Abstract
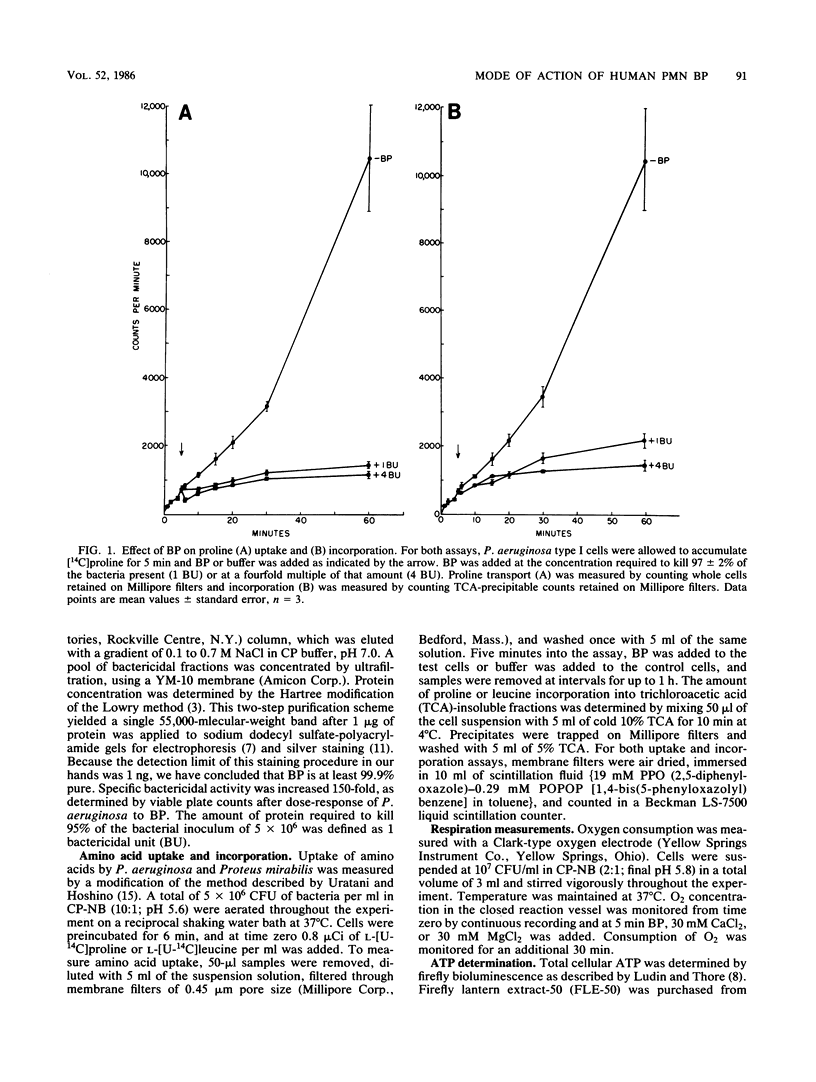
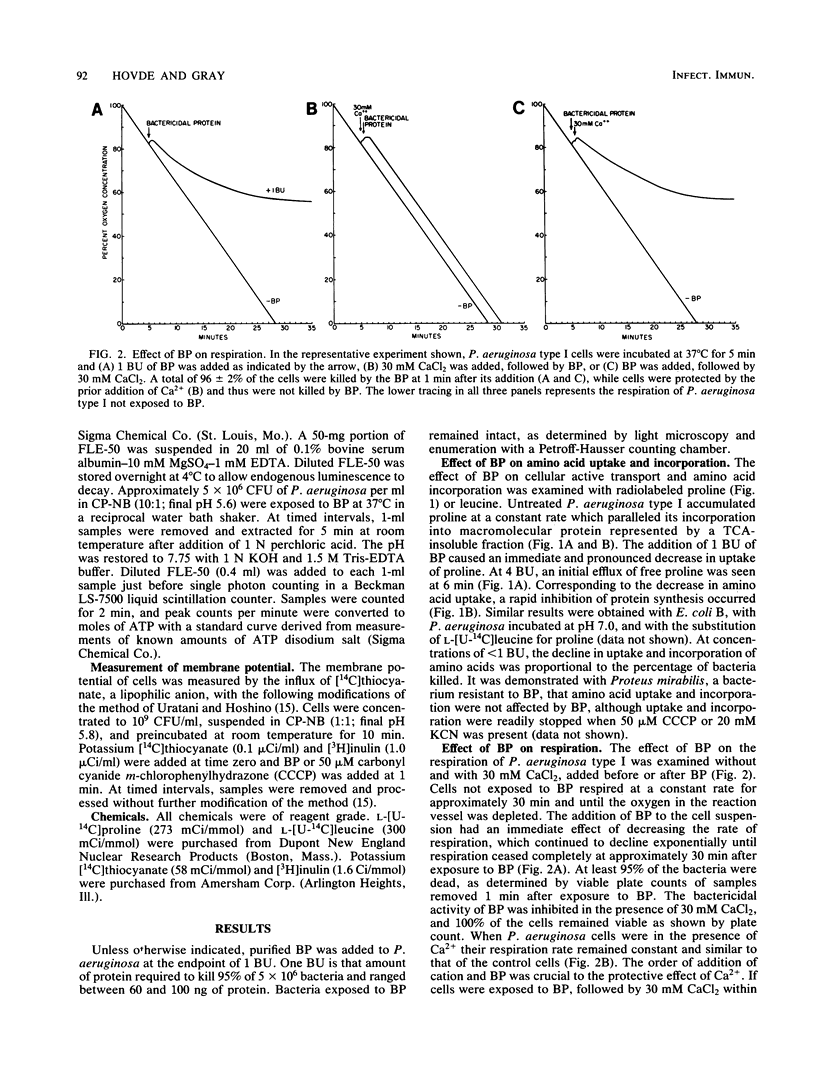
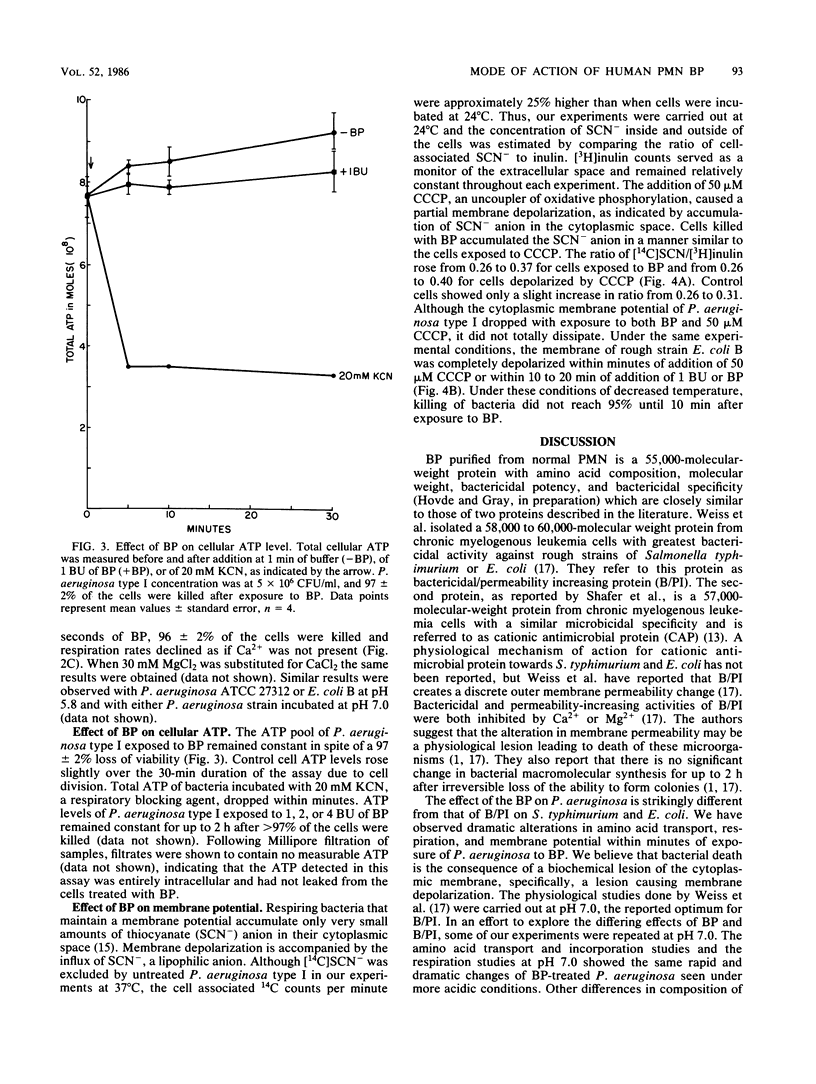
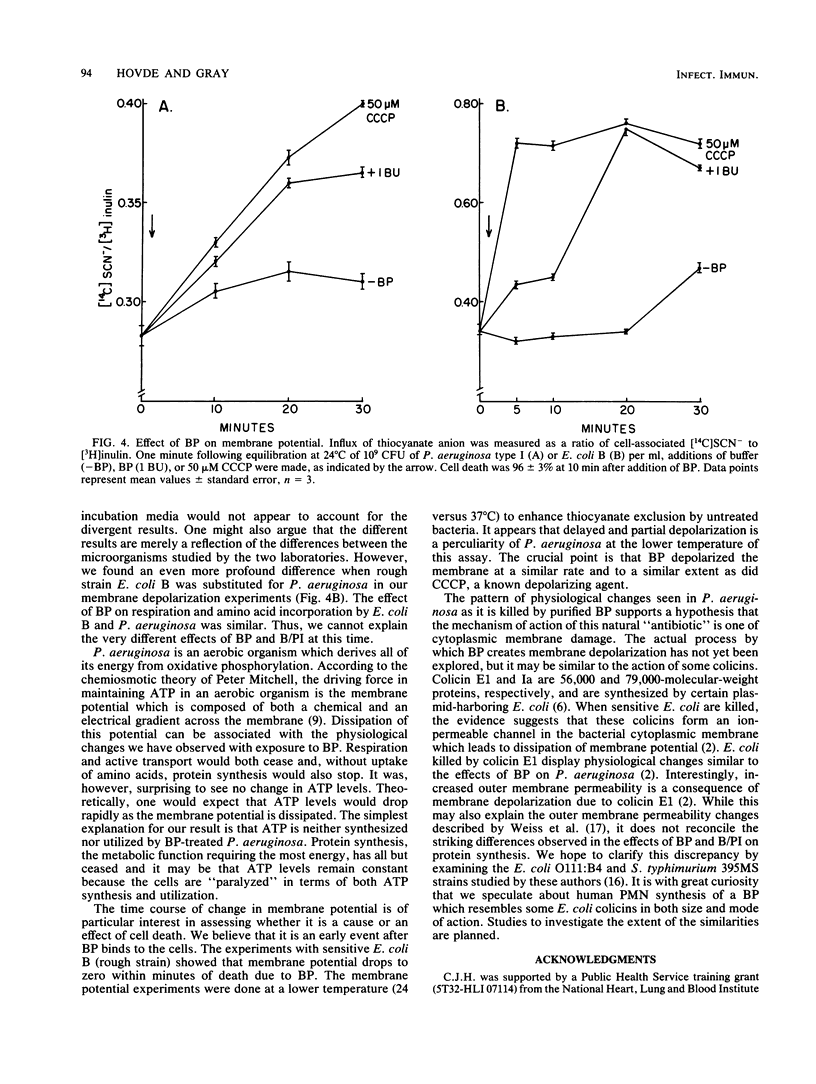
The physiological changes seen in Pseudomonas aeruginosa after exposure to a bactericidal protein (BP) from the granules of human polymorphonuclear leukocytes were studied. It was demonstrated, using radiolabeled proline or leucine, that both the rate of cellular uptake and amino acid incorporation into trichloroacetic acid-insoluble material were markedly decreased immediately after exposure to BP. The rate of O2 consumption by P. aeruginosa was decreased immediately after exposure to BP and continued to decline exponentially until it ceased completely 30 min after exposure to BP. In the presence of 30 mM CaCl2 or MgCl2, bacteria were protected from death due to BP and respiration rates were unaffected. The cellular ATP pool of P. aeruginosa remained constant for up to 2 h after exposure to BP. Membrane depolarization was measured by the influx of the lipophilic anion thiocyanate. It was shown that the cytoplasmic membrane of P. aeruginosa was partially depolarized after exposure to BP. Purified BP killed 95% of 5 X 10(6) CFU of P. aeruginosa at a concentration of 60 to 100 ng of protein per ml. Although the concentration of bacteria and BP varied with each type of experiment, the BP/bacteria ratio required to cause a 95 to 99% loss in viability remained constant. We propose that cytoplasmic membrane depolarization is the biochemical lesion responsible for the other physiological changes seen and ultimately for the death of P. aeruginosa induced by BP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elsbach P., Weiss J. A reevaluation of the roles of the O2-dependent and O2-independent microbicidal systems of phagocytes. Rev Infect Dis. 1983 Sep-Oct;5(5):843–853. doi: 10.1093/clinids/5.5.843. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cramer W. A. Studies on the depolarization of the Escherichia coli cell membrane by colicin E1. J Biol Chem. 1977 Aug 10;252(15):5491–5497. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundin A., Thore A. Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal Biochem. 1975 May 26;66(1):47–63. doi: 10.1016/0003-2697(75)90723-x. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The role of Pseudomonas aeruginosa in infections. J Antimicrob Chemother. 1983 May;11 (Suppl B):1–13. doi: 10.1093/jac/11.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984 Jul;45(1):29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K. Nonoxidative antimicrobial reactions of leukocytes. Contemp Top Immunobiol. 1984;14:283–343. doi: 10.1007/978-1-4757-4862-8_10. [DOI] [PubMed] [Google Scholar]
- Uratani Y., Hoshino T. Pyocin R1 inhibits active transport in Pseudomonas aeruginosa and depolarizes membrane potential. J Bacteriol. 1984 Feb;157(2):632–636. doi: 10.1128/jb.157.2.632-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]



