Abstract
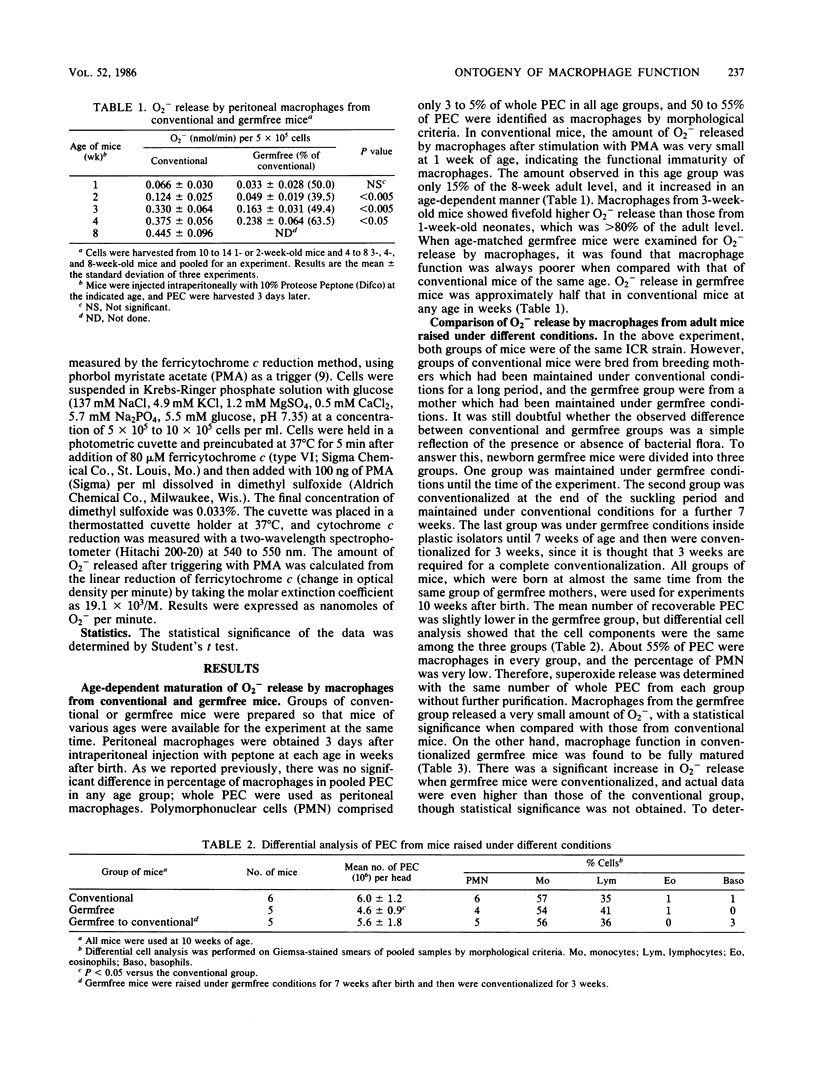
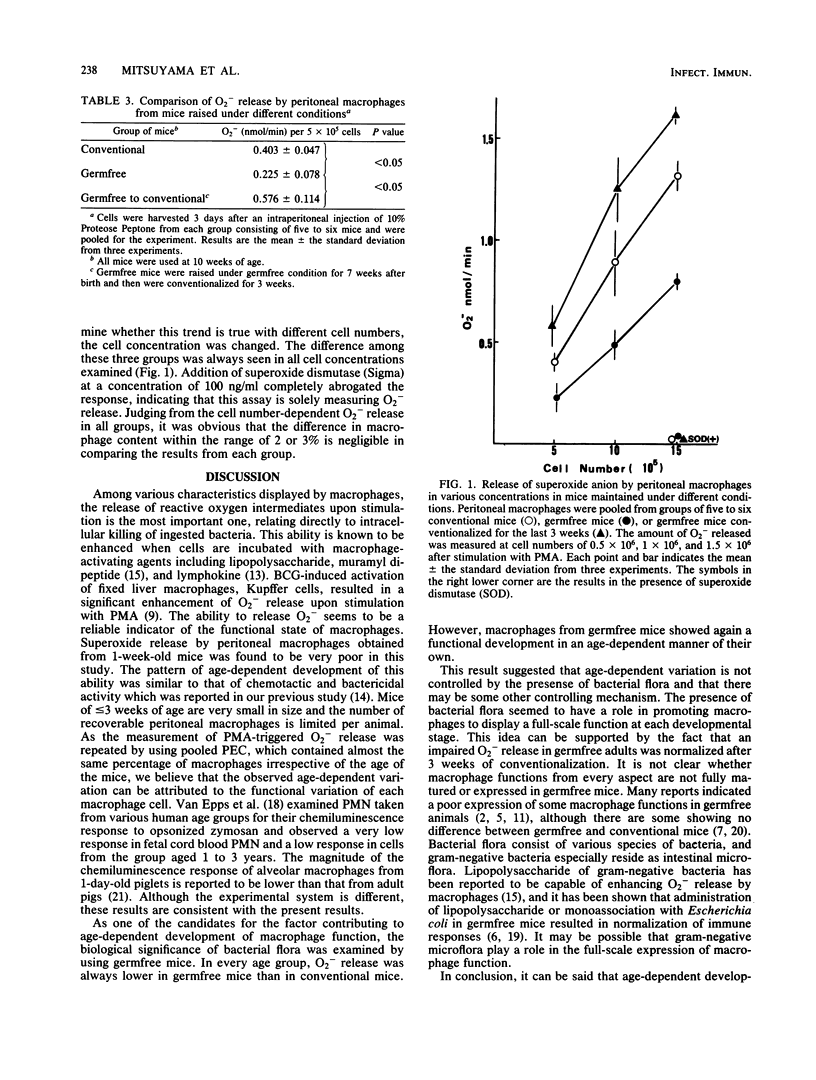
To determine whether the presence of bacterial flora contributes to the ontogenic development of macrophage function, the ability of macrophages to release superoxide anion (O2-) in response to stimulation with phorbol myristate acetate was compared in conventional and germfree mice of various ages after birth. One-week-old conventional mice showed a very low level of O2- release by their macrophages, and gradual increases were observed in 2-, 3-, and 4-week-old mice in an age-dependent manner. Macrophages from germfree mice always showed a significantly lower level of O2- release compared with conventional mice of the same age; however, age-dependent functional development was seen also in the germfree group. The poor level of O2- release by macrophages from adult germfree mice could be restored to more than the level by conventional mice when the mice were conventionalized for 3 weeks. These results suggested that the ontogenic development of macrophage function is not controlled by the presence of bacterial flora but that the full-scale expression of function at each age is under the influence of microflora.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., Challacombe D. N., Richardson J. M. The bacterial flora of the upper gastrointestinal tract in children both in health and disease. Soc Appl Bacteriol Symp Ser. 1974;3(0):197–203. [PubMed] [Google Scholar]
- Fornůsek L., Vetvicka V., Jarosková L., Stepánková R. Some properties of the plasma membrane of macrophages from germ-free rats. J Reticuloendothel Soc. 1983 Oct;34(4):331–340. [PubMed] [Google Scholar]
- Hardy B., Globerson A., Danon D. Ontogenic development of the reactivity of macrophages to antigenic stimulation. Cell Immunol. 1973 Nov;9(2):282–288. doi: 10.1016/0008-8749(73)90079-8. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Johnson W. J., Balish E. Macrophage function in germ-free, athymic (nu/nu), and conventional-flora (nu/+) mice. J Reticuloendothel Soc. 1980 Jul;28(1):55–66. [PubMed] [Google Scholar]
- Kiyono H., McGhee J. R., Michalek S. M. Lipopolysaccharide regulation of the immune response: comparison of responses to LPS in germfree, Escherichia coli-monoassociated and conventional mice. J Immunol. 1980 Jan;124(1):36–41. [PubMed] [Google Scholar]
- Knook D. L., Barkway C., Sleyster E. C. Lysosomal enzyme content of Kupffer and endothelial liver cells isolated from germfree and clean conventional rats. Infect Immun. 1981 Aug;33(2):620–622. doi: 10.1128/iai.33.2.620-622.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. Y., Unanue E. R. Ontogeny of murine macrophages: functions related to antigen presentation. Infect Immun. 1982 Apr;36(1):169–175. doi: 10.1128/iai.36.1.169-175.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo S., Nakagawara A., Ikeda K., Mitsuyama M., Nomoto K. Enhanced release of reactive oxygen intermediates by immunologically activated rat Kupffer cells. Clin Exp Immunol. 1985 Jan;59(1):203–209. [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Mørland B., Midtvedt T. Phagocytosis, peritoneal influx, and enzyme activities in peritoneal macrophages from germfree, conventional, and ex-germfree mice. Infect Immun. 1984 Jun;44(3):750–752. doi: 10.1128/iai.44.3.750-752.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., DeSantis N. M., Nogueira N., Nathan C. F. Lymphokines enhance the capacity of human monocytes to secret reactive oxygen intermediates. J Clin Invest. 1982 Nov;70(5):1042–1048. doi: 10.1172/JCI110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara R., Mitsuyama M., Miyata M., Nomoto K. Ontogeny of macrophage-mediated protection against Listeria monocytogenes. Infect Immun. 1985 Jun;48(3):763–768. doi: 10.1128/iai.48.3.763-768.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Goodwin J. S., Murphy S. Age-dependent variations in polymorphonuclear leukocyte chemiluminescence. Infect Immun. 1978 Oct;22(1):57–61. doi: 10.1128/iai.22.1.57-61.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemuehler M. J., Kiyono H., Babb J. L., Michalek S. M., McGhee J. R. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J Immunol. 1982 Sep;129(3):959–965. [PubMed] [Google Scholar]
- Woodward B. A study of the influence of the ambient microflora on the structure of lung alveolar macrophages and an ultrastructural comparison of lung and peritoneal macrophages in germ-free and conventionally reared mice. J Morphol. 1981 Sep;169(3):283–291. doi: 10.1002/jmor.1051690304. [DOI] [PubMed] [Google Scholar]
- Zeidler R. B., Kim H. D. Phagocytosis, chemiluminescence, and cell volume of alveolar macrophages from neonatal and adult pigs. J Leukoc Biol. 1985 Jan;37(1):29–43. doi: 10.1002/jlb.37.1.29. [DOI] [PubMed] [Google Scholar]


