Abstract
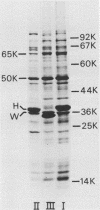
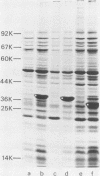
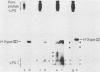
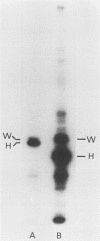
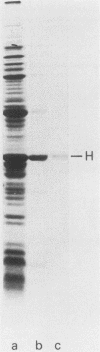
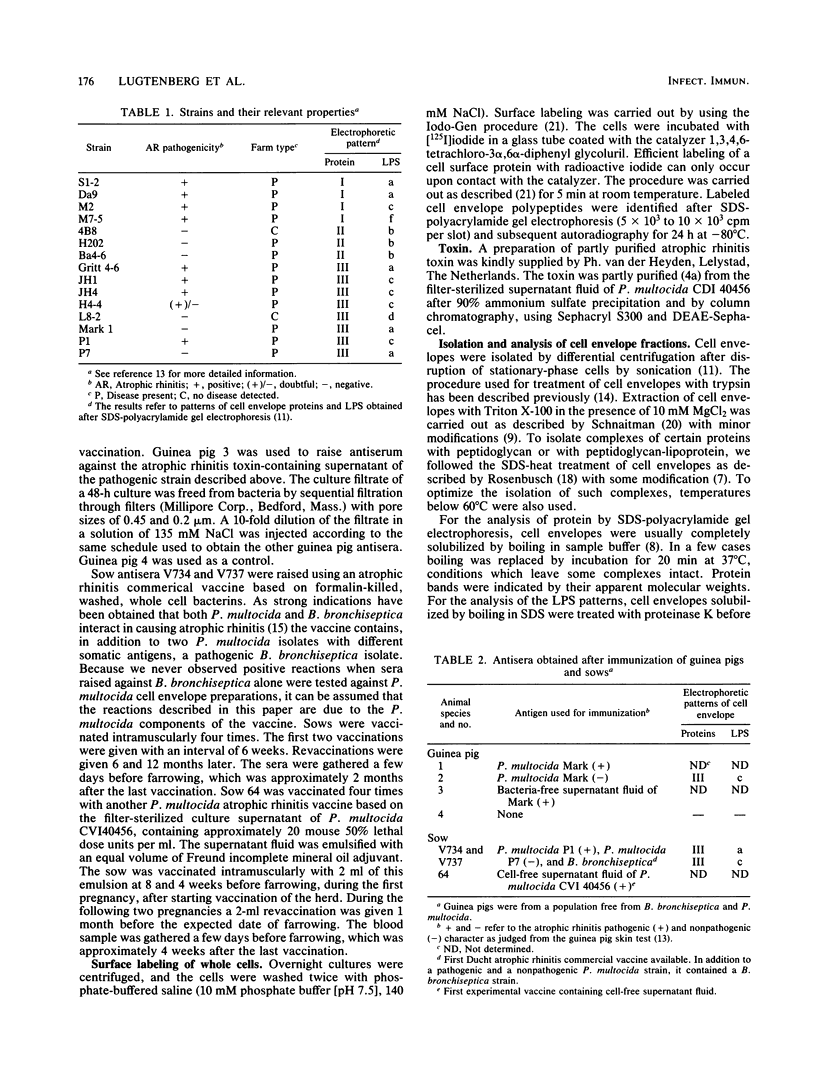
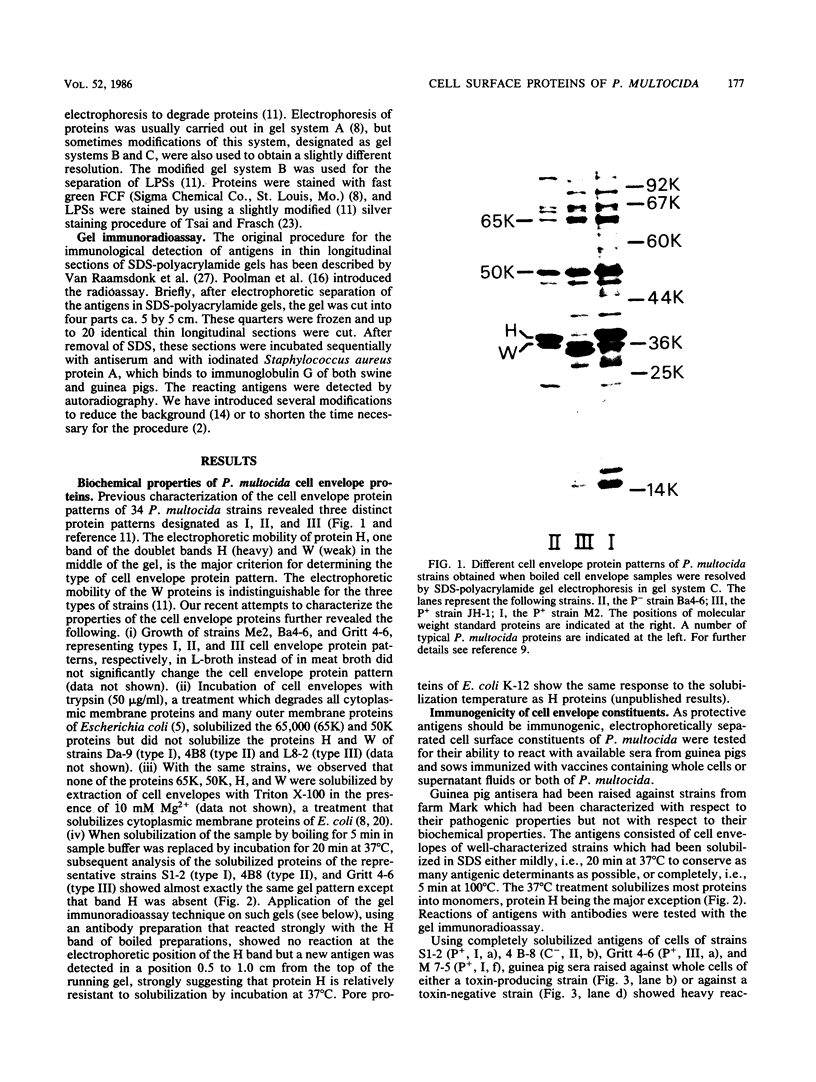
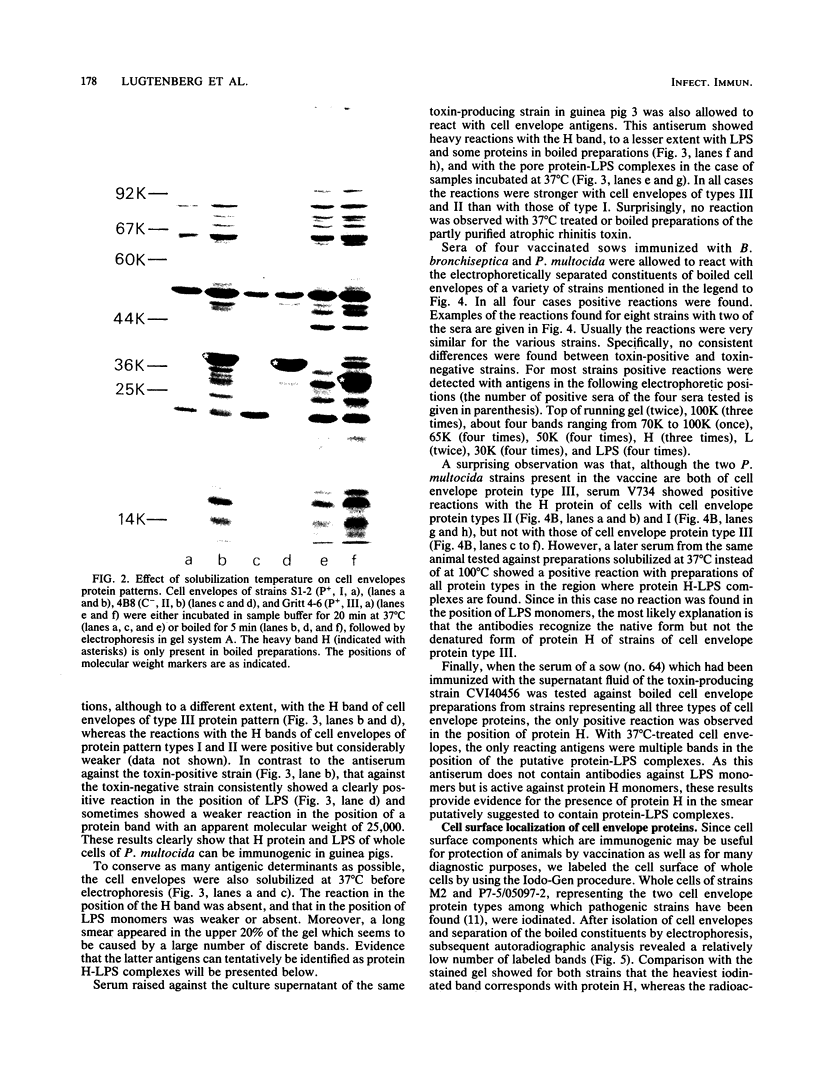
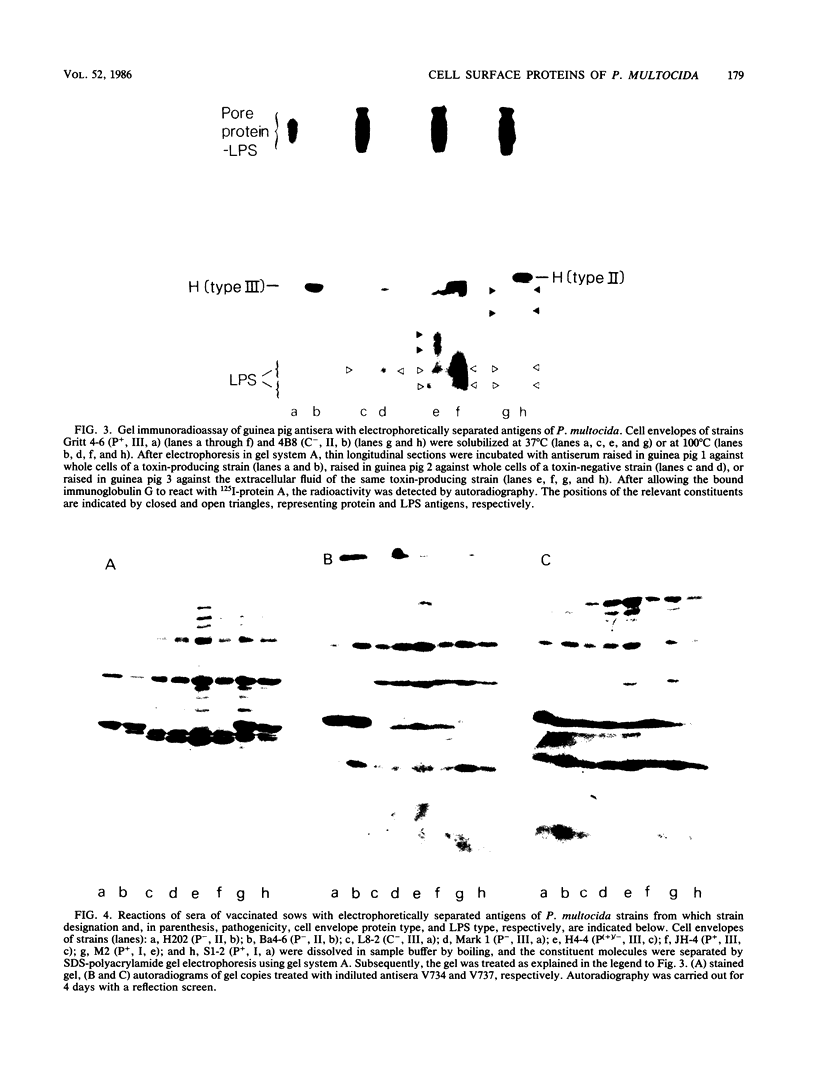
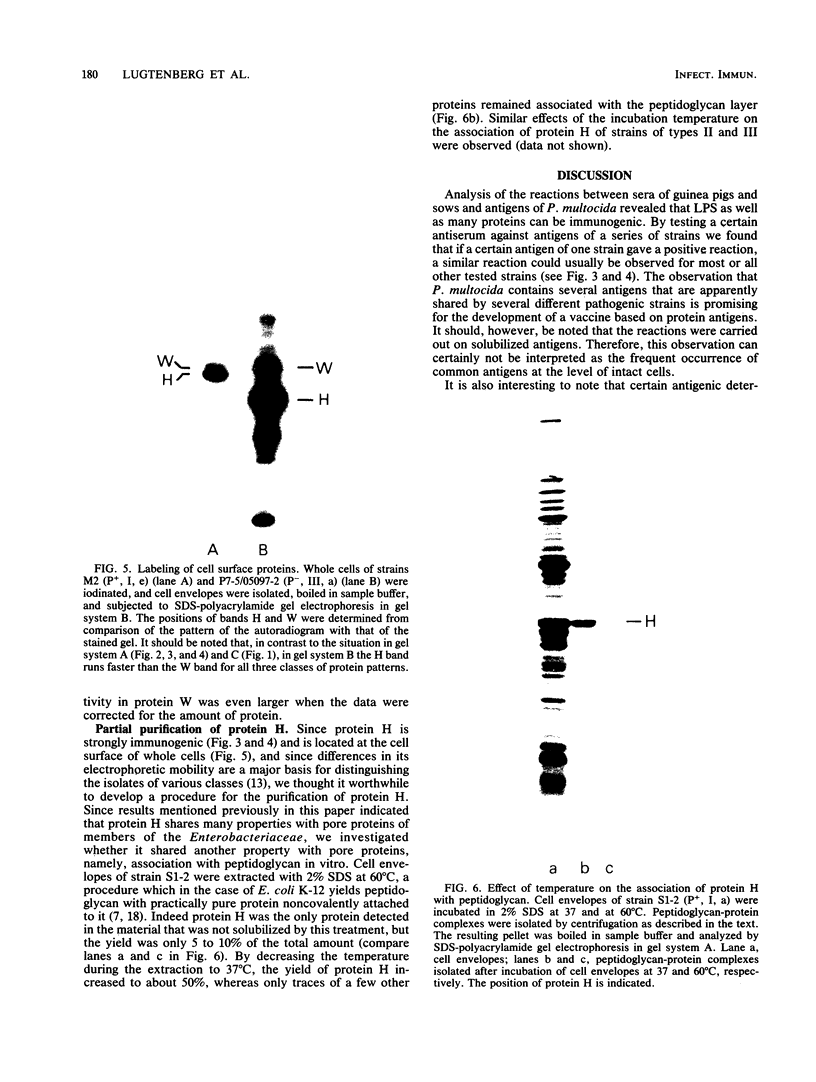
In a previous paper (B. Lugtenberg, R. van Boxtel, and M. de Jong, Infect. Immun., 46:48-54, 1984) we showed that among 34 isolates from swine the membrane protein and lipopolysaccharide (LPS) patterns, as analyzed by sodium dodecyl sulfate-gel electrophoresis, could be classified into three and six patterns, respectively. In all cases a certain LPS pattern was correlated with a certain protein pattern. Certain combinations of types of cell surface proteins and LPSs were correlated with pathogenicity, the latter property being judged by the guinea pig skin test. In the present paper the immunological and biochemical properties of cell surface constituents were analyzed. The reaction between electrophoretically separated cell surface constituents with guinea pig and sow antisera showed that LPS as well as several proteins were immunogenic. Among these is protein H, whose electrophoretic mobility is the main criterium for typing of cell envelope protein patterns. Protein H was the most heavily labeled component when whole cells were iodinated by the Iodo-Gen procedure showing its accessibility at the cell surface. These properties of protein H make it an attractive vaccine candidate. Further biochemical analyses revealed that protein H shares many properties with pore proteins of members of the family Enterobacteriaceae. One of these properties, association between pore proteins and peptidoglycan, was used as the basis for a simple procedure developed to partially purify protein H.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evenberg D., Versluis R., Lugtenberg B. Biochemical and immunological characterization of the cell surface of the fish pathogenic bacterium Aeromonas salmonicida. Biochim Biophys Acta. 1985 May 14;815(2):233–244. doi: 10.1016/0005-2736(85)90294-9. [DOI] [PubMed] [Google Scholar]
- Garavito R. M., Jenkins J., Jansonius J. N., Karlsson R., Rosenbusch J. P. X-ray diffraction analysis of matrix porin, an integral membrane protein from Escherichia coli outer membranes. J Mol Biol. 1983 Feb 25;164(2):313–327. doi: 10.1016/0022-2836(83)90079-7. [DOI] [PubMed] [Google Scholar]
- Garten W., Henning U. Cell envelope and shape of Escherichia coli K12. Isolation and preliminary characterization of the major ghost-membrane proteins. Eur J Biochem. 1974 Sep 1;47(2):343–352. doi: 10.1111/j.1432-1033.1974.tb03699.x. [DOI] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., van Boxtel R., de Jong M. Atrophic rhinitis in swine: correlation of Pasteurella multocida pathogenicity with membrane protein and lipopolysaccharide patterns. Infect Immun. 1984 Oct;46(1):48–54. doi: 10.1128/iai.46.1.48-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Major outer membrane proteins of Escherichia coli strains of human origin. J Gen Microbiol. 1980 Dec;121(2):373–380. doi: 10.1099/00221287-121-2-373. [DOI] [PubMed] [Google Scholar]
- Pedersen K. B., Barfod K. The aetiological significance of Bordetella bronchiseptica and Pasteurella multocida in atrophic rhinitis of swine. Nord Vet Med. 1981 Dec;33(12):513–522. [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunochemical characterization of Neisseria meningitidis serotype antigens by immunodiffusion and SDS-polyacrylamide gel electrophoresis immunoperoxidase techniques and the distribution of serotypes among cases and carriers. J Gen Microbiol. 1980 Feb;116(2):465–473. doi: 10.1099/00221287-116-2-465. [DOI] [PubMed] [Google Scholar]
- Prince G. H., Smith J. E. Antigenic studies on Pasteurella multocida using immunodiffusion techniques 3. Relationships between strains of Pasteurella multocida. J Comp Pathol. 1966 Jul;76(3):321–332. doi: 10.1016/0021-9975(66)90013-2. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. H., Williams R. P. Use of iodo-gen and iodine-125 to label the outer membrane proteins of whole cells of Neisseria gonorrhoeae. Anal Biochem. 1982 Mar 1;120(2):254–258. doi: 10.1016/0003-2697(82)90344-x. [DOI] [PubMed] [Google Scholar]
- Syuto B., Matsumoto M. Purification of a protective antigen from a saline extract of Pasteurella multocida. Infect Immun. 1982 Sep;37(3):1218–1226. doi: 10.1128/iai.37.3.1218-1226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wensink J., Witholt B. Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur J Biochem. 1981 May 15;116(2):331–335. doi: 10.1111/j.1432-1033.1981.tb05338.x. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Lugtenberg B., van Boxtel R., Hack A. M., Verhoef C., Havekes L. meoA is the structural gene for outer membrane protein c of Escherichia coli K12. Mol Gen Genet. 1979 Jan 31;169(2):147–155. doi: 10.1007/BF00271665. [DOI] [PubMed] [Google Scholar]
- van Raamsdonk W., Pool C. W., Heyting C. Detection of antigens and antibodies by an immuno-peroxidase method applied on thin longitudinal sections of SDS-polyacrylamide gels. J Immunol Methods. 1977;17(3-4):337–348. doi: 10.1016/0022-1759(77)90116-8. [DOI] [PubMed] [Google Scholar]
- van der Ley P., Amesz H., Tommassen J., Lugtenberg B. Monoclonal antibodies directed against the cell-surface-exposed part of PhoE pore protein of the Escherichia coli K-12 outer membrane. Eur J Biochem. 1985 Mar 1;147(2):401–407. doi: 10.1111/j.1432-1033.1985.tb08764.x. [DOI] [PubMed] [Google Scholar]