Abstract
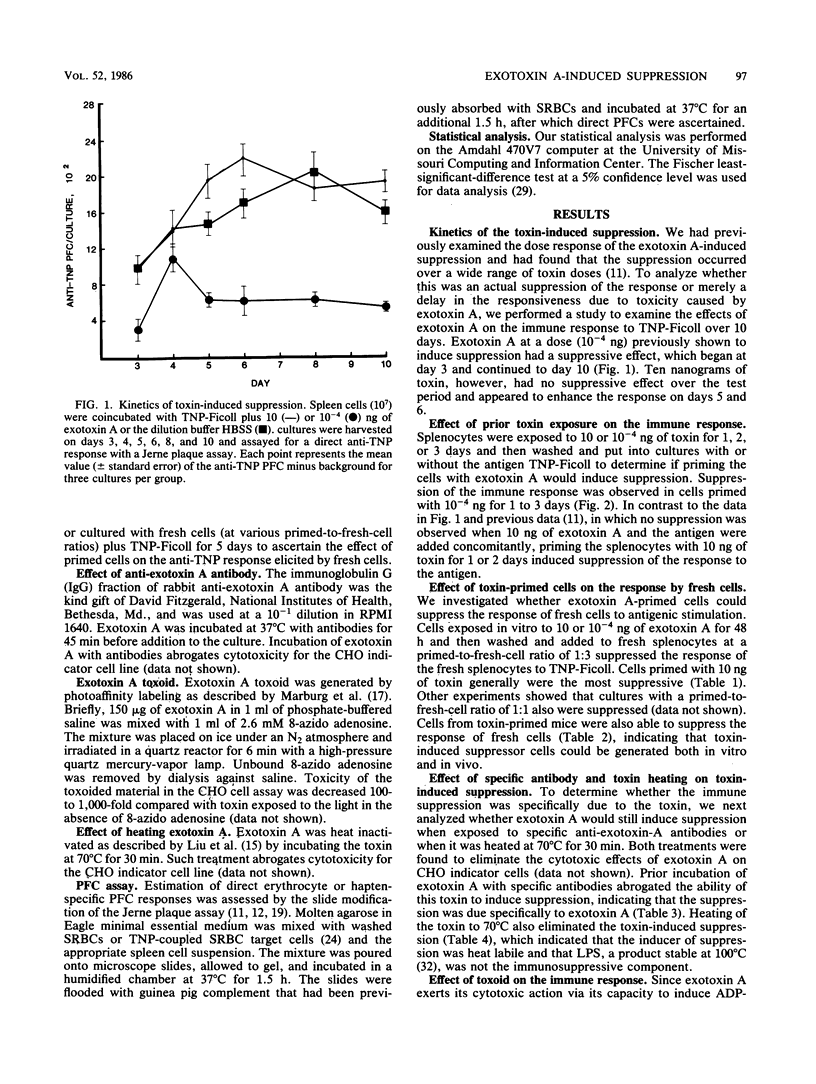
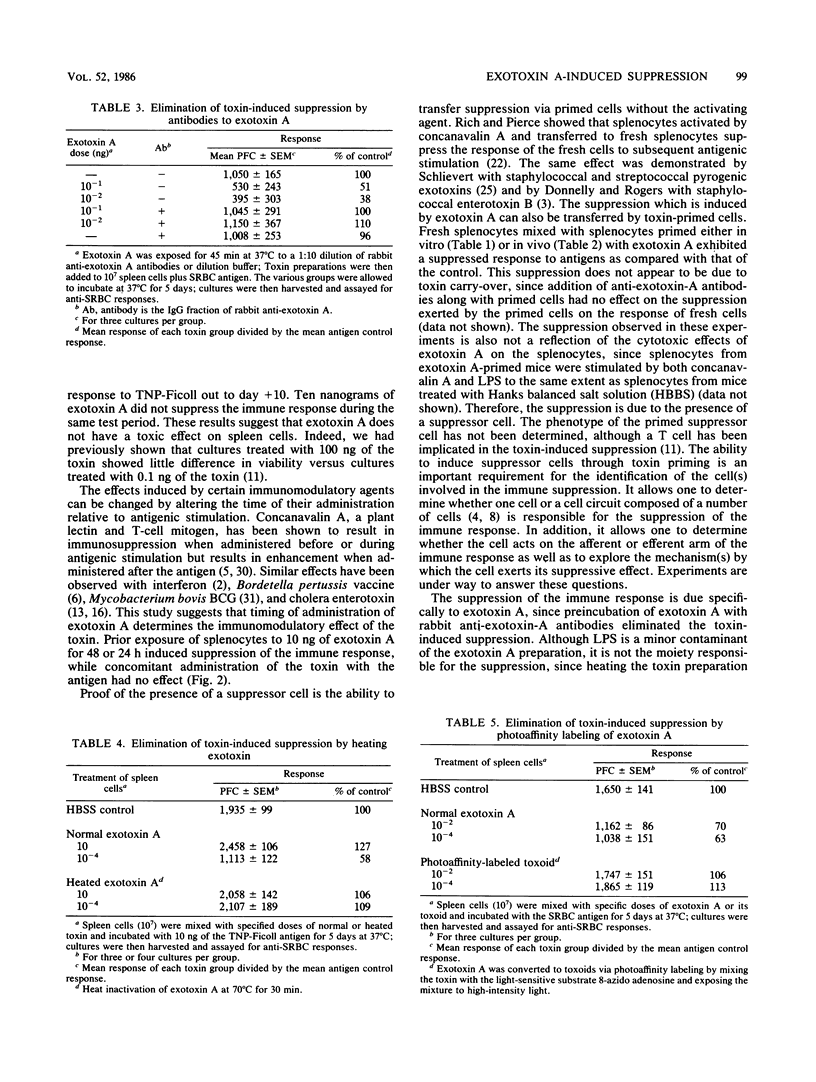
Pseudomonas exotoxin A has been shown previously to induce suppression of the murine immune response. In the present study, various parameters were examined which may have an effect on immunosuppression. The addition of 10(-4) ng of exotoxin A induced suppression of the immune response to trinitrophenylated Ficoll from days 3 to 10, while 10 ng of toxin exerted no suppressive effect over the same examination periods. When the toxin was administered 1 or 2 days before antigen stimulation, suppression of the response was observed with both 10 and 10(-4) ng. Priming splenocytes with toxin either in vivo or in vitro for 1 or 2 days suppressed the response of fresh cultured splenocytes to antigenic stimulation. Heated toxin, photoaffinity-labeled toxin, or preincubation of the toxin with rabbit anti-exotoxin A antiserum eliminated the toxin-induced suppression. These results suggest that Pseudomonas exotoxin A can generate multiple biological effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavaillon J. M., Leclerc C., Alouf J. E. Polyclonal antibody-forming cell activation and immunomodulation of the in vitro immune response induced by streptococcal extracellular products. Cell Immunol. 1983 Feb 15;76(1):200–206. doi: 10.1016/0008-8749(83)90362-3. [DOI] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J. Host genotype influences immunomodulation by interferon. Nature. 1980 Mar 13;284(5752):173–175. doi: 10.1038/284173a0. [DOI] [PubMed] [Google Scholar]
- Donnelly R. P., Rogers T. J. Immunosuppression induced by staphylococcal enterotoxin B. Cell Immunol. 1982 Sep 1;72(1):166–177. doi: 10.1016/0008-8749(82)90294-5. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. I. Characterization of the inhibitory cell activity. J Exp Med. 1972 Dec 1;136(6):1445–1460. doi: 10.1084/jem.136.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger H., Bartoschek M., Emmerling P. Time relationships between injection of antigen and adjuvant. II. Immunosuppression induced by Bordetella pertussis vaccine when given before secondary antigenic stimulation. Pathol Microbiol (Basel) 1972;38(2):93–102. [PubMed] [Google Scholar]
- Green D. R., Flood P. M., Gershon R. K. Immunoregulatory T-cell pathways. Annu Rev Immunol. 1983;1:439–463. doi: 10.1146/annurev.iy.01.040183.002255. [DOI] [PubMed] [Google Scholar]
- Hanna E. E., Watson D. W. Host-parasite relationships among group A streptococci. IV. Suppression of antibody response by streptococcal pyrogenic exotoxin. J Bacteriol. 1968 Jan;95(1):14–21. doi: 10.1128/jb.95.1.14-21.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. S., Misfeldt M. L. Alteration of murine immune response by Pseudomonas aeruginosa exotoxin A. Infect Immun. 1984 Jul;45(1):227–233. doi: 10.1128/iai.45.1.227-233.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. S., Misfeldt M. L. Induction of an immune response in athymic nude mice to thymus-dependent antigens by Pseudomonas aeruginosa exotoxin A. Cell Immunol. 1985 Oct 15;95(2):265–275. doi: 10.1016/0008-8749(85)90314-4. [DOI] [PubMed] [Google Scholar]
- Kateley J. R., Kasarov L., Friedman H. Modulation of in vivo antibody responses by cholera toxin. J Immunol. 1975 Jan;114(1 Pt 1):81–84. [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V., Yoshii S., Hsieh H. Exotoxins of Pseudomonas aeruginosa. II. Concentration, purification, and characterization of exotoxin A. J Infect Dis. 1973 Oct;128(4):514–519. doi: 10.1093/infdis/128.4.514. [DOI] [PubMed] [Google Scholar]
- Lyons S. F., Friedman H. Differential effects of cholera toxin pre-treatment on in vitro vs. in vivo immunocyte responses. Proc Soc Exp Biol Med. 1978 Apr;157(4):631–635. doi: 10.3181/00379727-157-40111. [DOI] [PubMed] [Google Scholar]
- Marburg S., Tolman R. L., Callahan L. T., 3rd Pseudomonas exotoxin A: toxoid preparation by photoaffinity inactivation. Proc Natl Acad Sci U S A. 1983 May;80(10):2870–2873. doi: 10.1073/pnas.80.10.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can J Microbiol. 1977 Feb;23(2):183–189. doi: 10.1139/m77-026. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Iglewski B. H., Pollack M. Mechanism of action of Pseudomonas aeruginosa exotoxin A in experimental mouse infections: adenosine diphosphate ribosylation of elongation factor 2. Infect Immun. 1978 Jan;19(1):29–33. doi: 10.1128/iai.19.1.29-33.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Anderson S. E., Jr Toxicity of Pseudomonas aeruginosa exotoxin A for human macrophages. Infect Immun. 1978 Mar;19(3):1092–1096. doi: 10.1128/iai.19.3.1092-1096.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M. Activation of murine T-suppressor lymphocytes by group A streptococcal and staphylococcal pyurogenic exotoxins. Infect Immun. 1980 Jun;28(3):876–880. doi: 10.1128/iai.28.3.876-880.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M., Schoettle D. J., Watson D. W. Nonspecific T-lymphocyte mitogenesis by pyrogenic exotoxins from group A streptococci and Staphylococcus aureus. Infect Immun. 1979 Sep;25(3):1075–1077. doi: 10.1128/iai.25.3.1075-1077.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Smith B. G., Johnson H. M. The effect of staphylococcal enterotoxins on the primary in vitro immune response. J Immunol. 1975 Aug;115(2):575–578. [PubMed] [Google Scholar]
- Taylor C. E., Stashak P. W., Caldes G., Prescott B., Fowlkes B. J., Baker P. J. Lectin-induced modulation of the antibody response to type III pneumococcal polysaccharide. Cell Immunol. 1984 Jan;83(1):26–33. doi: 10.1016/0008-8749(84)90221-1. [DOI] [PubMed] [Google Scholar]
- Turcotte R. Evidence for two distinct populations of suppressor cells in the spleens of Mycobacterium bovis BCG-Sensitized mice. Infect Immun. 1981 Nov;34(2):315–322. doi: 10.1128/iai.34.2.315-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


