigen incorporated radiolabeled glucose, mannose, and acetate. Also, an unlabeled 267K glycoprotein on nitrocellulose blots was detected by 125I-concanavalin A and 125I-wheat germ agglutinin, confirming the glycoprotein nature of the immunogen. Finally, of seven isolates used in this study, one possessed a cross-reactive 170K, rather than 267K, antigen. The data reinforce the idea that antigenic heterogeneity among T. vaginalis isolates may be a function of the presence or absence of high-molecular-weight glycoprotein immunogens from trichomonal membranes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun. 1983 Mar;39(3):1041–1047. doi: 10.1128/iai.39.3.1041-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Enzyme linked immunosorbent assay for detecting antibody to Trichomonas vaginalis: use of whole cells and aqueous extract as antigen. Br J Vener Dis. 1984 Jun;60(3):164–170. doi: 10.1136/sti.60.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Identification of immunogenic and antibody-binding membrane proteins of pathogenic Trichomonas vaginalis. Infect Immun. 1983 Apr;40(1):284–291. doi: 10.1128/iai.40.1.284-291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L., Smith J., Spence M. Heterogeneity of Trichomonas vaginalis and discrimination among trichomonal isolates and subpopulations with sera of patients and experimentally infected mice. Infect Immun. 1985 Sep;49(3):463–468. doi: 10.1128/iai.49.3.463-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly R. J., Torian B. E., Stibbs H. H. Identification of a surface antigen of Trichomonas vaginalis. Infect Immun. 1985 Aug;49(2):270–274. doi: 10.1128/iai.49.2.270-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Glass W. F., 2nd, Briggs R. C., Hnilica L. S. Use of lectins for detection of electrophoretically separated glycoproteins transferred onto nitrocellulose sheets. Anal Biochem. 1981 Jul 15;115(1):219–224. doi: 10.1016/0003-2697(81)90549-2. [DOI] [PubMed] [Google Scholar]
- Honigberg B. M., Livingston M. C., Frost J. K. Pathogenicity of fresh isolates of Trichomonas vaginalis: "the mouse assay" versus clinical and pathologic findings. Acta Cytol. 1966 Sep-Oct;10(5):353–361. [PubMed] [Google Scholar]
- KOTT H., ADLER S. A serological study of Trichomonas sp. parasitic in man. Trans R Soc Trop Med Hyg. 1961 Jul;55:333–344. doi: 10.1016/0035-9203(61)90102-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrison-Plummer J., Jones D. H., Baseman J. B. An ELISA to detect monoclonal antibodies specific for lipid determinants of Mycoplasma pneumoniae. J Immunol Methods. 1983 Nov 11;64(1-2):165–178. doi: 10.1016/0022-1759(83)90395-2. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Acquisition of alpha 1-Antitrypsin by a pathogenic strain of Trichomonas vaginalis. Infect Immun. 1983 May;40(2):640–646. doi: 10.1128/iai.40.2.640-646.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Iron uptake and increased intracellular enzyme activity follow host lactoferrin binding by Trichomonas vaginalis receptors. J Exp Med. 1984 Aug 1;160(2):398–410. doi: 10.1084/jem.160.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Selective acquisition of plasma proteins by Trichomonas vaginalis and human lipoproteins as a growth requirement for this species. Mol Biochem Parasitol. 1984 May;12(1):37–48. doi: 10.1016/0166-6851(84)90042-2. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Trichomonas vaginalis is dependent on uptake and degradation of human low density lipoproteins. J Exp Med. 1984 Nov 1;160(5):1261–1272. doi: 10.1084/jem.160.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su-Lin K. E., Honigberg B. M. Antigenic analysis of Trichomonas vaginalis strains by quantitative fluorescent antibody methods. Z Parasitenkd. 1983;69(2):161–181. doi: 10.1007/BF00926952. [DOI] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Stephens R. S., Stibbs H. H. Specific and common antigens of Trichomonas vaginalis detected by monoclonal antibodies. Infect Immun. 1984 Jan;43(1):270–275. doi: 10.1128/iai.43.1.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warton A., Honigberg B. M. Lectin analysis of surface saccharides in two Trichomonas vaginalis strains differing in pathogenicity. J Protozool. 1980 Nov;27(4):410–419. doi: 10.1111/j.1550-7408.1980.tb05386.x. [DOI] [PubMed] [Google Scholar]
- Wartoń A., Honigberg B. M. Analysis of surface saccharides in Trichomonas vaginalis strains with various pathogenicity levels by fluorescein-conjugated plant lectins. Z Parasitenkd. 1983;69(2):149–159. doi: 10.1007/BF00926951. [DOI] [PubMed] [Google Scholar]