Abstract
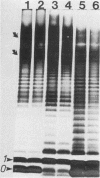
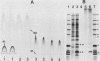
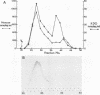
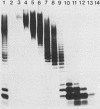
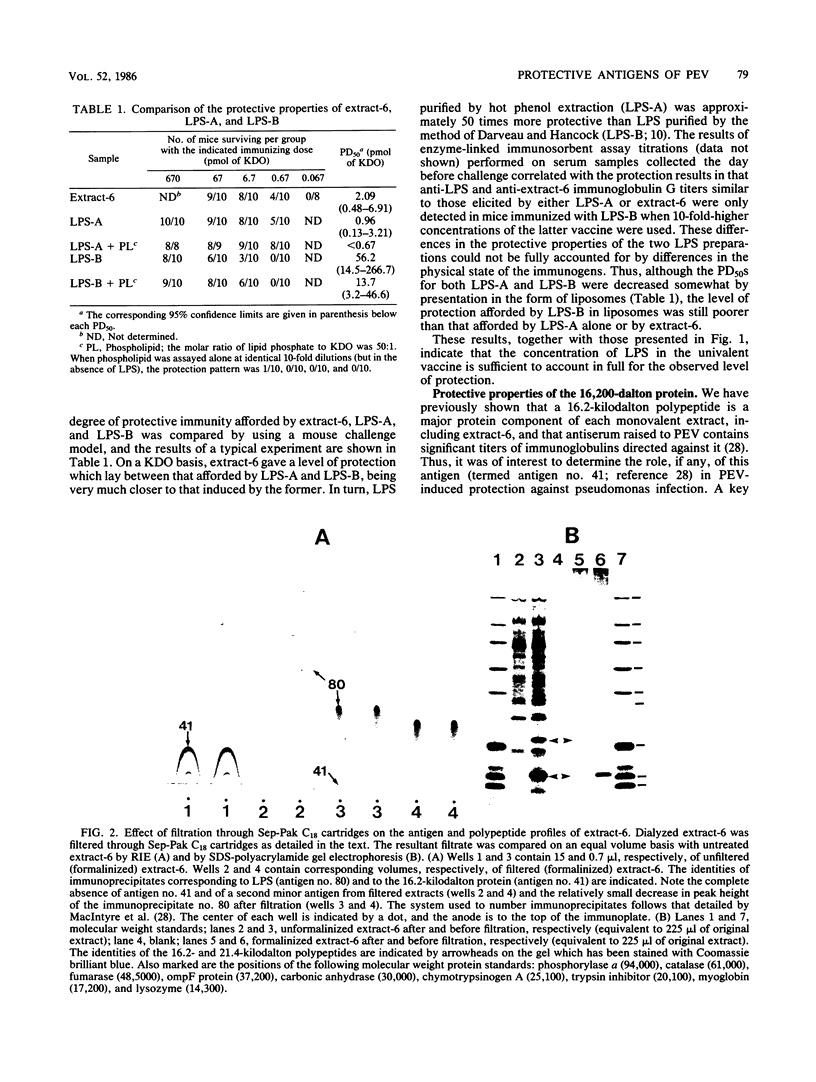
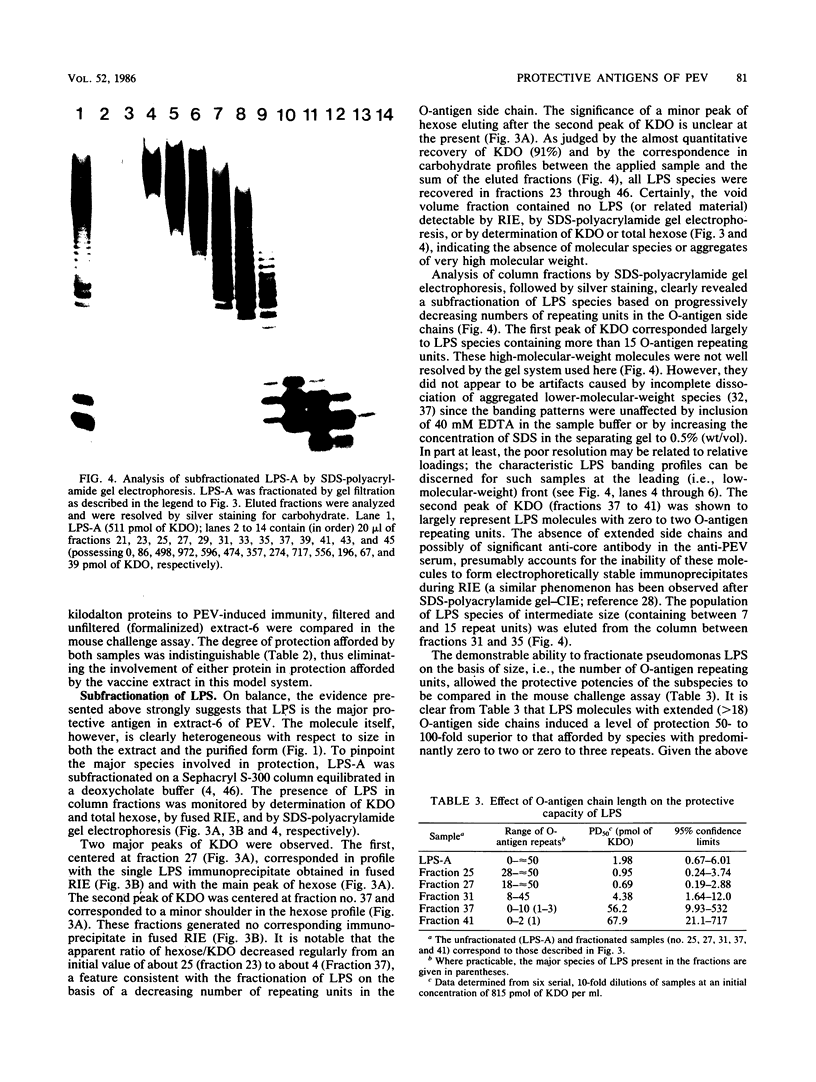
The nature of the protective antigen in one of the sixteen monovalent extracts (viz., extract-6) contributing to the pseudomonas polyvalent extract vaccine (PEV) was studied in a mouse challenge assay. Selective removal, by filtration through Sep-Pak C18 cartridges, of two major protein antigens with molecular weights of 16,200 and 21,000 had no effect on the protection afforded by extract-6. When analyzed on the basis of 2-keto-3-deoxyoctonate, lipopolysaccharide (LPS) purified by hot phenol extraction (LPS-A) from Pseudomonas aeruginosa (International Antigenic Typing System serotype 6) could account in full for the protective capacity of extract-6. Comparative analysis of LPS heterogeneity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by silver staining indicated that both extract-6 and LPS-A possessed similar spectra of smooth LPS molecules, containing between 10 and approximately equal to 50 O-antigen repeating units. Differences in the profiles of heterogeneity displayed by LPS in LPS-A and extract-6 were restricted to molecular species with short O-antigen chains. Subfractionation of LPS molecules on the basis of number of O-antigen repeating units was achieved by gel filtration in the presence of deoxycholate. Protection experiments performed on the subfractionated species of LPS-A revealed a relationship between O-antigen chain length and protective capacity; molecules with over 18 O-antigen repeating units being 50 to 100 times more protective than those with zero-two repeating units. The results indicate that most of the protection afforded by LPS-A and extract-6 can be accounted for by LPS molecules possessing extended (10 or more) O-antigen repeating units.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Bartell P. F. Determinants of the biologic activity of surface slime in experimental Pseudomonas aeruginosa infections. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S971–S978. doi: 10.1093/clinids/5.supplement_5.s971. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Buckley J. T., Halasa L. N., MacIntyre S. Purification and partial characterization of a bacterial phospholipid: cholesterol acyltransferase. J Biol Chem. 1982 Mar 25;257(6):3320–3325. [PubMed] [Google Scholar]
- Collins M. S., Roby R. E. Protective activity of an intravenous immune globulin (human) enriched in antibody against lipopolysaccharide antigens of Pseudomonas aeruginosa. Am J Med. 1984 Mar 30;76(3A):168–174. doi: 10.1016/0002-9343(84)90337-1. [DOI] [PubMed] [Google Scholar]
- Condon C., Cammack R., Patil D. S., Owen P. The succinate dehydrogenase of Escherichia coli. Immunochemical resolution and biophysical characterization of a 4-subunit enzyme complex. J Biol Chem. 1985 Aug 5;260(16):9427–9434. [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against Pseudomonas aeruginosa infection in a murine burn wound sepsis model by passive transfer of antitoxin A, antielastase, and antilipopolysaccharide. Infect Immun. 1983 Mar;39(3):1072–1079. doi: 10.1128/iai.39.3.1072-1079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against fatal Pseudomonas aeruginosa burn wound sepsis by immunization with lipopolysaccharide and high-molecular-weight polysaccharide. Infect Immun. 1984 Mar;43(3):795–799. doi: 10.1128/iai.43.3.795-799.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Meadow P. M., Fürer E., Germanier R. Protection against fatal Pseudomonas aeruginosa sepsis by immunization with smooth and rough lipopolysaccharides. Eur J Clin Microbiol. 1985 Apr;4(2):180–185. doi: 10.1007/BF02013594. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitracopoulos G., Bartell P. F. Slime glycolipoproteins and the pathogenicity of various strains of Pseudomonas aeruginosa in experimental infection. Infect Immun. 1980 Nov;30(2):402–408. doi: 10.1128/iai.30.2.402-408.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Parker M. G., Matthews J. M., Berg R. D. Use of a purified outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine in mice. Infect Immun. 1984 Apr;44(1):49–54. doi: 10.1128/iai.44.1.49-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Martin H. H. Phospholipid and lipopolysaccharide in Proteus mirabilis and its stable protoplast L-form. Difference in content and fatty acid composition. Eur J Biochem. 1976 Aug 16;67(2):487–494. doi: 10.1111/j.1432-1033.1976.tb10714.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Mouat E. C. Immunotherapeutic potential of monoclonal antibodies against Pseudomonas aeruginosa protein F. Eur J Clin Microbiol. 1985 Apr;4(2):224–227. doi: 10.1007/BF02013602. [DOI] [PubMed] [Google Scholar]
- Hedstrom R. C., Pavlovskis O. R., Galloway D. R. Antibody response of infected mice to outer membrane proteins of Pseudomonas aeruginosa. Infect Immun. 1984 Jan;43(1):49–53. doi: 10.1128/iai.43.1.49-53.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder I. A., Wheeler R., Montie T. C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect Immun. 1982 Jan;35(1):276–280. doi: 10.1128/iai.35.1.276-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. J. Antibody responses of burned patients immunized with a polyvalent Pseudomonas vaccine. J Hyg (Lond) 1979 Jun;82(3):453–462. doi: 10.1017/s0022172400053973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. J., Roe E. A., Lowbury E. J., Miler J. J., Spilsbury J. F. A new Pseudomonas vaccine: preliminary trial on human volunteers. J Hyg (Lond) 1976 Jun;76(3):429–439. doi: 10.1017/s0022172400055364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Knirel Y. A., Shashkov A. S., Dmitriev B. A., Kochetkov N. K. Structural studies of the Pseudomonas aeruginosa immunotype 1 antigen, containing the new sugar constituents 2-acetamido-2-deoxy-D-galacturonamide and 2-deoxy-2-formamido-D-galacturonic acid. Carbohydr Res. 1984 Oct 15;133(2):C12–C14. doi: 10.1016/0008-6215(84)85215-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacIntyre S., McVeigh T., Owen P. Immunochemical and biochemical analysis of the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986 Feb;51(2):675–686. doi: 10.1128/iai.51.2.675-686.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McManus A. T., Mason A. D., Jr, McManus W. F., Pruitt B. A., Jr Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur J Clin Microbiol. 1985 Apr;4(2):219–223. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- Miler J. M., Spilsbury J. F., Jones R. J., Roe E. A., Lowbury E. J. A new polyvalent Pseudomonas vaccine. J Med Microbiol. 1977 Feb;10(1):19–27. doi: 10.1099/00222615-10-1-19. [DOI] [PubMed] [Google Scholar]
- Mintz C. S., Apicella M. A., Morse S. A. Electrophoretic and serological characterization of the lipopolysaccharide produced by Neisseria gonorrhoeae. J Infect Dis. 1984 Apr;149(4):544–552. doi: 10.1093/infdis/149.4.544. [DOI] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Mutharia L. M., Nicas T. I., Hancock R. E. Outer membrane proteins of Pseudomonas aeruginosa serotype strains. J Infect Dis. 1982 Dec;146(6):770–779. doi: 10.1093/infdis/146.6.770. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Cohen M., Jennings H. Further purification and characterization of high-molecular-weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1983 Dec;42(3):936–941. doi: 10.1128/iai.42.3.936-941.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B. Immunochemistry of Pseudomonas aeruginosa lipopolysaccharides and high-molecular-weight polysaccharides. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S950–S956. doi: 10.1093/clinids/5.supplement_5.s950. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Pollack M., Cohen M. Immunochemical characterization of high-molecular-weight polysaccharide from Fisher immunotype 3 Pseudomonas aeruginosa. Infect Immun. 1984 Aug;45(2):309–313. doi: 10.1128/iai.45.2.309-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. High-molecular-weight polysaccharide antigen from Pseudomonas aeruginosa immunotype 2. Infect Immun. 1981 Nov;34(2):461–468. doi: 10.1128/iai.34.2.461-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Pier G. B., Prescott R. K. Immunization with Pseudomonas aeruginosa high-molecular-weight polysaccharides prevents death from Pseudomonas burn infections in mice. Infect Immun. 1984 Feb;43(2):759–760. doi: 10.1128/iai.43.2.759-760.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe E. A., Jones R. J. Immunization of burned patients against Pseudomonas aeruginosa infection at Safdarjang Hospital, New Delhi. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S922–S930. doi: 10.1093/clinids/5.supplement_5.s922. [DOI] [PubMed] [Google Scholar]
- Rowe P. S., Meadow P. M. Structure of the Core oligosaccharide from the lipopolysaccharide of Pseudomonas aeruginosa PAC1R and its defective mutants. Eur J Biochem. 1983 May 2;132(2):329–337. doi: 10.1111/j.1432-1033.1983.tb07366.x. [DOI] [PubMed] [Google Scholar]
- Sawada S., Suzuki M., Kawamura T., Fujinaga S., Masuho Y., Tomibe K. Protection against infection with Pseudomonas aeruginosa by passive transfer of monoclonal antibodies to lipopolysaccharides and outer membrane proteins. J Infect Dis. 1984 Oct;150(4):570–576. doi: 10.1093/infdis/150.4.570. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Chun P. W. The dispersion of gram-negative lipopolysaccharide by deoxycholate. Subunit molecular weight. J Biol Chem. 1980 Feb 10;255(3):1221–1226. [PubMed] [Google Scholar]