Abstract
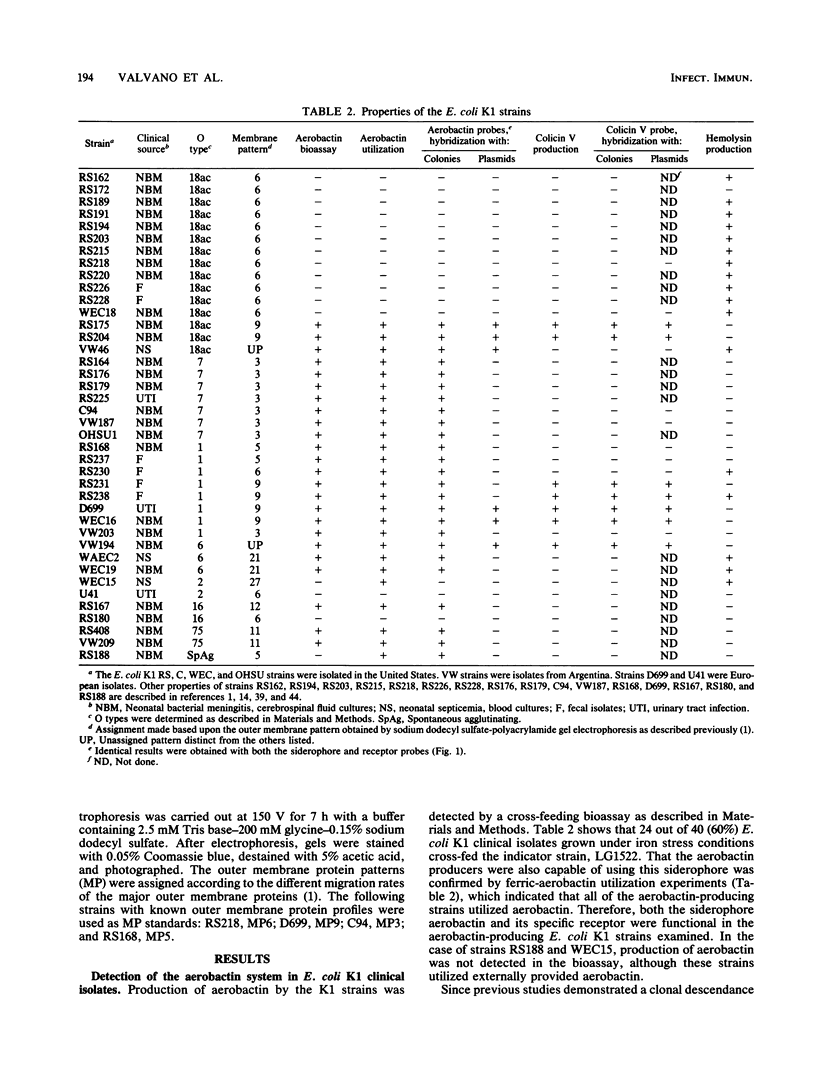
The incidence of the aerobactin system and the genetic location of aerobactin genes were investigated in Escherichia coli K1 neonatal isolates belonging to different clonal groups. A functional aerobactin system was found in all members of the O7 MP3, O1 MP5, O1 MP9, and O18 MP9 clonal groups examined and also in K1 strains having O6, O16, and O75 lipopolysaccharide types, which are less frequently associated with neonatal infections. In contrast, the aerobactin system was not detected in strains from the O18 MP6 clone. The combined results of plasmid and colony hybridization experiments showed that the aerobactin genes were located on the chromosome in the majority (75%) of the aerobactin-producing K1 isolates, the genetic location of the aerobactin genes was closely correlated with the outer membrane protein profile rather than the O lipopolysaccharide type, the K1 strains harboring a chromosome-mediated aerobactin system did not possess colicin V genes, and five of six K1 isolates possessing a plasmid-borne aerobactin system contained colicin V genes which were located on the same plasmids carrying the aerobactin genes. The comparison of hemolysin production with possession of the aerobactin system in virulent clones of E. coli K1 strains showed that all of the aerobactin-producing strains from the O18 MP9 and O7 MP3 clonal groups did not synthesize hemolysin, whereas 11 of 12 aerobactin-nonproducing O18 MP6 isolates were hemolytic. Of the K1 strains examined, 92.5% possessed either the aerobactin system or the ability to produce hemolysin or both.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Aerobactin genes in clinical isolates of Escherichia coli. J Bacteriol. 1985 Feb;161(2):727–735. doi: 10.1128/jb.161.2.727-735.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Cloning of the aerobactin-mediated iron assimilation system of plasmid ColV. J Bacteriol. 1983 Feb;153(2):1111–1113. doi: 10.1128/jb.153.2.1111-1113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Carbonetti N. H., Williams P. H. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun. 1984 Oct;46(1):7–12. doi: 10.1128/iai.46.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Schiewe M. H., Falkow S. Evidence for plasmid contribution to the virulence of fish pathogen Vibrio anguillarum. Infect Immun. 1977 Nov;18(2):509–513. doi: 10.1128/iai.18.2.509-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. The relationship of plasmid-mediated iron transport and bacterial virulence. Annu Rev Microbiol. 1984;38:69–89. doi: 10.1146/annurev.mi.38.100184.000441. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Gemski P., Sadoff J. C., Orskov F., Orskov I. The importance of the K1 capsule in invasive infections caused by Escherichia coli. J Infect Dis. 1984 Feb;149(2):184–193. doi: 10.1093/infdis/149.2.184. [DOI] [PubMed] [Google Scholar]
- Dietzman D. E., Fischer G. W., Schoenknecht F. D. Neonatal Escherichia coli septicemia--bacterial counts in blood. J Pediatr. 1974 Jul;85(1):128–130. doi: 10.1016/s0022-3476(74)80308-2. [DOI] [PubMed] [Google Scholar]
- Frick K. K., Quackenbush R. L., Konisky J. Cloning of immunity and structural genes for colicin V. J Bacteriol. 1981 Nov;148(2):498–507. doi: 10.1128/jb.148.2.498-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode M. P., Sutton A., Moxon E. R., Robbins J. B. Pathogenesis of neonatal Escherichia coli meningitis: induction of bacteremia and meningitis in infant rats fed E. coli K1. Infect Immun. 1977 Apr;16(1):75–80. doi: 10.1128/iai.16.1.75-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley N. D., Humphreys G. O., Anderson E. S. Molecular studies of R factor compatibility groups. J Bacteriol. 1973 Jul;115(1):387–398. doi: 10.1128/jb.115.1.387-398.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. J., Cheasty T., Rowe B. Isolation of bacteriophages specific for the K1 polysaccharide antigen of Escherichia coli. J Clin Microbiol. 1977 Dec;6(6):548–550. doi: 10.1128/jcm.6.6.548-550.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaijser B. Immunology of Escherichia coli: K antigen and its relation to urinary-tract infection. J Infect Dis. 1973 Jun;127(6):670–677. doi: 10.1093/infdis/127.6.670. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone W. J., Luirink J., Koningstein G., Oudega B., de Graaf F. K. Subcloning of the cloacin DF13/aerobactin receptor protein and identification of a pColV-K30-determined polypeptide involved in ferric-aerobactin uptake. J Bacteriol. 1983 Nov;156(2):945–948. doi: 10.1128/jb.156.2.945-948.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G. A rapid slide-agglutination method for typing pneumococci by means of specific antibody adsorbed to protein A-containing staphylococci. J Med Microbiol. 1973 May;6(2):187–190. doi: 10.1099/00222615-6-2-187. [DOI] [PubMed] [Google Scholar]
- Lawlor K. M., Payne S. M. Aerobactin genes in Shigella spp. J Bacteriol. 1984 Oct;160(1):266–272. doi: 10.1128/jb.160.1.266-272.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. J. Transferable drug resistance and other transferable agents in strains of Escherichia coli from two human populations. Lancet. 1968 Jun 29;1(7557):1389–1393. doi: 10.1016/s0140-6736(68)91973-9. [DOI] [PubMed] [Google Scholar]
- Linggood M. A., Ingram P. L. The role of alpha haemolysin in the virulence of Escherichia coli for mice. J Med Microbiol. 1982 Feb;15(1):23–30. doi: 10.1099/00222615-15-1-23. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- McDougall S., Neilands J. B. Plasmid- and chromosome-coded aerobactin synthesis in enteric bacteria: insertion sequences flank operon in plasmid-mediated systems. J Bacteriol. 1984 Jul;159(1):300–305. doi: 10.1128/jb.159.1.300-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer A. A., Morelli G., Heuzenroeder M., Kamke M., Achtman M. Conservation of plasmids among Escherichia coli K1 isolates of diverse origins. Infect Immun. 1984 Dec;46(3):649–657. doi: 10.1128/iai.46.3.649-657.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Moseley S. L., Huq I., Alim A. R., So M., Samadpour-Motalebi M., Falkow S. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J Infect Dis. 1980 Dec;142(6):892–898. doi: 10.1093/infdis/142.6.892. [DOI] [PubMed] [Google Scholar]
- Nimmich W., Zingler G. Biochemical characteristics, phage patterns, and O1 factor analysis of Escherichia coli O1:K1:H7:F11 and O1:K1:H-:F9 strains isolated from patients with urinary tract infections. Med Microbiol Immunol. 1984;173(2):75–85. doi: 10.1007/BF02124821. [DOI] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Evidence for clonal population structure in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(1):198–201. doi: 10.1073/pnas.81.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M. Synthesis and utilization of siderophores by Shigella flexneri. J Bacteriol. 1980 Sep;143(3):1420–1424. doi: 10.1128/jb.143.3.1420-1424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Casal J. F., Crosa J. H. Aerobactin iron uptake sequences in plasmid ColV-K30 are flanked by inverted IS1-like elements and replication regions. J Bacteriol. 1984 Oct;160(1):256–265. doi: 10.1128/jb.160.1.256-265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Achtman M. Degree of antibody-independent activation of the classical complement pathway by K1 Escherichia coli differs with O antigen type and correlates with virulence of meningitis in newborns. Infect Immun. 1984 Feb;43(2):684–692. doi: 10.1128/iai.43.2.684-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mercer A., Kusećek B., Pohl A., Achtman M. Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infect Immun. 1983 Feb;39(2):599–608. doi: 10.1128/iai.39.2.599-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Schiffer M. S., Oliveira E., Glode M. P., McCracken G. H., Jr, Sarff L. M., Robbins J. B. A review: relation between invasiveness and the K1 capsular polysaccharide of Escherichia coli. Pediatr Res. 1976 Feb;10(2):82–87. doi: 10.1203/00006450-197602000-00002. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Aaronson W., Sutton A., Schneerson R. Comparative analysis of plasmids and some metabolic characteristics of Escherichia coli K1 from diseased and healthy individuals. Infect Immun. 1980 Jul;29(1):200–206. doi: 10.1128/iai.29.1.200-206.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stuart S. J., Greenwood K. T., Luke R. K. Hydroxamate-mediated transport of iron controlled by ColV plasmids. J Bacteriol. 1980 Jul;143(1):35–42. doi: 10.1128/jb.143.1.35-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano M. A., Crosa J. H. Aerobactin iron transport genes commonly encoded by certain ColV plasmids occur in the chromosome of a human invasive strain of Escherichia coli K1. Infect Immun. 1984 Oct;46(1):159–167. doi: 10.1128/iai.46.1.159-167.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner P. J., Williams P. H., Bindereif A., Neilands J. B. ColV plasmid-specific aerobactin synthesis by invasive strains of Escherichia coli. Infect Immun. 1981 Aug;33(2):540–545. doi: 10.1128/iai.33.2.540-545.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Frick K., Neilands J. B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976 Apr;126(1):7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Warner P. J. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect Immun. 1980 Aug;29(2):411–416. doi: 10.1128/iai.29.2.411-416.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]